Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Enzymes and Chemicals
2.2. Plant Material Characterization
2.2.1. Moisture and Dry Matter
2.2.2. pH
2.2.3. Cell Wall Polysaccharides Composition
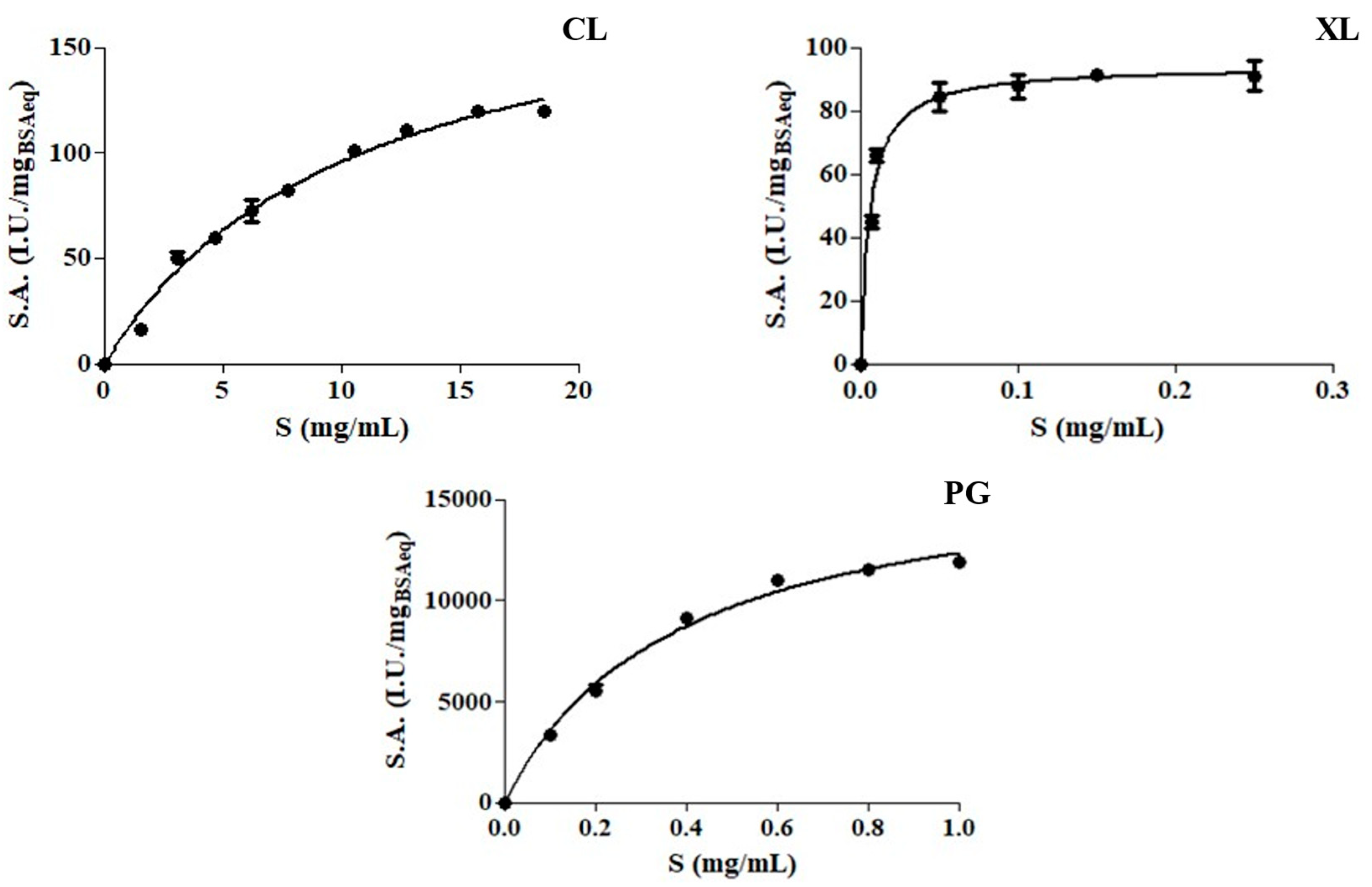
2.3. Characterization of Enzyme Preparations
2.4. Extraction Process Optimization—Response Surface Methodology
2.5. Chlorophyll Recovery by Enzyme Assisted Extraction
2.6. Chlorophyll Content and Colorimetric Characterization
2.7. Application of Chlorophyll-Based Colorant in Real Food
2.8. Statistical Analysis
3. Results and Discussion
3.1. Plant Material and Enzyme Preparations
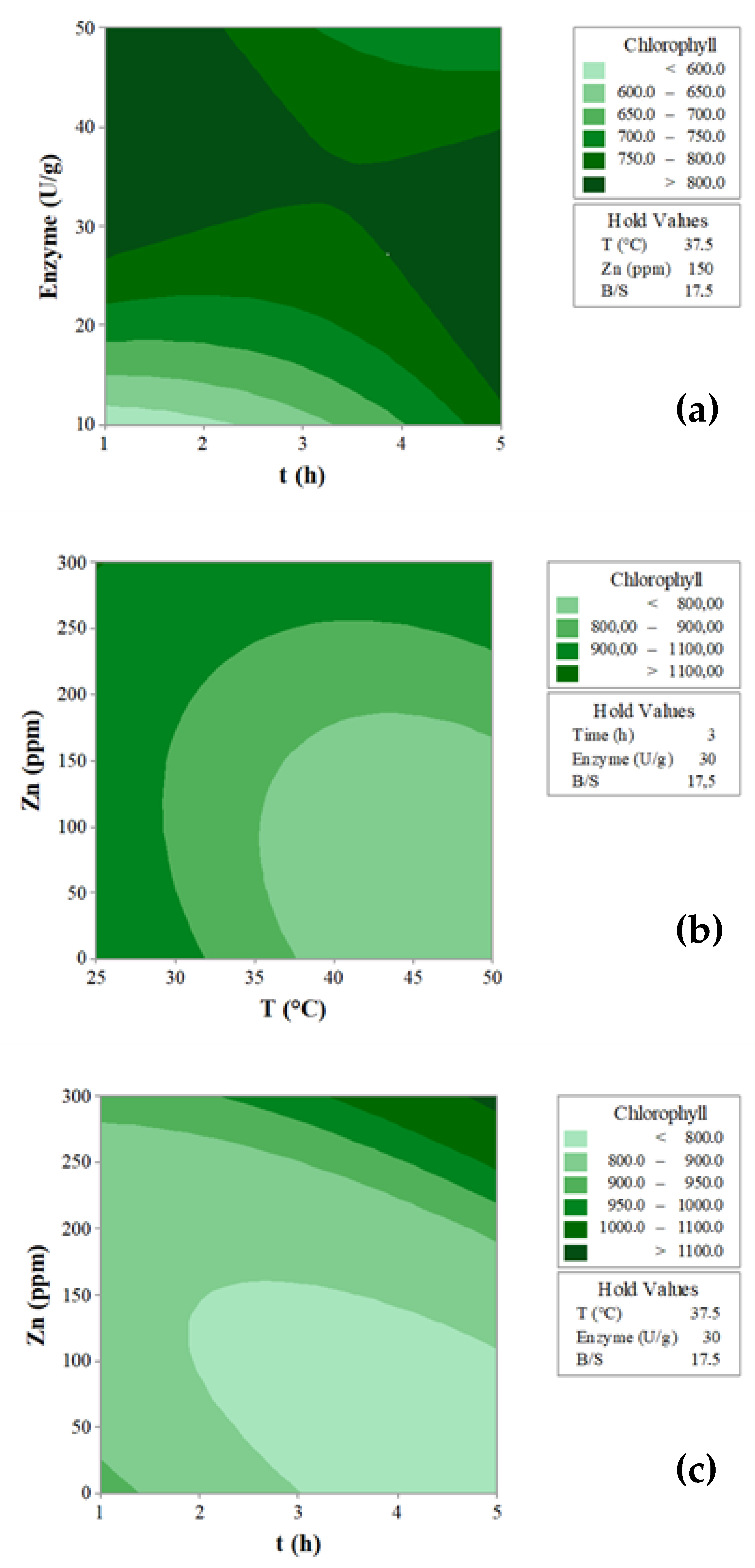
3.2. Enzyme-Assisted Extraction for Chlorophyll Recovery
3.3. Colorimetric Properties of the Extracts
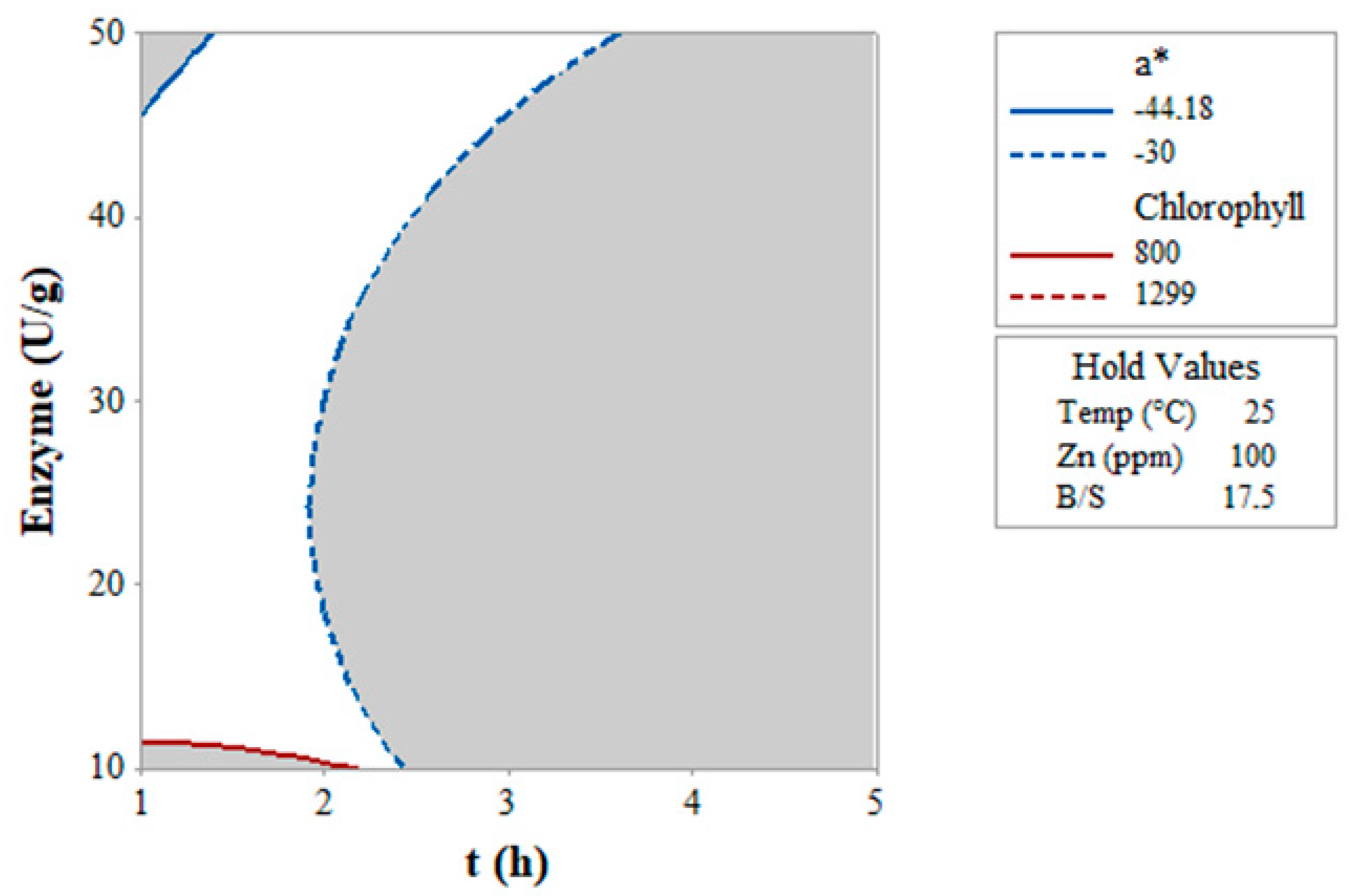
3.4. Overlay Plot of Chlorophyll and a* Value
3.5. Application of Green Colorant in a Food Matrix
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piirsalu, E.; Moora, H.; Väli, K.; Värnik, R.; Aro, K.; Lillemets, J. The Generation of Food Waste and Food Loss in the Estonian Food Supply Chain; Stockholm Environment Institute: Stockholm, Sweden, 2022. [Google Scholar]
- Directive (EU) 2018/851 of the European Parliament and European Council, 2018 of 30 May 2018 amending Directive 2008/98/EC on waste. Off. J. Eur. Union 2018, 150, 109–140.
- Zhang, Q.; Dhir, A.; Kaur, P. Circular economy and the food sector: A systematic literature review. Sustain. Prod. Consum. 2022, 32, 655–668. [Google Scholar] [CrossRef]
- Lombardelli, C.; Liburdi, K.; Benucci, I.; Esti, M. Tailored and synergistic enzyme-assisted extraction of carotenoid-containing chromoplasts from tomatoes. Food Bioprod. Process. 2020, 121, 43–53. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. Green Enzymatic Recovery of Functional Bioactive Compounds from Unsold Vegetables: Storability and Potential Health Benefits. Appl. Sci. 2022, 12, 12249. [Google Scholar] [CrossRef]
- Porrarud, S.; Pranee, A. Microencapsulation of Zn-chlorophyll pigment from Pandan leaf by spray drying and its characteristic. Int. Food Res. J. 2010, 17, 1031–1042. [Google Scholar]
- Özkan, G.; Bilek, S.E. Enzyme-assisted extraction of stabilized chlorophyll from spinach. Food Chem. 2015, 176, 152–157. [Google Scholar] [CrossRef]
- Francis, F. Lesser-known food colorants. Food Technol. 1987, 41, 62–68. [Google Scholar]
- Ilker, R. In vitro pigment production: An alternative to color synthesis. Food Technol. 1987, 41, 70–72. [Google Scholar]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Carrillo, C.; Nieto, G.; Martinez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel approaches for the recovery of natural pigments with potential health effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a sustainable purification protocol for lutein and chlorophyll from spinach by-products by a saponification procedure using Box Behnken design and desirability function. Food Bioprod. Proc. 2019, 116, 54–62. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Leite, A.C.; Coutinho, J.A.; Freire, M.G. Chlorophylls extraction from spinach leaves using aqueous solutions of surface-active ionic liquids. Sustain. Chem. 2021, 2, 764–777. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Marquez, U.M.L.; Sinnecker, P. Chlorophylls in Foods: Sources and Stability. In Food Colorants: Chemical and Functional Properties; CRC Press: Boca Raton, FL, USA, 2007; p. 195. [Google Scholar]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Wani, F.A.; Rashid, R.; Jabeen, A.; Brochier, B.; Yadav, S.; Aijaz, T.; Makroo, H.A.; Dar, B.N. Valorisation of food wastes to produce natural pigments using non-thermal novel extraction methods: A review. Int. J. Food Sci. Technol. 2021, 56, 4823–4833. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Valanciene, E.; Augustiniene, E.; Malys, N. Valorization of bilberry (Vaccinium myrtillus L.) Pomace by enzyme-assisted extraction: Process optimization and comparison with conventional solid-liquid extraction. Antioxidants 2021, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Ratrey, P.; Datta, B. Reusable nanobiocatalysts for the efficient extraction of pigments from orange peel. J. Food Sci. Technol. 2016, 53, 3013–3019. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. A novel process for the recovery of betalains from unsold red beets by low-temperature enzyme-assisted extraction. Foods 2021, 10, 236. [Google Scholar] [CrossRef]
- Senklang, P.; Anprung, P. Optimizing enzymatic extraction of Zn–chlorophyll derivatives from pandan leaf using response surface methodology. J. Food Process. Preserv. 2009, 34, 759–776. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Liburdi, K.; Spinelli, S.E.; Benucci, I.; Lombardelli, C.; Esti, M. A preliminary study of continuous milk coagulation using Cynara cardunculus flower extract and calf rennet immobilized on magnetic particles. Food Chem. 2018, 239, 157–164. [Google Scholar] [CrossRef]
- Liburdi, K.; Benucci, I.; Esti, M. Effect of microwave power and blanching time in relation to different geometric shapes of vegetables. LWT 2019, 99, 497–504. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Zia-ur-Rehman, Z.; Islam, M.; Shah, W.H. Effect of microwave and conventional cooking on insoluble dietary fibre components of vegetables. Food Chem. 2003, 80, 237–240. [Google Scholar] [CrossRef]
- Herranz, J.; Vidal-Valverde, C.; Rojas-Hidalgo, E. Cellulose, hemicellulose and lignin content of raw and cooked processed vegetables. J. Food Sci. 1983, 48, 274–275. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Pitterou, I.; Tzani, A.; Detsi, A.; Frutos, M.J. Novel chitosan/alginate hydrogels as carriers of phenolic-enriched extracts from saffron floral by-products using natural deep eutectic solvents as green extraction media. Curr. Res. Food Sci. 2023, 6, 100469. [Google Scholar] [CrossRef]
- Hou, X.J.; Chen, W. Optimization of extraction process of crude polysaccharides from wild edible BaChu mushroom by response surface methodology. Carbohydr. Polym. 2008, 72, 67–74. [Google Scholar]
- Rastogi, N.K.; Rashmi, K.R. Optimisation of enzymatic liquefaction of mango pulp by response surface methodology. Eur. Food Res. Technol. 1999, 209, 57–62. [Google Scholar] [CrossRef]
| Treatment Trial | Temperature (°C) | Time (h) | Enzyme Mix (U/g) | Zn (ppm) | B/S | Chl (mg/g) |
|---|---|---|---|---|---|---|
| X1 (x1) | X2 (x2) | X3 (x3) | X4 (x4) | X5 (x5) | ||
| 1 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 791 |
| 2 | 25 (−1) | 5 (+1) | 50 (+1) | 300 (+1) | 5 (−1) | 1299 |
| 3 | 25 (−1) | 5 (+1) | 10 (−1) | 300 (+1) | 30 (+1) | 877 |
| 4 | 25 (−1) | 1 (−1) | 10 (−1) | 300 (+1) | 5 (−1) | 783 |
| 5 | 25 (−1) | 5 (+1) | 50 (+1) | 0 (−1) | 30 (+1) | 626 |
| 6 | 25 (−1) | 1 (−1) | 10 (−1) | 0 (−1) | 30 (+1) | 597 |
| 7 | 50 (+1) | 5 (+1) | 50 (+1) | 0 (−1) | 5 (−1) | 350 |
| 8 | 50 (+1) | 1 (−1) | 50 (+1) | 300 (+1) | 5 (−1) | 1283 |
| 9 | 50 (+1) | 5 (+1) | 10 (−1) | 300 (+1) | 5 (−1) | 993 |
| 10 | 50 (+1) | 1 (−1) | 50 (+1) | 0 (−1) | 30 (+1) | 509 |
| 11 | 25 (−1) | 5 (+1) | 10 (−1) | 0 (−1) | 5 (−1) | 869 |
| 12 | 50 (+1) | 5 (+1) | 50 (+1) | 300 (+1) | 30 (+1) | 817 |
| 13 | 50 (+1) | 5 (+1) | 10 (−1) | 0 (−1) | 30 (+1) | 590 |
| 14 | 50 (+1) | 1 (−1) | 10 (−1) | 300 (+1) | 30 (+1) | 331 |
| 15 | 25 (−1) | 1 (−1) | 50 (+1) | 300 (+1) | 30 (+1) | 636 |
| 16 | 25 (−1) | 1 (−1) | 50 (+1) | 0 (−1) | 5 (−1) | 1286 |
| 17 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 791 |
| 18 | 50 (+1) | 1 (−1) | 10 (−1) | 0 (−1) | 5 (−1) | 621 |
| 19 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 791 |
| 20 | 50 (+1) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 718 |
| 21 | 37.5 (0) | 5 (+1) | 30 (0) | 150 (0) | 17.5 (0) | 752 |
| 22 | 37.5 (0) | 3 (0) | 50 (+1) | 150 (0) | 17.5 (0) | 716 |
| 23 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 714 |
| 24 | 37.5 (0) | 3 (0) | 30 (0) | 300 (+1) | 17.5 (0) | 970 |
| 25 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 5 (−1) | 755 |
| 26 | 25 (−1) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 1130 |
| 27 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 30 (+1) | 659 |
| 28 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 739 |
| 29 | 37.5 (0) | 3 (0) | 30 (0) | 150 (0) | 17.5 (0) | 742 |
| 30 | 37.5 (0) | 3 (0) | 30 (0) | 0 (−1) | 17.5 (0) | 895 |
| 31 | 37.5 (0) | 1 (−1) | 30 (0) | 150 (0) | 17.5 (0) | 999 |
| 32 | 37.5 (0) | 3 (0) | 10 (−1) | 150 (0) | 17.5 (0) | 750 |
| Moisture (%) | Dry Matter (%) | pH | Cellulose (g/100 gdwb) | Hemicellulose (g/100 gdwb) | Pectin (g/100 gdwb) |
|---|---|---|---|---|---|
| 93.6 ± 1.2 | 6.4 ± 0.9 | 6.29 ± 0.02 | 5.20 ± 0.08 | 5.35 ± 0.08 | 2.43 ± 0.14 |
| Protein Content (mgBSAeq/mL) | Vmax (I.U./mgBSAeq) | KM (mg/mL) | kcat (min−1) | Ka (min−1/mg mL−1) | R2 | |
|---|---|---|---|---|---|---|
| CL | 0.024 ± 0.002 | 195.8 ± 9.94 | 10.41 ± 1.07 | 3.24 × 106 ± 9.94 | 3.11 × 105 ± 3.3 × 103 | 0.98 |
| XL | 0.027 ± 0.003 | 93.84 ± 2.02 | 0.0057 ± 0.0007 | 4.14 × 105 ± 2.02 | 7.29 × 107 ± 1.1 × 105 | 0.97 |
| PG | 0.024 ± 0.001 | 16950 ± 620 | 0.375 ± 0.034 | 6.13 × 108 ± 620 | 1.63 × 109 ± 1.3 × 107 | 0.99 |
| Regression Coefficient | Chl |
|---|---|
| b0 | +794.3 |
| b1 | −105.1 ** |
| b2 | +7.0 |
| b3 | +61.7 * |
| b4 | +91.5 ** |
| b5 | −144.2 *** |
| b11 | +90.5 |
| b22 | +41.9 |
| b33 | −100.9 |
| b44 | +99.0 |
| b55 | −126.7 |
| b12 | −22.6 |
| b13 | −18.5 |
| b14 | +71.1 * |
| b15 | +31.4 |
| b23 | −101.2 ** |
| b24 | +95.6 ** |
| b25 | +81.2 ** |
| b34 | +59.9 |
| b35 | −47.5 |
| b45 | −55.8 |
| Model p-value | 0.003 |
| R2 | 0.93 |
| R2 adj | 0.77 |
| Treatment Trial | L* | a* | b* | C* | h° | Color |
|---|---|---|---|---|---|---|
| 1 | 47.14 f | −20.26 i | 74.5 g | 77.2 hij | 105.2 ij | |
| 2 | 28.21 f | −12.59 f | 47.2 p | 48.9 o | 104.9 ij | |
| 3 | 48.05 f | −34.09 l | 73.4 gh | 80.9 fg | 114.9 l | |
| 4 | 65.07 c | −26.68 j | 33.4 q | 42.7 p | 128.6 a | |
| 5 | 55.89 e | −27.86 j | 84.1 d | 88.6 d | 108.3 gh | |
| 6 | 72.4 b | −39.27 m | 95.5 b | 103.2 a | 112.4 e | |
| 7 | 42.75 gh | −1.71 b | 54.62 o | 54.6 n | 91.8 n | |
| 8 | 23.29 j | −14.11 f | 45.65 p | 47.8 o | 107.2 gh | |
| 9 | 30.95 i | −3.48 bc | 51.92 o | 52.0 n | 93.8 mn | |
| 10 | 63.86 cd | −26.72 j | 92.67 b | 96.4 bc | 106.1 jh | |
| 11 | 31.19 i | −5.56 d | 52.72 o | 53.0 n | 96.0 l | |
| 12 | 38.96 g | −0.18 a | 65.31 l | 65.3 m | 90.2 n | |
| 13 | 52.7 f | −10.97 f | 82.75 d | 83.5 ef | 97.6 l | |
| 14 | 77.51 a | −36.47 l | 98.42 a | 105.0 a | 110.3 fg | |
| 15 | 60.62 d | −44.18 n | 85.24 d | 96.0 b | 117.4 d | |
| 16 | 40.74 g | −43.38 n | 62.93 l | 76.4 ij | 124.6 b | |
| 17 | 48.14 f | −19.26 i | 73.48 gh | 76.0 ij | 104.7 j | |
| 18 | 58.51 d | −27.68 j | 87.73 c | 92.0 c | 107.5 hi | |
| 19 | 50.17 f | −20.98 i | 71.22 ijk | 74.2 j | 106.4 hi | |
| 20 | 42.86 i | −3.32 bc | 70.53 k | 70.6 k | 92.7 mn | |
| 21 | 43.86 g | −5.78 cd | 71.35 jk | 71.6 k | 94.6 m | |
| 22 | 49.32 g | −17.03 h | 77.75 f | 79.6 fjk | 102.4 j | |
| 23 | 49.25 f | −15.8 gh | 77.67 f | 79.3 fjk | 101.5 j | |
| 24 | 39.1 f | −14.25 f | 64.26 l | 65.8 l | 102.5 j | |
| 25 | 46.74 gh | −19.72 i | 72.94 hi | 75.6 j | 105.1 j | |
| 26 | 35.78 f | −30.49 k | 57.01 n | 64.7 l | 118.1 d | |
| 27 | 53.07 h | −19.13 i | 82.32 de | 84.5 e | 103.1 j | |
| 28 | 48.98 f | −16.02 gh | 76.99 fg | 78.6 ghi | 101.8 k | |
| 29 | 47.22 f | −14.99 g | 76.78 fg | 78.2 ghi | 101.0 k | |
| 30 | 40.36 f | −11.6 e | 66.47 l | 67.5 l | 99.9 k | |
| 31 | 38.77 f | −37.51 m | 60.88 m | 71.5 k | 121.6 c | |
| 32 | 52.76 g | −31.77 k | 79.15 e | 85.3 de | 111.9 f |
| Colorant (%) | L* | a* | b* | Sample |
|---|---|---|---|---|
| 0% | 38.4 a | 2.64 a | 18.16 a |  |
| 1.0% | 36.43 b | −1.53 b | 18.98 a |  |
| 2.5% | 23.94 d | −2.09 c | 15.99 b |  |
| 5.0% | 22.01 e | −3.92 d | 13.83 c |  |
| 7.5% | 26.75 c | −3.98 d | 13.93 c |  |
| 10% | 26.15 c | −4.41 d | 11.37 c |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzocchi, C.; Benucci, I.; Lombardelli, C.; Esti, M. Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant. Foods 2023, 12, 3440. https://doi.org/10.3390/foods12183440
Mazzocchi C, Benucci I, Lombardelli C, Esti M. Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant. Foods. 2023; 12(18):3440. https://doi.org/10.3390/foods12183440
Chicago/Turabian StyleMazzocchi, Caterina, Ilaria Benucci, Claudio Lombardelli, and Marco Esti. 2023. "Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant" Foods 12, no. 18: 3440. https://doi.org/10.3390/foods12183440
APA StyleMazzocchi, C., Benucci, I., Lombardelli, C., & Esti, M. (2023). Enzyme-Assisted Extraction for the Recovery of Food-Grade Chlorophyll-Based Green Colorant. Foods, 12(18), 3440. https://doi.org/10.3390/foods12183440








