A Comparative Analysis of Phenolic Content, Antioxidant Activity, Antimicrobial Activity, and Chemical Profile of Coffea robusta Extracts Using Subcritical Fluid Extraction and Supercritical Carbon Dioxide Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents
2.2. Raw Materials
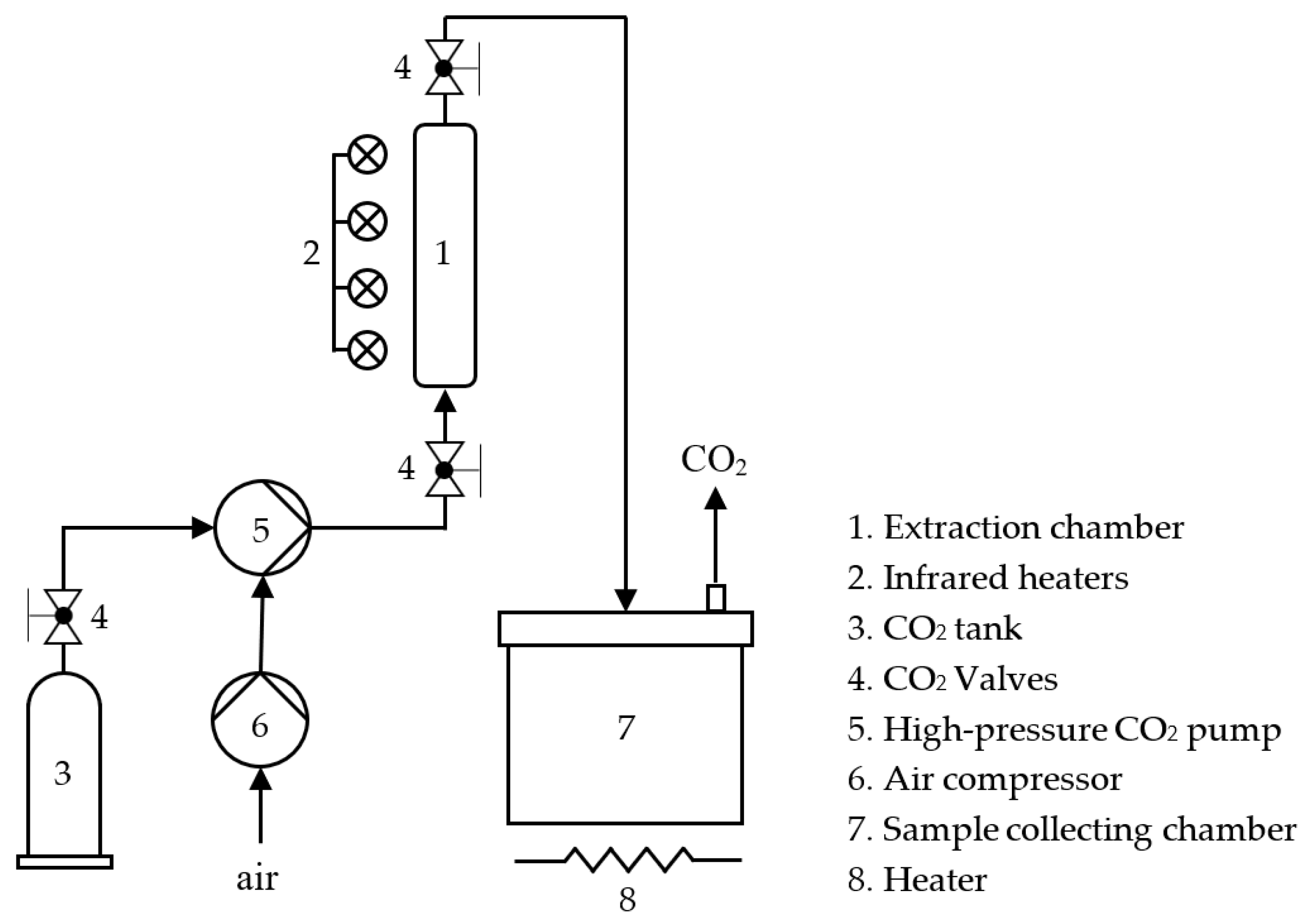
2.3. Extraction Process
2.3.1. Subcritical Fluid Extraction Using HFC-134a and HCFC-22
2.3.2. SCCO2 Extraction
2.3.3. Overall Extraction Yield
2.4. Determination of Total Phenolic Contents
2.5. Determination of Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. FRAP Assay
2.6. Antibacterial Activity
2.6.1. Inoculum Preparation
2.6.2. Determination of Inhibition Zone Using Disk Diffusion Assay
2.7. Analysis of Chemical Profiles Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.8. Data Analysis
3. Results
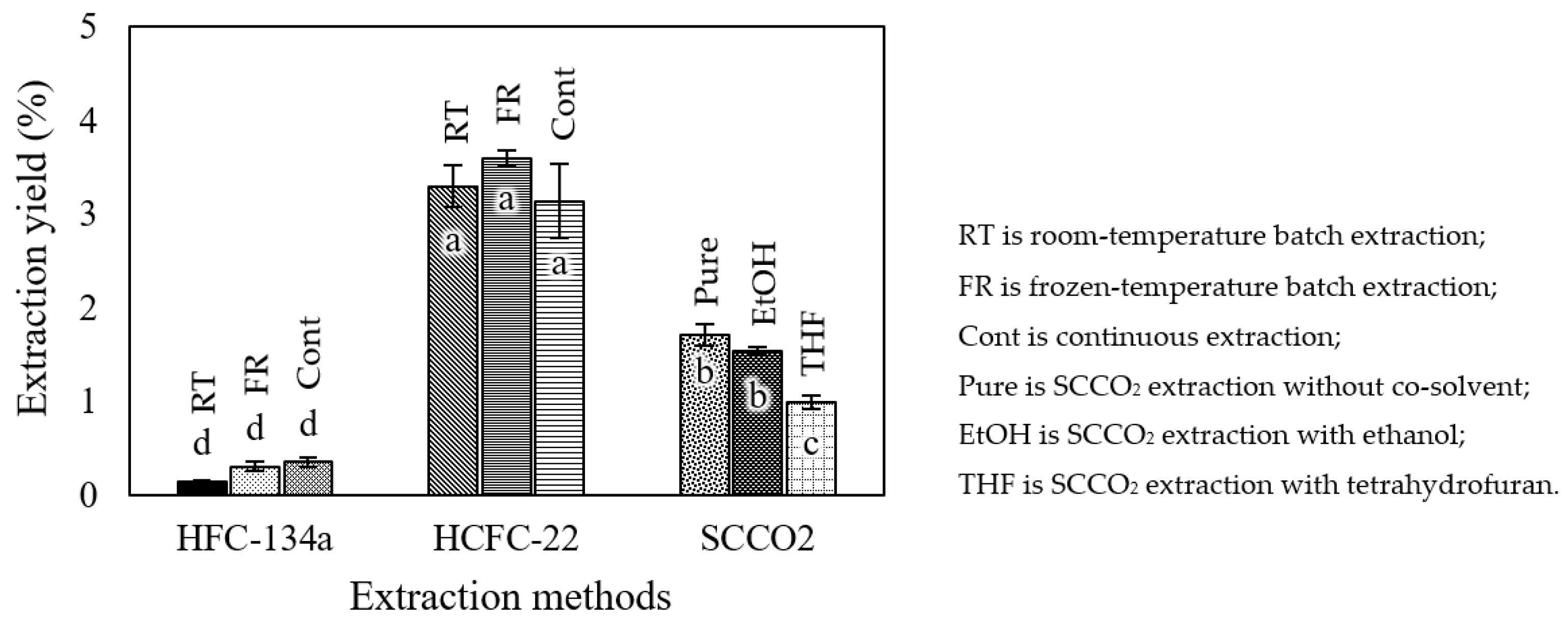
3.1. Extraction Yields
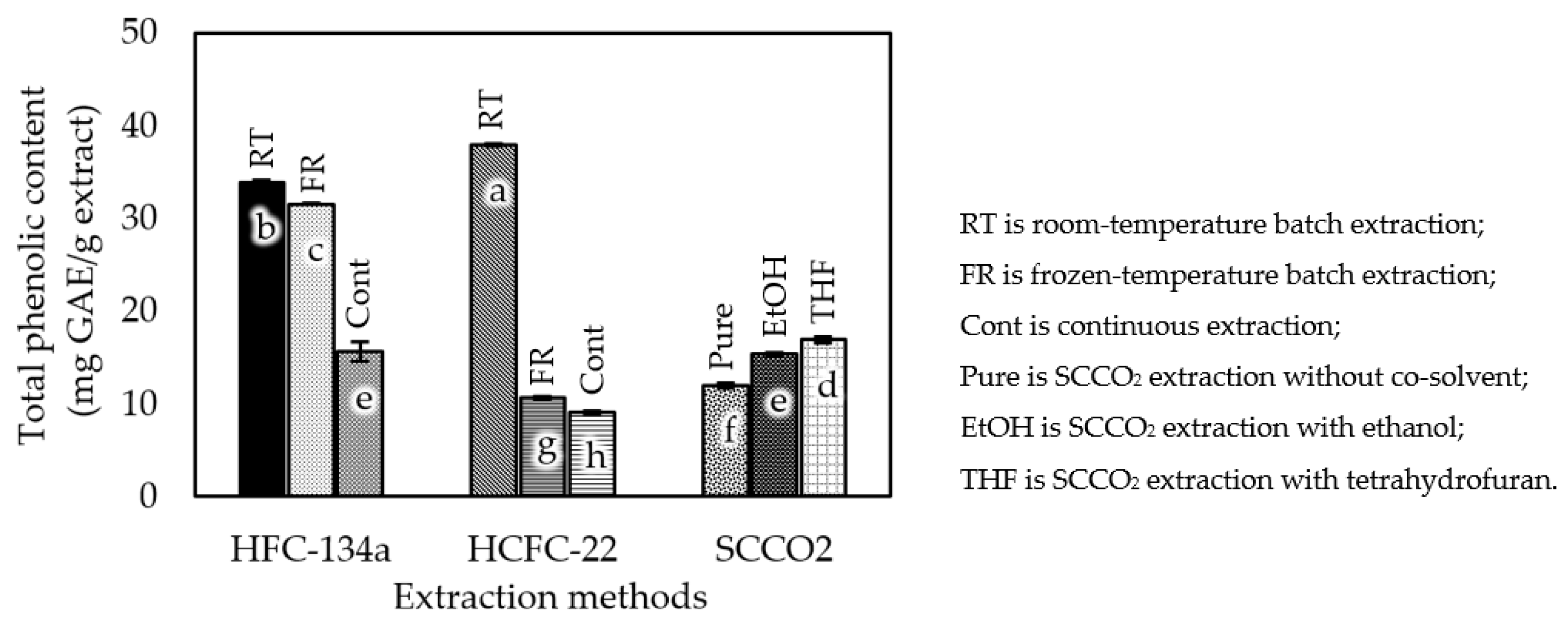
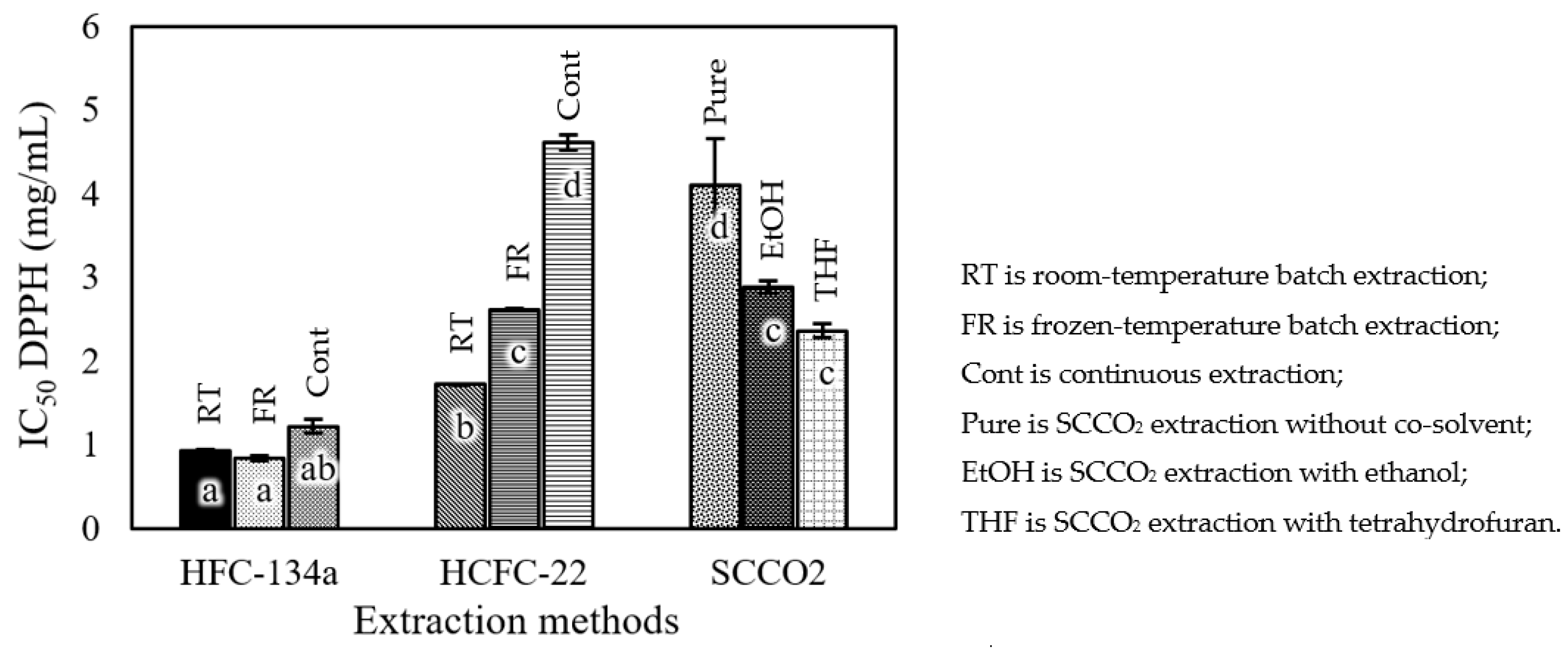
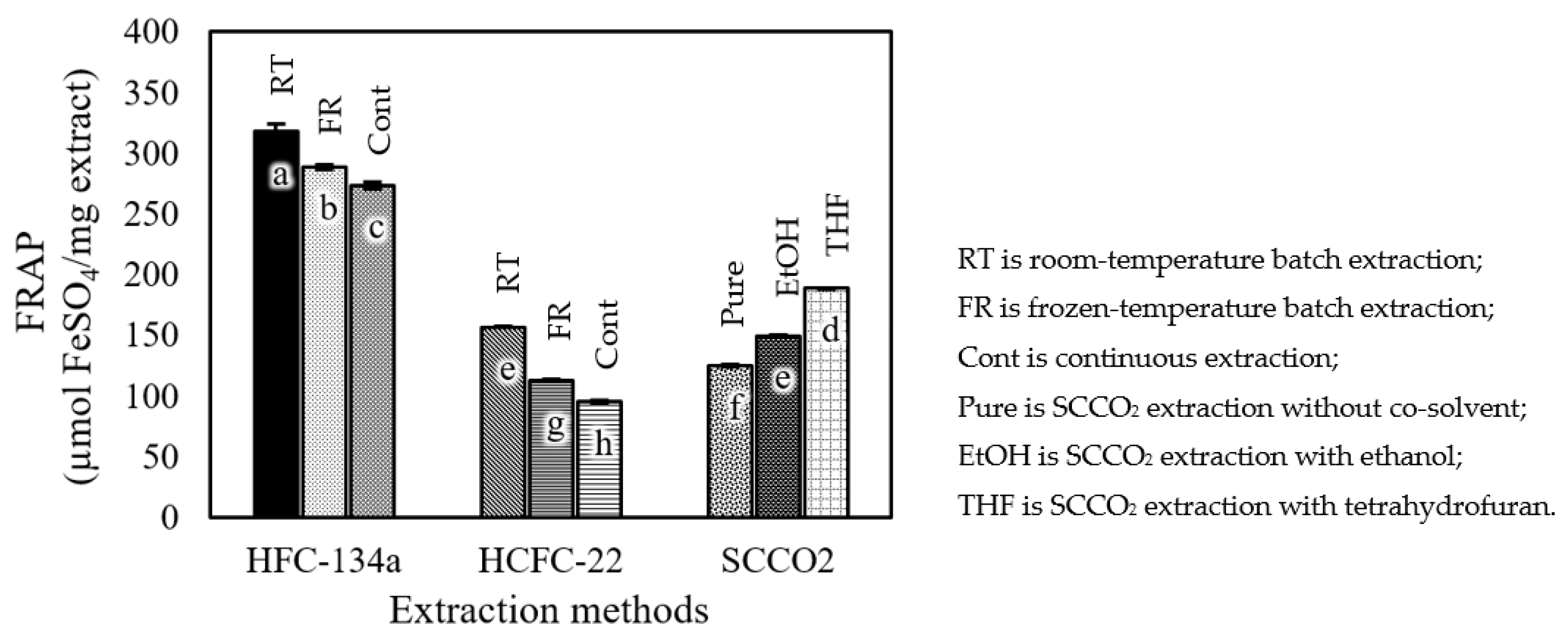
3.2. Total Phenolic Content and Antioxidant Activity
3.3. Antibacterial Activity of Roasted Coffee Extracts
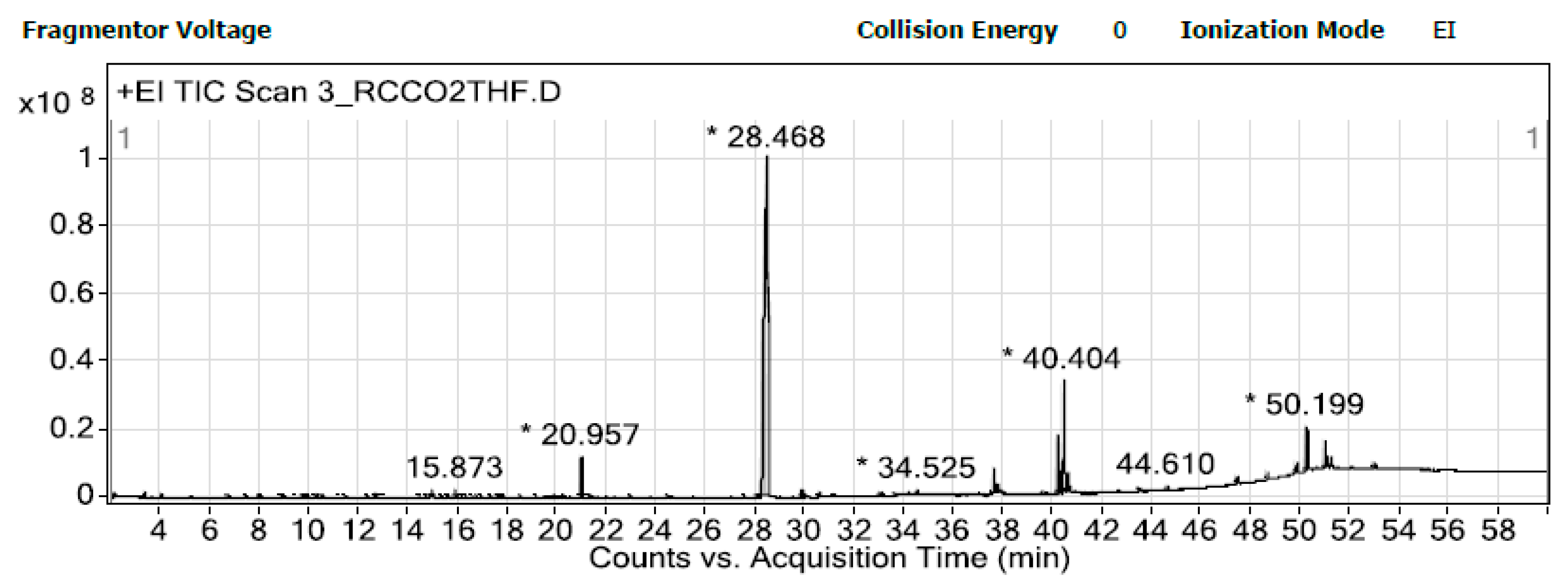
3.4. Tentative Chemical Profiles
4. Discussion
4.1. Comparison of Extraction Yields
4.2. Total Phenolic Content and Antioxidant Activity
4.3. Antimicrobial Activity
4.4. Chemical Profiles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Hao, X.; Li, X.; Wang, W. Effect of chitosan coating combined with sulfur dioxide fumigation on the storage quality of fresh areca nut. J. Food Process. Preserv. 2017, 41, e12974. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Fu, M.-R. Inhibitory effect of chlorine dioxide (ClO2) fumigation on growth and patulin production and its mechanism in Penicillum expansum. LWT 2018, 96, 335–343. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Fu, M.; Chen, Q.; Muzammil, J.M. Chlorine dioxide fumigation generated by a solid releasing agent enhanced the efficiency of 1-MCP treatment on the storage quality of strawberry. J. Food Sci. Technol. 2018, 55, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Speranza, B.; Perricone, M.; Sinigaglia, M.; Corbo, M.R. Bioactivity of essential oils towards fungi and bacteria: Mode of action and mathematical tools. In Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 231–246. [Google Scholar]
- Bosquez-Molina, E.; Ronquillo-de Jesús, E.; Bautista-Baños, S.; Verde-Calvo, J.; Morales-López, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Mehdi, M.; Asgar, A.; Alderson, P.G. Effect of cinnamon oil on incidence of anthracnose disease and postharvest quality of bananas during storage. Int. J. Agric. Biol. 2010, 12, 516–520. [Google Scholar]
- Singh, P.; Pandey, A.K.; Sonker, N.; Tripathi, N. Preservation of Buchnania lanzan Spreng. seeds by Ocimum canum Sims. essential oil. Ann. Plant Prot. 2011, 19, 407–410. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Raba, D.N.; Poiana, M.-A.; Borozan, A.B.; Stef, M.; Radu, F.; Popa, M.-V. Investigation on crude and high-temperature heated coffee oil by ATR-FTIR spectroscopy along with antioxidant and antimicrobial properties. PLoS ONE 2015, 10, e0138080. [Google Scholar] [CrossRef]
- Bessada, S.M.; Alves, R.C.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Han, B.; Nazary-Vannani, A.; Talaei, S.; Clark, C.C.; Rahmani, J.; Rasekhmagham, R.; Kord-Varkaneh, H. The effect of green coffee extract supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 2918–2926. [Google Scholar] [CrossRef]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical profile and antioxidant capacity of coffee plant organs compared to green and roasted coffee beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.N.; Tuan, N.N.; Thang, T.D.; Kuo, P.-C.; Thanh, N.B.; Tam, L.N.; Tuoi, L.H.; Nguyen, T.H.; Vu, D.C.; Ho, T.L.; et al. Chemical Composition Analysis and Antioxidant Activity of Coffea robusta Monofloral Honeys from Vietnam. Foods 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, S.; Hanasasmita, N.; Sunarharum, W. Effect of different aroma extraction methods combined with GC-MS on the aroma profiles of coffee. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012044. [Google Scholar]
- Essien, S.O.; Young, B.; Baroutian, S. Technology, Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Cornelio-Santiago, H.P.; Gonçalves, C.B.; de Oliveira, N.A.; de Oliveira, A.L. Supercritical CO2 extraction of oil from green coffee beans: Solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J. Supercrit. Fluids 2017, 128, 386–394. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, L.; Feng, Z. Technical Study on Extraction of Procyanidins in Lycium ruthenicum Murr. Using Sub-critical Fluid 1, 1, 1, 2-Tetrafluoroethane (R134a) and Content Determination from Different Producing Areas. J. Med. Plant 2018, 9, 36–44. [Google Scholar]
- Mustapa, A.; Manan, Z.; Azizi, C.M.; Setianto, W.; Omar, A.M. Extraction of β-carotenes from palm oil mesocarp using sub-critical R134a. Food Chem. 2011, 125, 262–267. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Q.; Wang, L.; Xue, C. Extraction of astaxanthin from Euphausia pacific using subcritical 1,1,1,2-tetrafluoroethane. J. Ocean Univ. China 2012, 11, 562–568. [Google Scholar] [CrossRef]
- Kwon, H.-L.; Chung, M.-S. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015, 185, 58–64. [Google Scholar] [CrossRef]
- Corr, S. 1, 1, 1, 2-tetrafluoroethane (R-134a): A selective solvent for the generation of flavor and fragrance ingredients. In Natural Flavors and Fragrances; ACS Publications: Washington, DC, USA, 2005; Volume 908, pp. 41–59. [Google Scholar]
- Sang, J.; Wang, H.; Jin, J.; Meng, H. Comparison and modelling of rutin solubility in supercritical carbon dioxide and subcritical 1, 1, 1, 2-tetrafluoroethane. J. CO2 Util. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Srisook, K.; Salee, P.; Charoensuk, Y.; Srisook, E. In vitro anti-oxidant and anti-tyrosinase activities of the rhizomal extractsfrom Amomum biflorum Jack. Thai J. Bot. 2010, 2, 143–150. [Google Scholar]
- Rodglin, C.; Srisook, E.; Srisook, K. Effects of Extraction Conditions on Total Phenolic Content, Total Flavonoid Content and Antioxidant Activities of Different Parts of Citrus aurantium L. Burapha Sci. J. 2017, 22, 211–225. [Google Scholar]
- Kantawong, F.; Singhatong, S.; Srilamay, A.; Boonyuen, K.; Mooti, N.; Wanachantararak, P.; Kuboki, T. Properties of macerated herbal oil. BioImpacts BI 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Klaokwan, S.; Doungnapa, B.; Rattiya, B.; Panadda, S.; Yaowaluck, C.; Ekaruth, S. Antioxidant and anti-inflammatory activities of hot water extract from Pluchea indica Less. herbal tea. J. Med. Plant Res. 2012, 6, 4077–4408. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Pokorná, J.; Venskutonis, P.; Kraujalyte, V.; Kraujalis, P.; Dvořák, P.; Tremlová, B.; Kopřiva, V.; Ošťádalová, M. Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Alimentaria 2015, 44, 454–460. [Google Scholar] [CrossRef]
- The European Society of Clinical Microbiology and Infectious Diseases. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method; Version 7.0; European Society of Clinical Microbiology and Infectious Diseases: Växjö, Sweden, 2019. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, D.; del Pilar Sánchez-Camargo, A. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Prokopchuk, D.; Kostenko, M.; Pokrovskiy, O. Spontaneous Precipitation of Caffeine from Supercritical Extracts of Roasted Coffee Beans. Theor. Found. Chem. Eng. 2021, 55, 1010–1015. [Google Scholar] [CrossRef]
- Muangrat, R.; Pongsirikul, I. Recovery of spent coffee grounds oil using supercritical CO2: Extraction optimisation and physicochemical properties of oil. CYTA J. Food 2019, 17, 334–346. [Google Scholar] [CrossRef]
- Setapar, S.H.M.; Khatoon, A.; Ahmad, A.; Yunus, M.-A.C.; Zaini, M.A.A. Use of supercritical CO2 and R134a as a solvent for extraction of β-carotene and α-tocopherols from crude palm oil. Asian J. Chem. 2014, 26, 5911–5916. [Google Scholar] [CrossRef]
- Li, S.; Ong, C.; Lee, M.; Lee, H. Supercritical fluid extraction and chromatography of steroids with Freon-22. J. Chromatogr. 1990, 515, 515–520. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Han, Y.; Xue, C. Optimization of subcritical fluid extraction of carotenoids and chlorophyll a from Laminaria japonica Aresch by response surface methodology. J. Sci. Food Agric. 2014, 94, 139–145. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Solubility of R22, R23, R32, R134a, R152a, R125 and R744 refrigerants in water by using equations of state. Int. J. Refrig. 2013, 36, 1681–1688. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Composition, phenolic content, antioxidant and antimicrobial activity of Pistacia atlantica subsp. kurdica hulls’ essential oil. Food Biosci. 2020, 34, 100510. [Google Scholar] [CrossRef]
- Sihite, N.W.; Rusmarilin, H.; Rotua, M. Analysis of The Phytochemical Characteristics of Jasmine Flower Against Escherichia coli and Staphylococcus aureus. Microbiol. Indones. 2021, 15, 4. [Google Scholar] [CrossRef]
- Sulewska, A.M.; Larsen, F.H.; Sørensen, J.K.; Pedersen, A.H. Advanced instrumental characterization of the coffee extracts produced by pilot scale instant coffee process. Eur. Food Res. Technol. 2021, 247, 1379–1387. [Google Scholar] [CrossRef]
- Thammarat, P.; Kulsing, C.; Wongravee, K.; Leepipatpiboon, N.; Nhujak, T. Identification of volatile compounds and selection of discriminant markers for elephant dung coffee using static headspace gas chromatography—Mass spectrometry and chemometrics. Molecules 2018, 23, 1910. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Viegas, M.C.; De Conti, A.J.; Corso, M.P.; de Toledo Benassi, M. Steam pressure treatment of defective Coffea canephora beans improves the volatile profile and sensory acceptance of roasted coffee blends. Food Res. Int. 2018, 105, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Dan, M.; Kovacs, M.H.; Kovacs, E.D.; Levei, E.A.; Cadar, O. Analysis of Volatile Compounds, Composition, and Thermal Behavior of Coffee Beans According to Variety and Roasting Intensity. Foods 2022, 11, 3146. [Google Scholar] [CrossRef] [PubMed]
- Burdan, F. Caffeine in coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Cambridge, MA, USA, 2015; pp. 201–207. [Google Scholar]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Technology, Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Shibamoto, T. Volatile chemicals from thermal degradation of less volatile coffee components. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Cambridge, MA, USA, 2015; pp. 129–135. [Google Scholar]


| Microbial Strain | Antibiotic Control | HFC-134a (25% Coffee Extracts) | HCFC-22 (50% Coffee Extracts) | SCCO2 (33% Coffee Extracts) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chloram- phenicol | Tetra- cycline | RT | FR | Cont | RT | FR | Cont | SCCO2 | SCCO2 + EtOH | SCCO2 + THF | |
| Gram-positive | |||||||||||
| B. cereus | 27.0 | 33.0 | 8.0 | 7.0 | 8.0 | 6.0 | 7.0 | 8.0 | 10.0 | 9.0 | 9.0 |
| C. diphtheriae | 38.0 | 35.0 | 20.0 | 15.0 | 16.0 | 12.0 | 10.0 | NI | 16.0 | 18.0 | 19.0 |
| E. faecalis | 24.0 | 20.0 | NI | NI | NI | NI | NI | 8.0 | 8.0 | 8.0 | 8.0 |
| E. faecium | 29.0 | 30.0 | NI | 7.0 | NI | 7.0 | 7.0 | NI | 10.0 | 9.0 | 9.0 |
| L. innocua | 33.0 | 40.0 | 10.0 | 9.3 | 8.0 | 7.0 | 8.7 | 7.3 | 10.0 | 9.0 | 9.0 |
| L. monocytogenes | 33.0 | 39.0 | NI | NI | NI | NI | NI | NI | 8.0 | 8.0 | 8.0 |
| S. aureus | 26.0 | 33.0 | 8.0 | 8.0 | 8.0 | 6.0 | 8.0 | NI | 8.0 | 8.0 | 8.0 |
| Gram-negative | |||||||||||
| E. tarda | 21.0 | 15.0 | 8.0 | 7.0 | 7.0 | 7.0 | 7.0 | 9.0 | 10.0 | 8.0 | 8.0 |
| E. coli | 21.3 | 11.0 | 6.0 | NI | NI | NI | NI | NI | NI | NI | NI |
| K. pneumonia | 25.0 | 20.0 | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| S. typhimurium | 26.0 | 26.0 | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| S. marcescens | 22.0 | 13.0 | NI | NI | NI | NI | NI | NI | 8.0 | NI | NI |
| P. mirabillis | 20.0 | NI | NI | 8.0 | 7.0 | NI | NI | 11.0 | NI | NI | NI |
| P. aeruginosa | 18.0 | 17.0 | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| Y. enterocolitica | 22.0 | 24.0 | 8.0 | 7.0 | 8.0 | NI | 7.0 | 7.0 | 11.0 | 10.0 | 10.0 |
| Name | RT (Min) | LRI | CAS No. | Content (%) | |||
|---|---|---|---|---|---|---|---|
| Exp a | Ref b | HFC-134a | HCFC-22 | SCCO2 w/THF | |||
| 2-Hexanol 2 | 3.35 | 809 | 803 | 108-11-2 | 0.10 | 0.31 | 0.19 |
| Butanoic acid, 3-methyl- 1 | 3.65 | 826 | 827 | 503-74-2 | 0.02 | - | - |
| 2-Furanmethanol 6 | 4.04 | 849 | 845 | 98-00-0 | 0.09 | 0.10 | 0.16 |
| Phenol 11 | 6.74 | 978 | 978 | 108-95-2 | 0.07 | 0.02 | 0.08 |
| 2-Furanmethanol, acetate 6 | 7.14 | 995 | 998 | 623-17-6 | 0.01 | 0.02 | - |
| Pyrazine, 2-ethyl-6-methyl- 13 | 7.23 | 999 | 997 | 13925-03-6 | 0.01 | 0.02 | - |
| Pyrazine, trimethyl- 13 | 7.34 | 1003 | 1008 | 14667-55-1 | 0.01 | - | - |
| 1H-Pyrrole-2-carboxaldehyde 16 | 7.44 | 1007 | 1009 | 1003-29-8 | 0.01 | - | 0.03 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- 9 | 7.94 | 1025 | 1022 | 80-71-7 | 0.03 | - | - |
| D-Limonene 17 | 8.06 | 1029 | 1029 | 5989-27-5 | - | - | 0.03 |
| Ethanone, 1-(1H-pyrrol-2-yl)- 16 | 8.85 | 1059 | 1060 | 1072-83-9 | 0.10 | - | 0.11 |
| 1H-Pyrrole, 3-ethyl-2,4-dimethyl- 16 | 9.27 | 1074 | 1075 | 517-22-6 | 0.02 | - | - |
| Pyrazine, 3-ethyl-2,5-dimethyl- 13 | 9.41 | 1079 | 1078 | 13360-65-1 | 0.04 | 0.02 | - |
| Pyrazine, 2-ethyl-3,5-dimethyl- 13 | 9.62 | 1087 | 1085 | 13925-07-0 | 0.02 | - | - |
| Phenol, 2-methoxy- 11 | 9.69 | 1089 | 1092 | 90-05-1 | 0.20 | 0.05 | 0.08 |
| 3-Pyridinol 14 | 9.93 | 1098 | NR | 109-00-2 | - | - | 0.12 |
| Pyrazine, (1-methylethenyl)- 13 | 10.17 | 1107 | 1092 | 38713-41-6 | 0.04 | - | |
| Maltol 15 | 10.28 | 1111 | 1110 | 118-71-8 | 0.10 | - | 0.13 |
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- 9 | 10.48 | 1118 | 1121 | 21835-01-8 | 0.09 | - | 0.08 |
| Nicotinyl alcohol 14 | 10.57 | 1121 | 1119 | 100-55-0 | 0.03 | - | 0.03 |
| Methyl nicotinate 14 | 11.06 | 1139 | 1140 | 93-60-7 | 0.02 | - | - |
| 5H-5-Methyl-6,7-dihydrocyclopentapyrazine 13 | 11.11 | 1140 | 1147 | 23747-48-0 | 0.02 | - | - |
| 3-Pyridinol, 6-methyl- 14 | 11.44 | 1152 | NR | 1121-78-4 | - | - | 0.04 |
| 2,4,6-Trimethyl-1,3-phenylenediamine 3 | 11.60 | 1158 | 1159 | 3102-70-3 | 0.04 | - | - |
| 1H-Pyrrole, 1-(2-furanylmethyl)- 16 | 12.32 | 1184 | 1185 | 1438-94-4 | 0.11 | - | - |
| Pyrazine, 2-methyl-3-(2-propenyl)- 13 | 12.61 | 1194 | NR | 55138-62-0 | 0.06 | - | - |
| Catechol 11 | 12.67 | 1196 | 1200 | 120-80-9 | - | - | 0.19 |
| Ethanone, 1-(2,5-dihydroxyphenyl)- 11 | 14.84 | 1276 | NR | 490-78-8 | 0.15 | - | 0.10 |
| Phenol, 4-ethyl-2-methoxy- 11 | 14.95 | 1280 | 1287 | 2785-89-9 | 0.90 | 0.15 | 0.35 |
| Indole 8 | 15.32 | 1293 | 1295 | 120-72-9 | 0.09 | 0.02 | 0.05 |
| Furan, 2,2’-[oxybis(methylene)]bis- 6 | 15.57 | 1303 | 1305 | 4437-22-3 | 0.11 | 0.01 | - |
| Ethanone, 1-(2-hydroxy-5-methylphenyl)- 11 | 15.88 | 1314 | 1316 | 1450-72-2 | 1.20 | 0.14 | 0.42 |
| 5-Aminoindole 8 | 16.26 | 1329 | NR | 5192-03-0 | 0.05 | - | 0.02 |
| 2-Naphthalenol, 5-amino- 18 | 16.73 | 1347 | NR | 86-97-5 | 0.04 | - | 0.02 |
| 4-Hydroxy-7-methyl-1,8-naphthylidine 10 | 16.91 | 1354 | NR | 1569-18-2 | 0.10 | - | - |
| 4-Ethylcatechol 11 | 17.64 | 1382 | 1392 | 1124-39-6 | - | 0.01 | 0.11 |
| 2-Cyclohexen-1-one, 4-hydroxy-3,5,5-trimethyl-4-(3-oxo-1-butenyl)- 17 | 17.80 | 1388 | NR | 7070-24-8 | 0.17 | - | 0.07 |
| p-tert-Butyl catechol 11 | 20.26 | 1487 | 1493 | 98-29-3 | 0.06 | - | - |
| Butylated Hydroxytoluene 11 | 20.96 | 1516 | 1514 | 128-37-0 | - | 0.04 | 2.01 |
| 5-tert-Butylpyrogallol 11 | 21.25 | 1528 | 1526 | 20481-17-8 | 0.05 | - | - |
| 4-(2,4-Dimethoxyphenyl)butan-2-one 11 | 23.73 | 1635 | NR | 93467-61-9 | 0.04 | - | - |
| Benzenemethanol, 3-hydroxy-5-methoxy- 11 | 27.55 | 1812 | NR | 30891-29-3 | 0.08 | - | - |
| 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-, methyl ester 11 | 28.13 | 1840 | 1854 | 2309-07-1 | 0.11 | - | 0.03 |
| Caffeine 12 | 28.63 | 1865 | 1848 | 58-08-2 | 73.43 | 22.87 | 68.51 |
| Hexadecanoic acid, methyl ester 4 | 29.86 | 1927 | 1926 | 112-39-0 | 1.25 | 0.15 | 0.38 |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- 16 | 30.07 | 1938 | 1908 | 5654-86-4 | 0.38 | 0.02 | 0.14 |
| n-Hexadecanoic acid 1 | 30.57 | 1964 | 1963 | 57-10-3 | 0.20 | - | 0.29 |
| Hexadecanoic acid, ethyl ester 4 | 31.17 | 1995 | 1993 | 628-97-7 | 0.12 | 0.01 | 0.08 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester 4 | 33.04 | 2095 | 2092 | 112-63-0 | 0.62 | 0.05 | 0.18 |
| Oleic acid 1 | 33.14 | 2101 | 2102 | 112-80-1 | 0.27 | - | 0.07 |
| Methyl stearate 4 | 33.61 | 2128 | 2128 | 112-61-8 | 0.18 | 0.03 | 0.07 |
| Linoleic acid ethyl ester 4 | 34.24 | 2163 | 2163 | 544-35-4 | 0.17 | - | 0.07 |
| Hexadecanamide 10 | 34.53 | 2179 | 2182 | 629-54-9 | 0.11 | 0.10 | 0.27 |
| Eicosanoic acid, methyl ester 4 | 37.07 | 2329 | 2335 | 1120-28-1 | 0.18 | - | - |
| 9-Octadecenamide, (Z)- 10 | 37.76 | 2371 | 2375 | 301-02-0 | 0.33 | 0.59 | 0.77 |
| 5-Pregnen-3.beta.-ol-20-one, propionate 17 | 40.18 | 2525 | 2487 | 1000368-37-1 | 2.85 | 4.34 | 3.53 |
| 5-Pregnen-3.beta-ol-20-one, butyrate 17 | 40.42 | 2541 | 2546 | 1000368-37-2 | 8.14 | 7.73 | 8.07 |
| Squalene 17 | 44.61 | 2832 | 2847 | 111-02-4 | 0.25 | - | - |
| Stigmasterol 17 | 50.20 | 3268 | 3249 | 83-48-7 | - | - | 3.00 |
| Acids 1 | 0.49 | - | 0.36 | ||||
| Alcohols 2 | 0.10 | 0.31 | 0.19 | ||||
| Anilines 3 | 0.04 | - | - | ||||
| Esters 4 | 2.52 | 0.24 | 0.78 | ||||
| Furanones 5 | - | - | - | ||||
| Furans 6 | 0.21 | 0.13 | 0.16 | ||||
| Hydrocarbons 7 | - | - | - | ||||
| Indoles 8 | 0.14 | 0.02 | 0.07 | ||||
| Ketones 9 | 0.12 | - | 0.08 | ||||
| Miscellaneous nitrogen compounds 10 | 0.54 | 0.69 | 1.04 | ||||
| Phenolic compounds 11 | 2.86 | 0.41 | 3.37 | ||||
| Purines 12 | 73.43 | 22.87 | 68.51 | ||||
| Pyrazines 13 | 0.20 | 0.04 | - | ||||
| Pyridines 14 | 0.05 | - | 0.19 | ||||
| Pyrones 15 | 0.10 | - | 0.13 | ||||
| Pyrroles 16 | 0.62 | 0.02 | 0.28 | ||||
| Terpenes and terpenoids 17 | 11.41 | 12.07 | 14.70 | ||||
| Others 18 | 0.04 | - | 0.02 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supanivatin, P.; Thipayarat, A.; Siriwattanayotin, S.; Ekkaphan, P.; Deepatana, A.; Wongwiwat, J. A Comparative Analysis of Phenolic Content, Antioxidant Activity, Antimicrobial Activity, and Chemical Profile of Coffea robusta Extracts Using Subcritical Fluid Extraction and Supercritical Carbon Dioxide Extraction. Foods 2023, 12, 3443. https://doi.org/10.3390/foods12183443
Supanivatin P, Thipayarat A, Siriwattanayotin S, Ekkaphan P, Deepatana A, Wongwiwat J. A Comparative Analysis of Phenolic Content, Antioxidant Activity, Antimicrobial Activity, and Chemical Profile of Coffea robusta Extracts Using Subcritical Fluid Extraction and Supercritical Carbon Dioxide Extraction. Foods. 2023; 12(18):3443. https://doi.org/10.3390/foods12183443
Chicago/Turabian StyleSupanivatin, Pattarin, Aluck Thipayarat, Suwit Siriwattanayotin, Paweena Ekkaphan, Anat Deepatana, and Jakrapop Wongwiwat. 2023. "A Comparative Analysis of Phenolic Content, Antioxidant Activity, Antimicrobial Activity, and Chemical Profile of Coffea robusta Extracts Using Subcritical Fluid Extraction and Supercritical Carbon Dioxide Extraction" Foods 12, no. 18: 3443. https://doi.org/10.3390/foods12183443
APA StyleSupanivatin, P., Thipayarat, A., Siriwattanayotin, S., Ekkaphan, P., Deepatana, A., & Wongwiwat, J. (2023). A Comparative Analysis of Phenolic Content, Antioxidant Activity, Antimicrobial Activity, and Chemical Profile of Coffea robusta Extracts Using Subcritical Fluid Extraction and Supercritical Carbon Dioxide Extraction. Foods, 12(18), 3443. https://doi.org/10.3390/foods12183443






