Sea Cucumber Peptide Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium by Alleviating Gut Microbiota Imbalance and Regulating miR-155/SOCS1 Axis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sea Cucumber Peptide
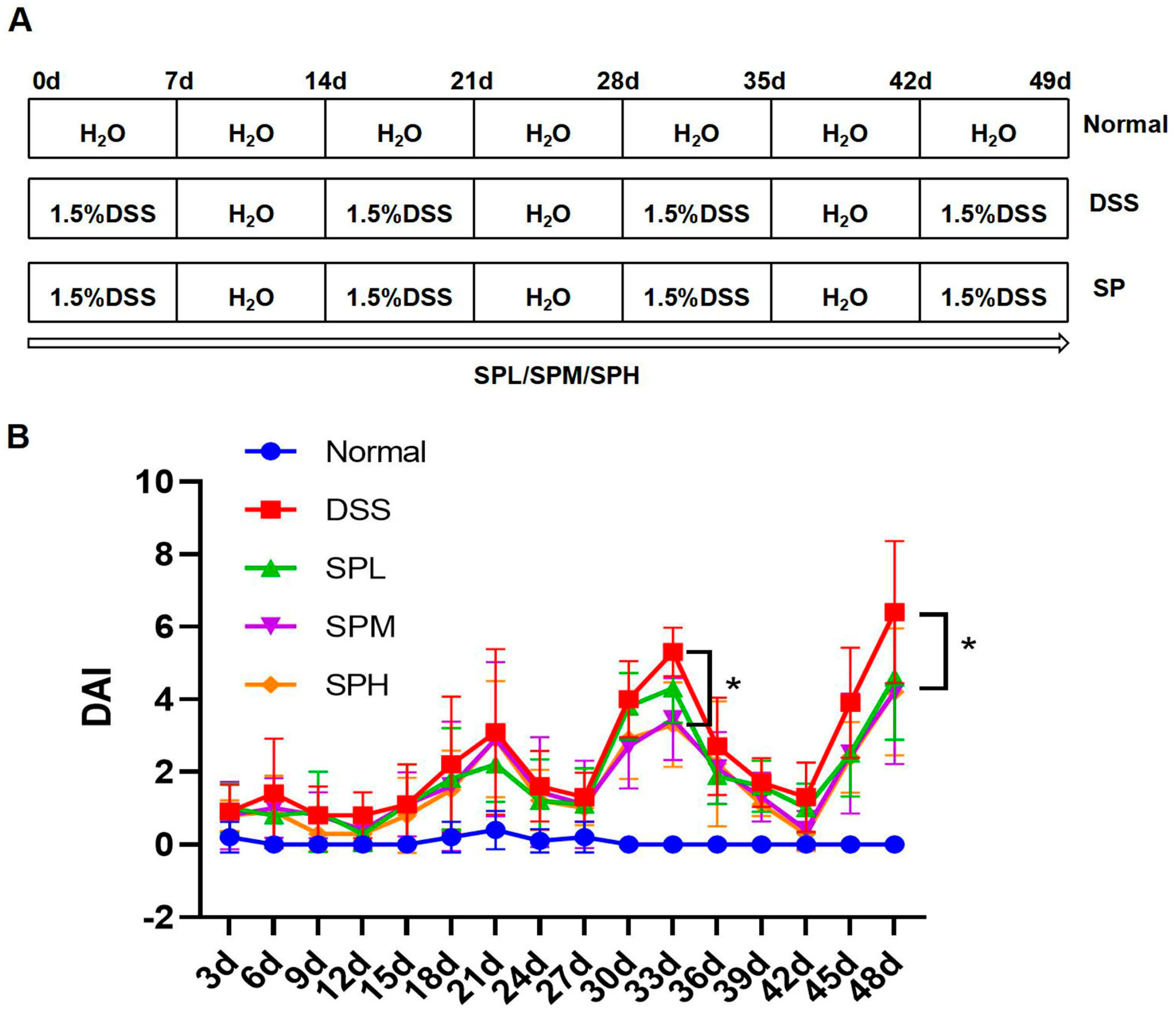
2.2. Animals and Experimental Design
2.3. ELISA Assay
2.4. Determination of SCFA Concentration
2.5. Histopathological Examination
2.6. RT-PCR
2.7. Western Blot
2.8. High-Throughput Sequencing of Gut Microbiota
2.9. Statistical Analysis
3. Results
3.1. SP Alleviated DSS-Induced UC
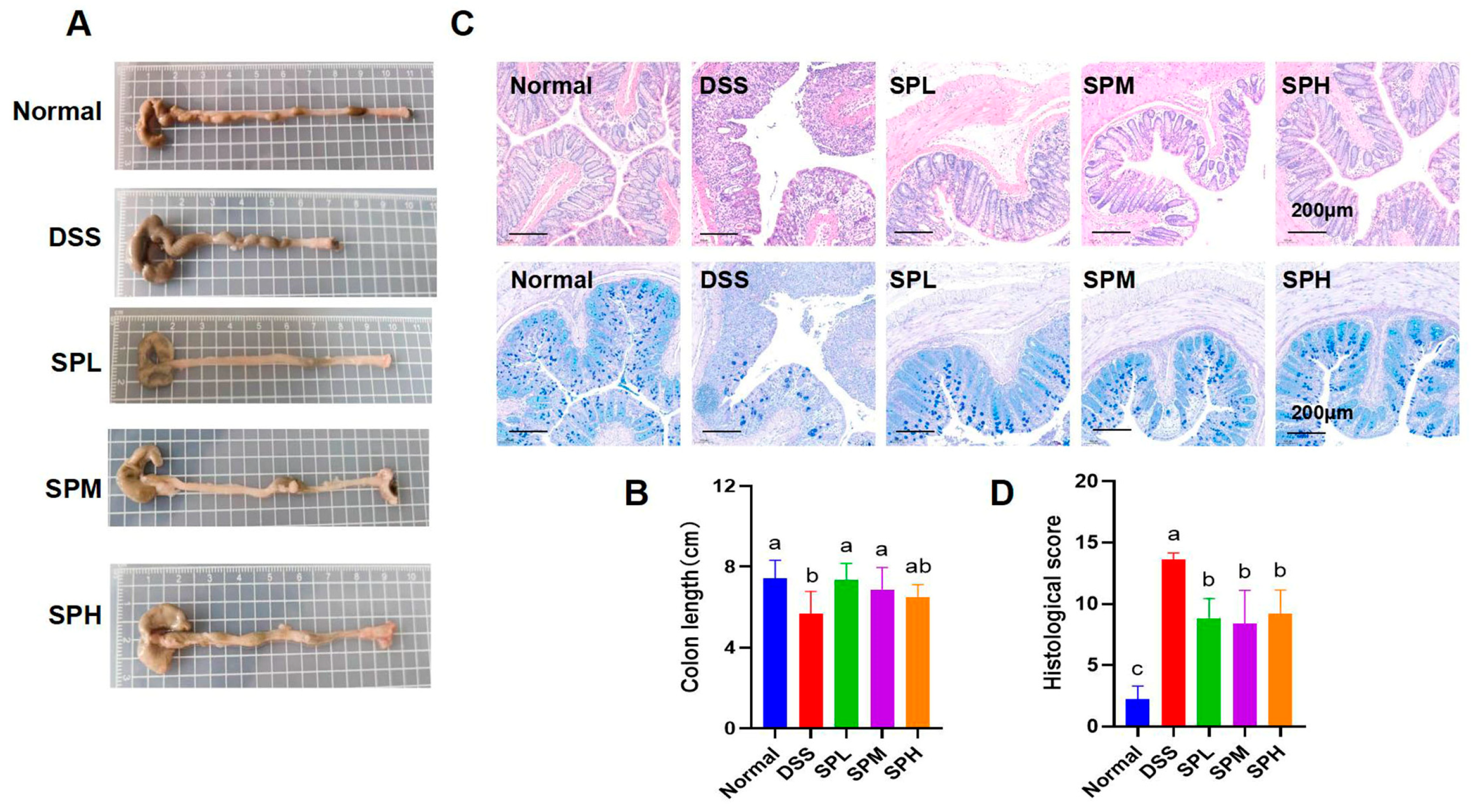
3.2. SP Alleviated DSS-Induced Colon Tissue Injury
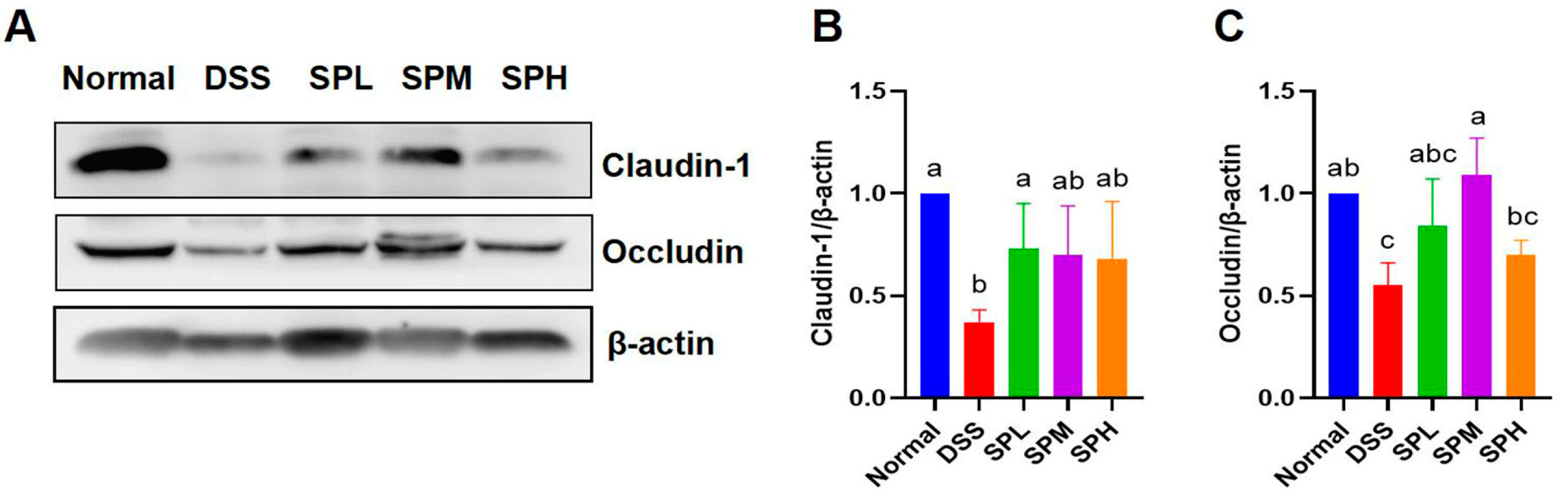
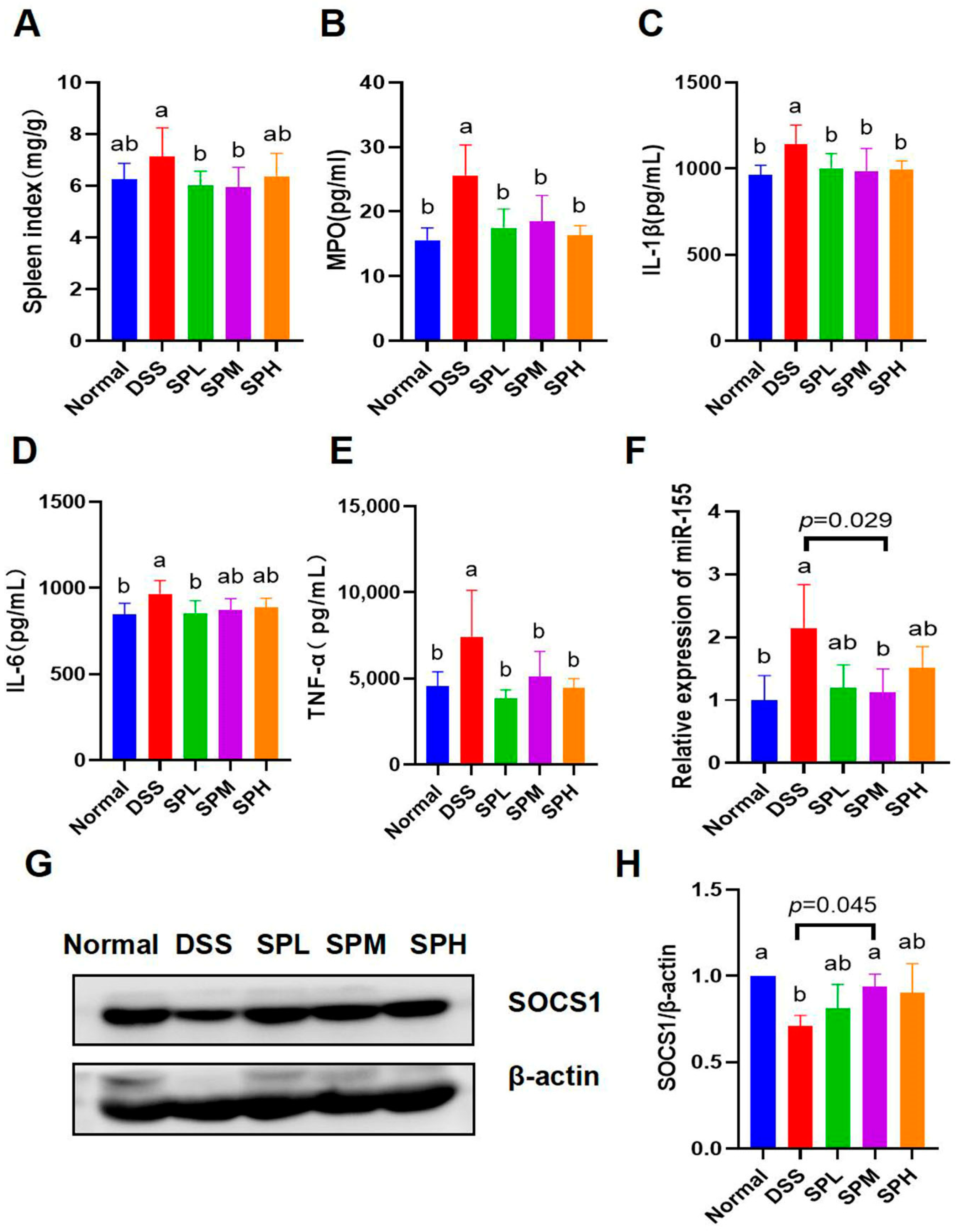
3.3. Anti-Inflammatory Effects of SP
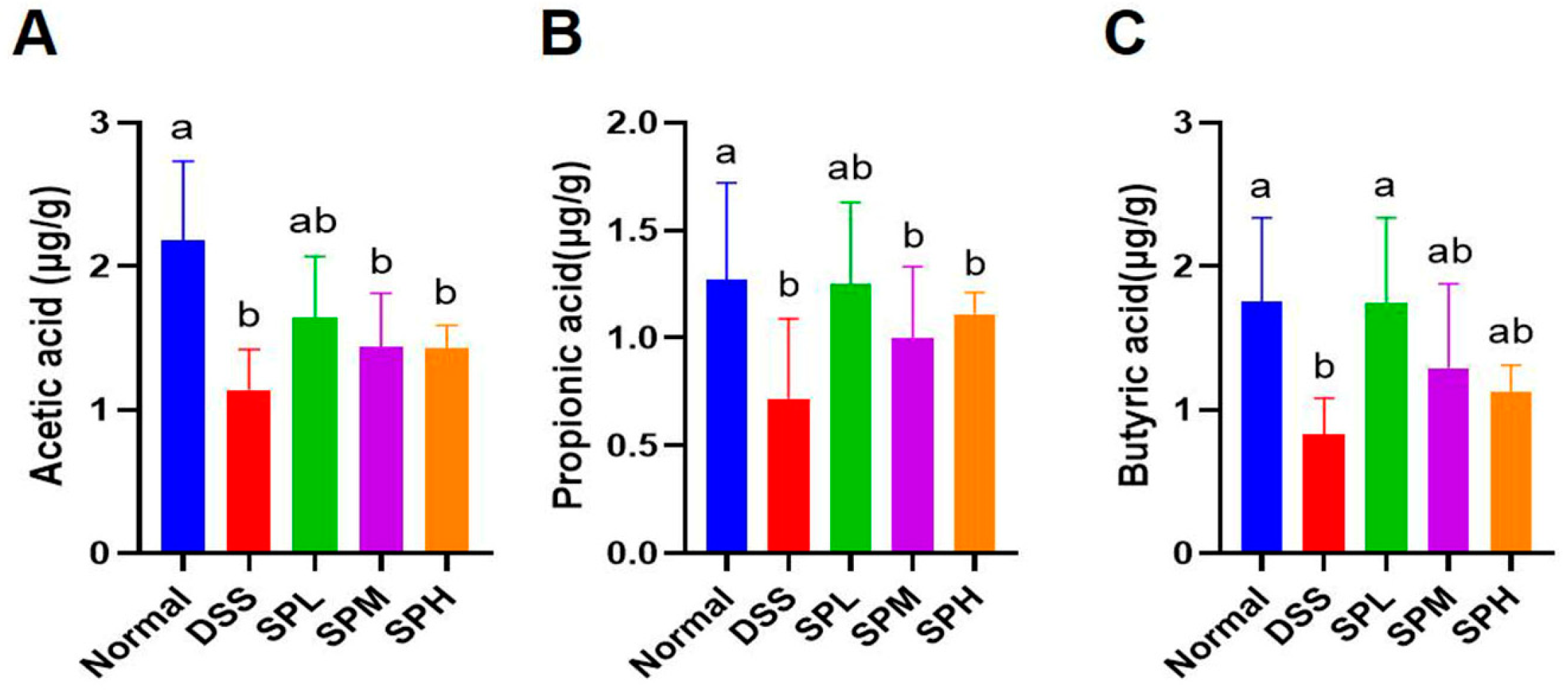
3.4. Effect of SP on the Content of SCFA in Mouse Feces
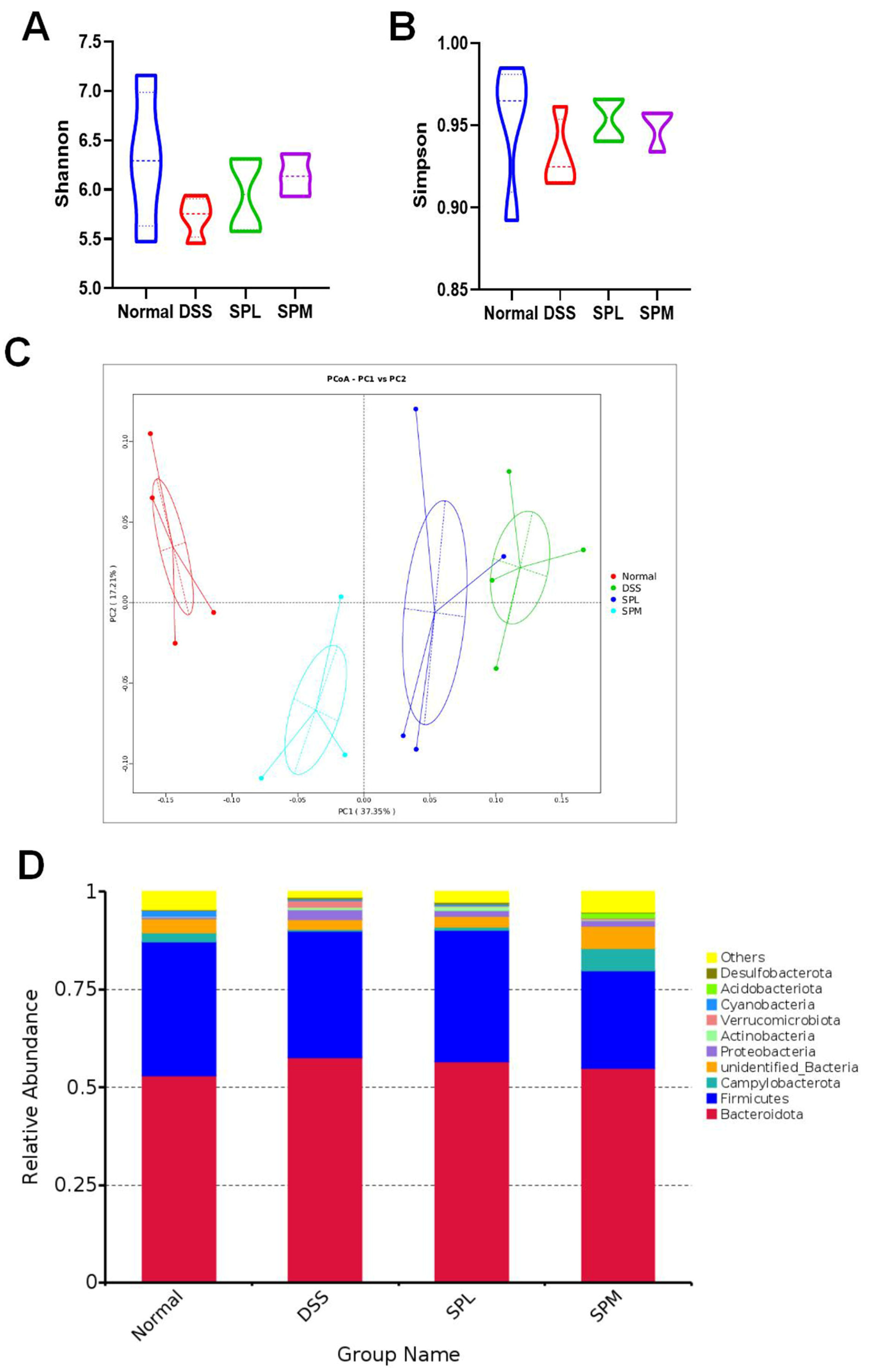
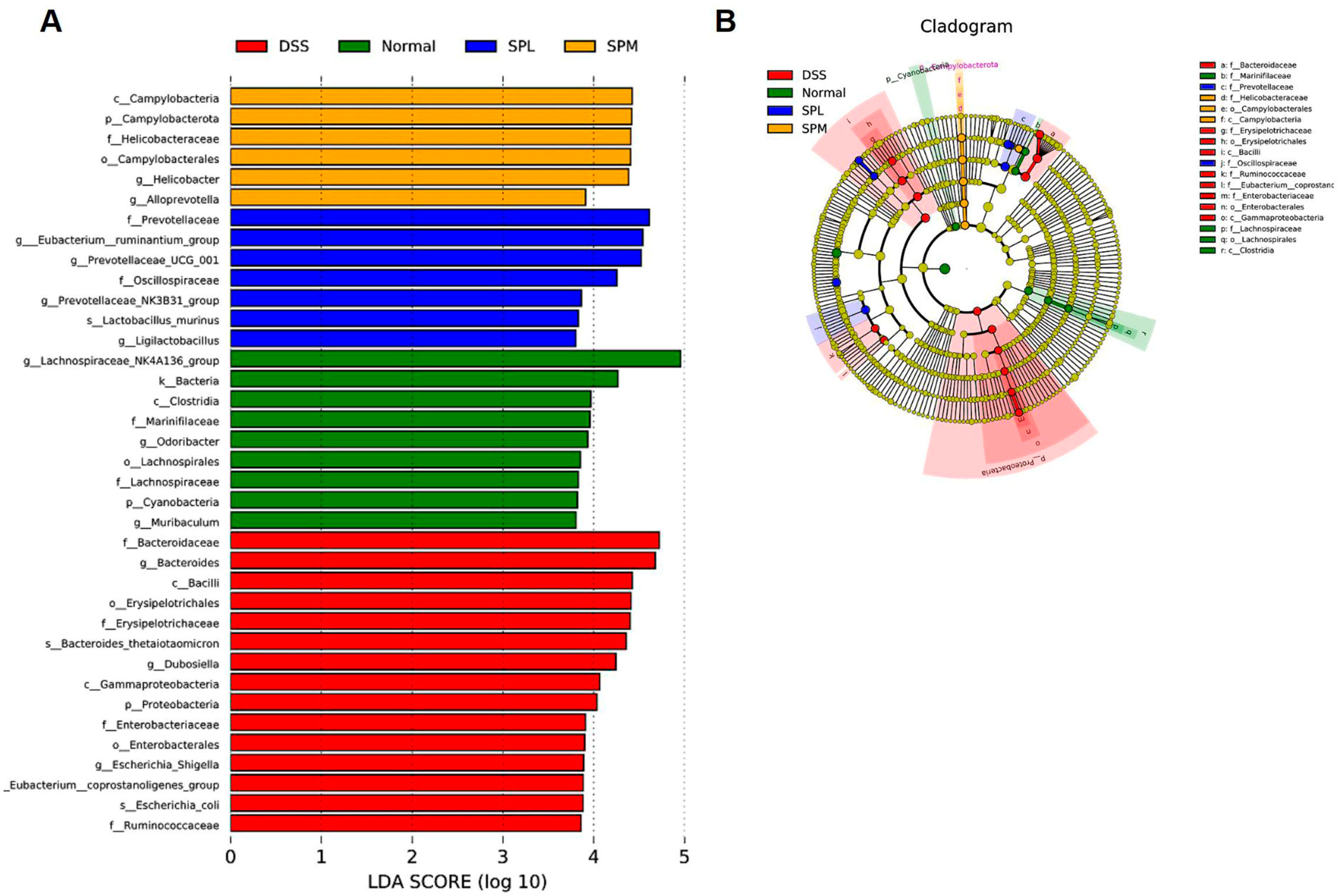
3.5. SP Regulates the Gut Microbiota
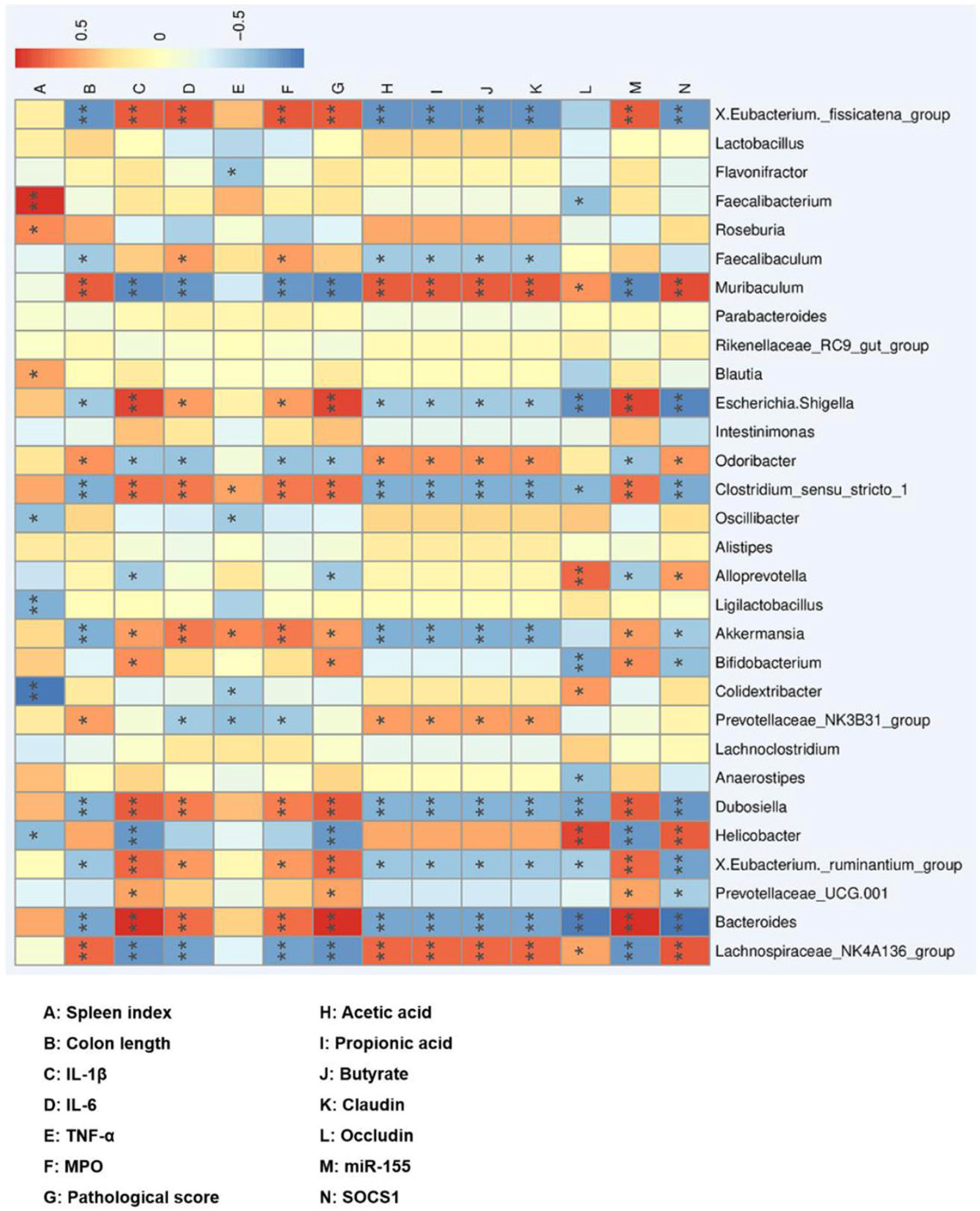
3.6. Correlation between the Gut Microbiota and UC
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Wu, J.C.; Chan, F.K.; Sung, J.J.; Kaplan, G. The Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21 st Century: A Systematic Review of Population-Based Studies. Gastroenterology 2017, 152, S970–S971. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.C.; Lyra, A.C.; Rocha, R.; Santana, G.O. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 2014, 20, 9458–9467. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Cai, N.; Luo, W.; Yao, L.; Li, X.; Wang, Z.; Xu, H.; Li, H.; Hu, Z.; Bao, W.; Xu, X. Activation of murine RAW264.7 macrophages by oligopeptides from sea cucumber (Apostichopus japonicus) and its molecular mechanisms. J. Funct. Foods 2020, 75, 104229. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Z.; Liu, X.; Zhao, M. Anti-diabetic effects of sea cucumber (Holothuria nobilis) hydrolysates in streptozotocin and high-fat-diet induced diabetic rats via activating the PI3K/Akt pathway. J. Funct. Foods 2020, 75, 104224. [Google Scholar] [CrossRef]
- Xu, X.; Liang, R.; Li, D.; Jiang, C.; Lin, S. Evaluation of sea cucumber peptides-assisted memory activity and acetylation modification in hippocampus of test mice based on scopolamine-induced experimental animal model of memory disorder. J. Funct. Foods 2020, 68, 103909. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Zheng, L.; Zhao, M.; Fan, J.; Liu, S. Preparation of sea cucumber (Stichopus variegates) peptide fraction with desired organoleptic property and its anti-aging activity in fruit flies and D-galactose-induced aging mice. J. Funct. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Li, M.; Ge, Q.; Du, H.; Jiang, P.; Bao, Z.; Chen, D.; Lin, S. Potential Mechanisms Mediating the Protective Effects of Tricholoma matsutake-Derived Peptides in Mitigating DSS-Induced Colitis. J. Agric. Food Chem. 2021, 69, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Vigorito, E.; Kohlhaas, S.; Lu, D.; Leyland, R. miR-155: An ancient regulator of the immune system. Immunol. Rev. 2013, 253, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Croker, B.A.; Kiu, H.; Nicholson, S.E. SOCS regulation of the JAK/STAT signalling pathway. Semin. Cell Dev. Biol. 2008, 19, 414–422. [Google Scholar] [CrossRef]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Inca, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palu, G.; et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, L.; Tang, X.; Hu, W.; Zhou, P. Major yolk protein from sea cucumber (Stichopus japonicus) attenuates acute colitis via regulation of microbial dysbiosis and inflammatory responses. Food Res. Int. 2022, 151, 110841. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, Y.; Wang, H.; Li, Z.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. The effects of live and pasteurized Akkermansia muciniphila on DSS-induced ulcerative colitis, gut microbiota, and metabolomics in mice. Food Funct. 2023, 14, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li, X.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-kappaB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, F.; Zhang, H.; Ye, Y.; Liu, P.; Lin, D.; Zhou, H.; Li, M.; Yang, B. Polysaccharides from Agaricus blazei Murrill ameliorate dextran sulfate sodium-induced colitis via attenuating intestinal barrier dysfunction. J. Funct. Foods 2022, 92, 105072. [Google Scholar] [CrossRef]
- McKenzie, C.V.; Colonne, C.K.; Yeo, J.H.; Fraser, S.T. Splenomegaly: Pathophysiological bases and therapeutic options. Int. J. Biochem. Cell Biol. 2018, 94, 40–43. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Shin-Ya, M.; Naito, Y.; Kishida, T.; Ito, R.; Suzuki, N.; Yasuda, H.; Sakagami, J.; Imanishi, J.; Kataoka, K.; et al. Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice. J. Gastroenterol. 2010, 45, 608–617. [Google Scholar] [CrossRef]
- Hering, N.A.; Fromm, M.; Schulzke, J.D. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol. 2012, 590, 1035–1044. [Google Scholar] [CrossRef]
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B.; Abraham, C.; Turner, J.R. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 2009, 136, 551–563. [Google Scholar] [CrossRef]
- Wang, D.; Cai, M.; Wang, T.; Liu, T.; Huang, J.; Wang, Y.; Granato, D. Ameliorative effects of L-theanine on dextran sulfate sodium induced colitis in C57BL/6J mice are associated with the inhibition of inflammatory responses and attenuation of intestinal barrier disruption. Food Res. Int. 2020, 137, 109409. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol Supplementation Attenuates DSS-Induced Colitis via Mediating Gut Barrier Integrity, Inflammatory Responses, and Reshaping Gut Microbiome. J. Agric. Food Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef]
- Zeng, L.; Tan, J.; Xue, M.; Liu, L.; Wang, M.; Liang, L.; Deng, J.; Chen, W.; Chen, Y. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-kB pathway. J. Transl. Med. 2020, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2017, 8, 1932. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ouyang, Y.; Xu, X.; Yuan, Z.; Liu, C.; Zhu, Z. MiR-155 promotes colitis-associated intestinal fibrosis by targeting HBP1/Wnt/beta-catenin signalling pathway. J. Cell. Mol. Med. 2021, 25, 4765–4775. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Murphy, A.E.; Enos, R.T.; Shamran, H.A.; Singh, N.P.; Guan, H.; Hegde, V.L.; Fan, D.; Price, R.L.; Taub, D.D.; et al. miR-155 deficiency protects mice from experimental colitis by reducing T helper type 1/type 17 responses. Immunology 2014, 143, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Wirtz, S.J.; Neurath, M.F. MiR-155 Regulates TH17 Differentiation in Experimental Colitis. Gastroenterology 2012, 142, S-723. [Google Scholar] [CrossRef]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef]
- Durchschein, F.; Petritsch, W.; Hammer, H.F. Diet therapy for inflammatory bowel diseases: The established and the new. World J. Gastroenterol. 2016, 22, 2179–2194. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shi, J. Vitamin A supplementation ameliorates ulcerative colitis in gut microbiota-dependent manner. Food Res. Int. 2021, 148, 110568. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Pu-erh Tea Extract Promotes Gut Microbiota Resilience against Dextran Sulfate Sodium Induced Colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Liu, Y.; Sun, K.; Luo, L.; Zeng, L. Aged Ripe Pu-erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes. J. Agric. Food Chem. 2021, 69, 10592–10605. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef]

| Gene Symbol | Primer | Primer Sequence (5′-3′) |
|---|---|---|
| miR-155 | RT primer | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTACCCCTAT |
| Forward | TGCGGTTAATGCTAATTGTGA | |
| Reverse | CAGTGCGTGTCGTGGAGT | |
| U6 | RT primer | AAAATATGGAACGCTTCACGA |
| Forward Reverse | CGCTTCGGCAGCACATATAC AAAATATGGAACGCTTCACGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Zhao, Y.; Wang, L.; Wu, T.; Jin, Y.; Meng, J.; Zhang, M. Sea Cucumber Peptide Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium by Alleviating Gut Microbiota Imbalance and Regulating miR-155/SOCS1 Axis in Mice. Foods 2023, 12, 3434. https://doi.org/10.3390/foods12183434
Mao J, Zhao Y, Wang L, Wu T, Jin Y, Meng J, Zhang M. Sea Cucumber Peptide Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium by Alleviating Gut Microbiota Imbalance and Regulating miR-155/SOCS1 Axis in Mice. Foods. 2023; 12(18):3434. https://doi.org/10.3390/foods12183434
Chicago/Turabian StyleMao, Jing, Yunjiao Zhao, Lechen Wang, Tao Wu, Yan Jin, Jing Meng, and Min Zhang. 2023. "Sea Cucumber Peptide Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium by Alleviating Gut Microbiota Imbalance and Regulating miR-155/SOCS1 Axis in Mice" Foods 12, no. 18: 3434. https://doi.org/10.3390/foods12183434






