Influence of Variety, Enzyme Addition and Destemming on Yield and Bioactive Compounds of Juices from Selected Hybrid Grape Varieties Cultivated in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapes
2.2. Reagents, Enzymes and Standards
2.3. Juice Processing
2.4. Dry Matter, Total Soluble Solid, Titratable Acidity and Pectin
2.5. Color (L*a*b)
2.6. Turbidity and Viscosity
2.7. Antioxidant Capacity
2.8. Total Phenolic Content
2.9. Identification and Quantification of Polyphenols
2.10. Statistical Analysis
3. Results
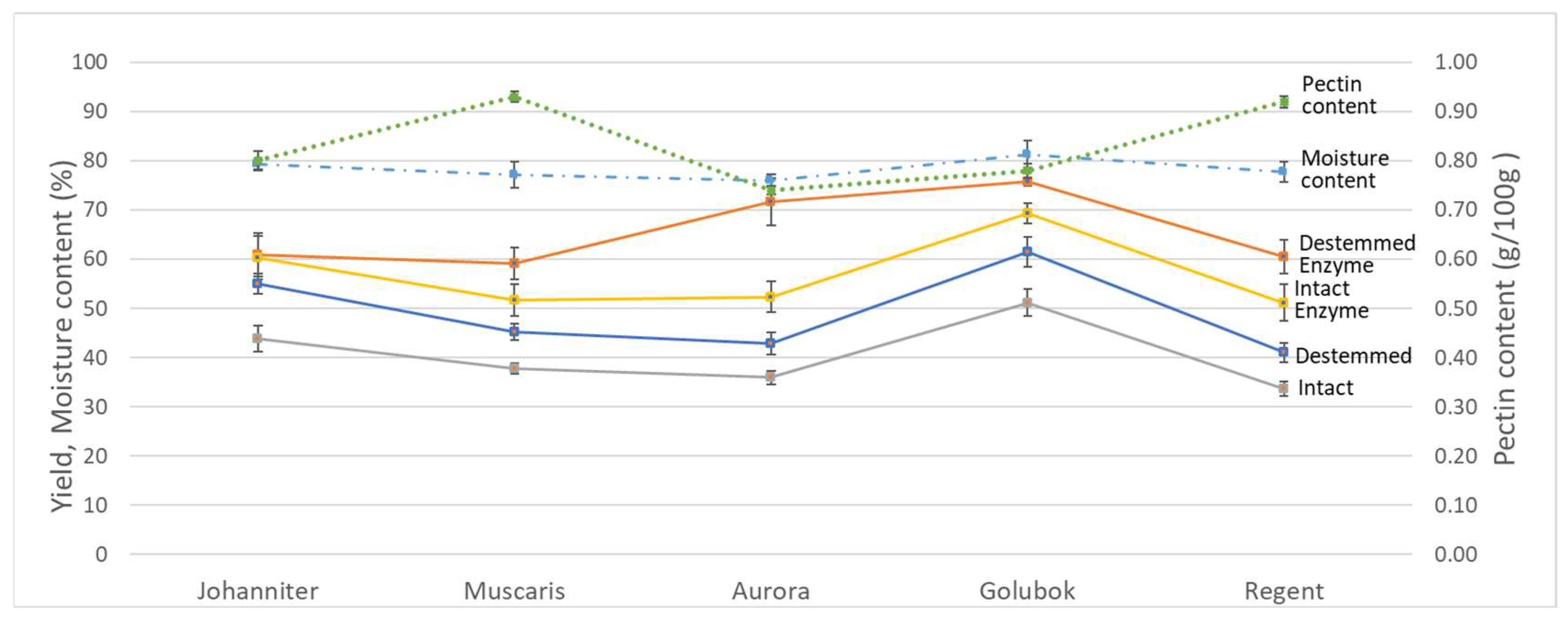
3.1. Yield, Initial Moisture Content and Pectin Content
3.2. Physicochemical Properties
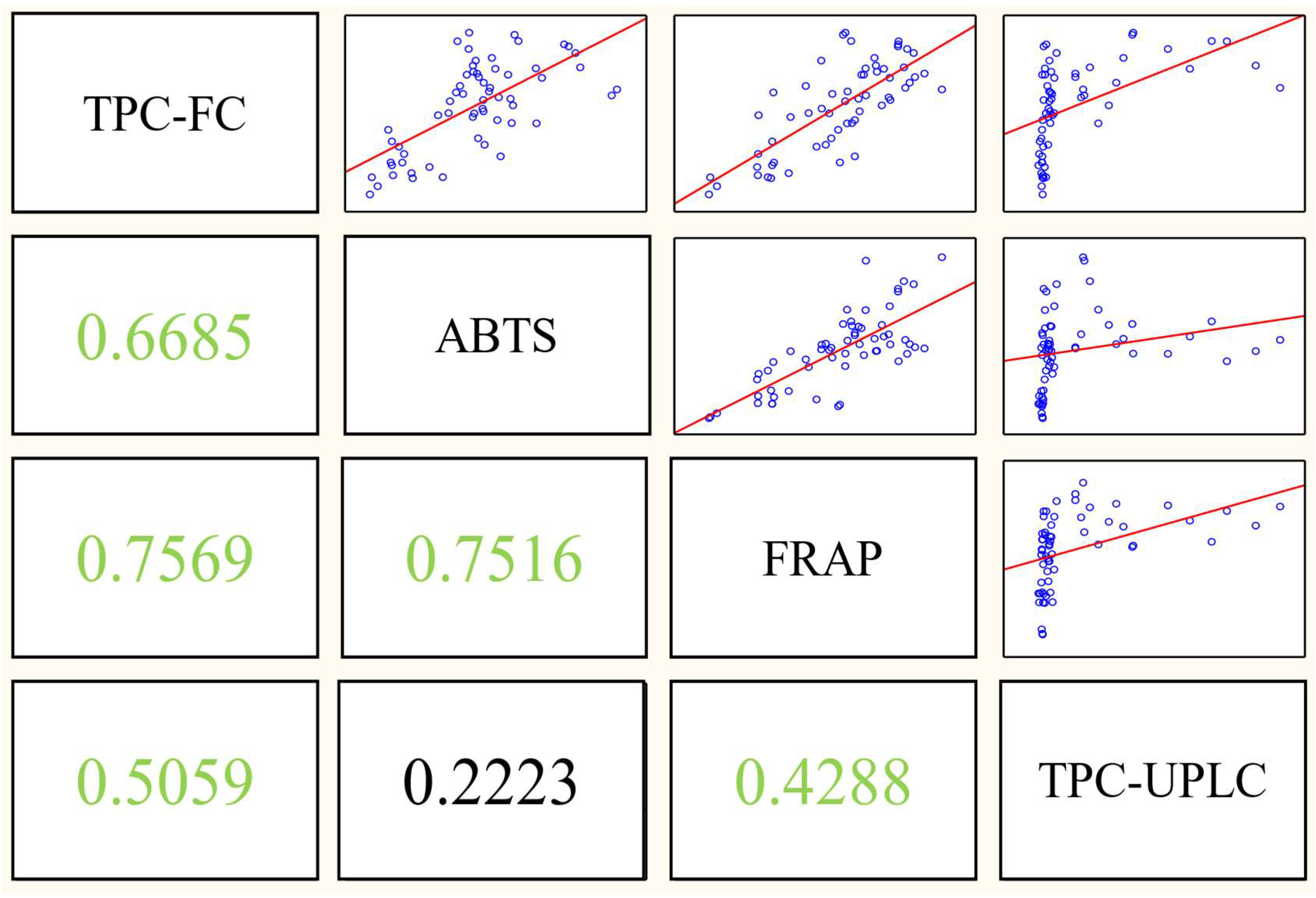
3.3. Total Polyphenol and Antioxidant Capacity
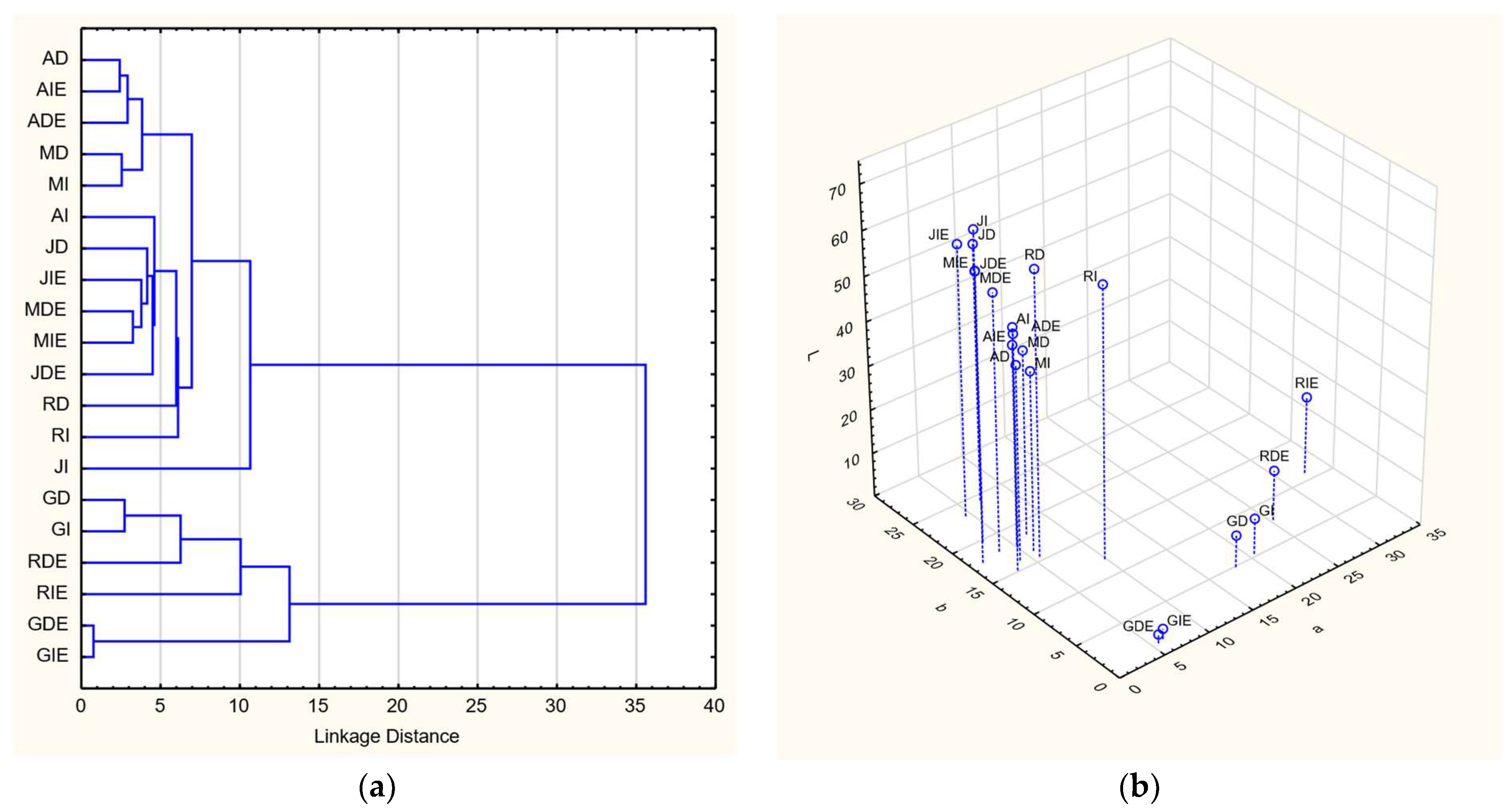
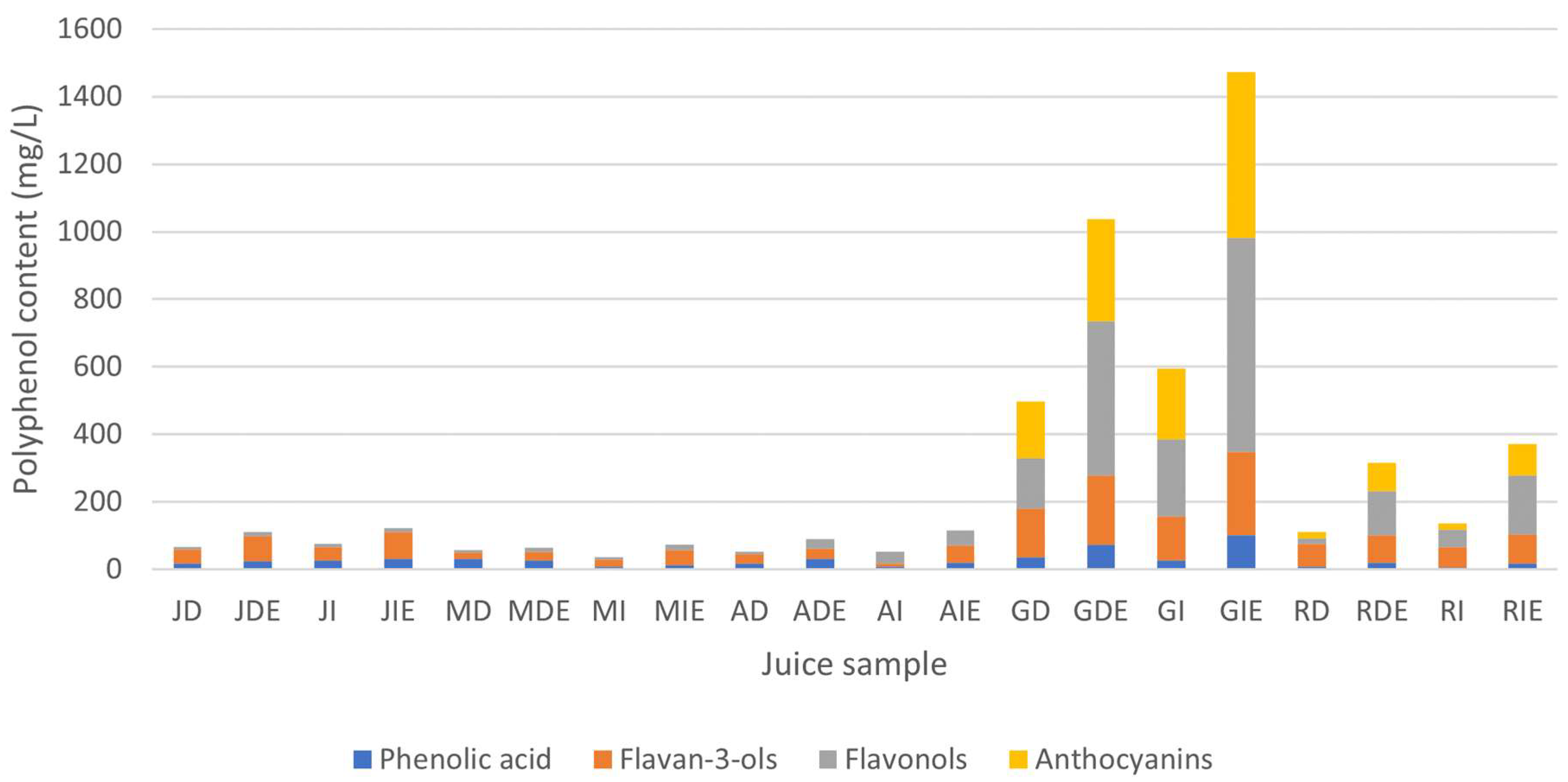
3.4. Individual Polyphenol Content
4. Conclusions
- (1)
- Regardless of the variety, enzyme addition and the destemming process improved the yield by 20–30% and 10–15%, respectively.
- (2)
- The addition of enzymes increased polyphenol content but, at the same time, altered the color of the juice. Meanwhile, the destemming process eliminated the potential health benefits present in the stems.
- (3)
- Although similar treatments were applied, the total polyphenol content and antioxidant capacity of red grape juice were higher than those of white grape juice. This indicates that the characteristics of each variety play an important role in determining the health properties of the juice.
- (4)
- A total of 25 polyphenolic compounds were identified in the grape juices. However, the polyphenolic profile was dependent on variety and processing.
- (5)
- In summary, this study contributes significantly to understanding how specific grape varieties and processing techniques collectively influence the quality of grape juice. These insights can potentially guide and advance the production of grape juice, leading to improved quality and health-enhancing benefits, especially relevant in challenging climatic conditions similar to those encountered in Poland.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Freitas Laiber Pascoal, G.; de Almeida Sousa Cruz, M.A.; Pimentel de Abreu, J.; Santos, M.C.B.; Bernardes Fanaro, G.; Júnior, M.R.M.; Freitas Silva, O.; Moreira, R.F.A.; Cameron, L.C.; Simões Larraz Ferreira, M.; et al. Evaluation of the Antioxidant Capacity, Volatile Composition and Phenolic Content of Hybrid Vitis vinifera L. Varieties Sweet Sapphire and Sweet Surprise. Food Chem. 2022, 366, 130644. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Pitucha, I.; Woznica, A. Comparison of Bioactive Compound Contents in Red Wines from Different European Countries. Zywn. Nauk. Technol. Jakosc 2009, 4, 142–148. [Google Scholar]
- Samoticha, J.; Wojdyło, A.; Golis, T. Phenolic Composition, Physicochemical Properties and Antioxidant Activity of Interspecific Hybrids of Grapes Growing in Poland. Food Chem. 2017, 215, 263–273. [Google Scholar] [CrossRef]
- Council Regulation (EC) 2165/2005 of 20 December 2005 Amending Regulation (EC) No 1493/1999 on the Common Organisation of the Market in Wine. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2165 (accessed on 3 May 2023).
- Jackson, D.; Schuster, D. The Production of Grapes & Wine in Cool Climates; Dunmore Press Ltd.: Auckland, New Zealand, 2001. [Google Scholar]
- Czaplicka, M.; Parypa, K.; Szewczuk, A.; Gudarowska, E.; Rowińska, M.; Zubaidi, M.A.; Nawirska-Olszańska, A. Assessment of Selected Parameters for Determining the Internal Quality of White Grape Cultivars Grown in Cold Climates. Appl. Sci. 2022, 12, 5534. [Google Scholar] [CrossRef]
- Leis, D.; Renner, W.; Leitner, E. Characterisation of Wines Produced from Fungus Resistant Grape Varieties. In BT-Flavour Science; Verlag der Technischen Universität Graz: Graz, Austria, 2018; pp. 511–514. [Google Scholar]
- Porro, D.; Wolf, M.; Pedò, S. Evaluation of Mechanical Properties of Berries on Resistant or Tolerant Varieties of Grapevine. Bio Web Conf. 2019, 13, 01005. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Robak, J.; Fortuna, T.; Buksa, K. Characterization of Polish Wines Produced from the Multispecies Hybrid and Vitis vinifera L. Grapes. Int. J. Food Prop. 2015, 18, 699–713. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.d.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the Bioactive Compounds and the Antioxidant Capacity of Grape Pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of Polish Wines Produced from the Interspecific Hybrid Grapes Grown in South-East Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Bieniasz, M.; Kostecka-Gugała, A. The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’as a Factor Determining the Method of Producing Straw Wines. Foods 2022, 11, 1027. [Google Scholar] [CrossRef]
- Stój, A.; Kapusta, I.; Domagała, D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342. [Google Scholar] [CrossRef]
- Dal Magro, L.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Identification of Bioactive Compounds from Vitis labrusca L. Variety Concord Grape Juice Treated with Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioprocess Technol. 2016, 9, 365–377. [Google Scholar] [CrossRef]
- Souquet, J.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- PN-90/A-75101/04; Fruit and Vegetable Products. Preparation of Samples and Test Methods. Determination of Total Acidity. Polski Komitet Normalizacyjny: Warsaw, Poland; p. 1990.
- Pijanowski, E.; Mrożewski, S.; Horubała, A.; Jarczyk, A. Technologia Produktów Owocowych i Warzywnych; Państwowe Wydawnictwa Rolnicze i Leśne: Warsaw, Poland, 1973; Volume 1. [Google Scholar]
- Chettri, S.; Sharma, N.; Mohite, A.M. Utilization of Lima Bean Starch as an Edible Coating Base Material for Sapota Fruit Shelf-Life Enhancement. J. Agric. Food Res. 2023, 12, 100615. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical Composition and Antioxidant Capacity of Different Anatomical Parts of Pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of Vacuum Impregnation with Apple-Pear Juice on Content of Bioactive Compounds and Antioxidant Activity of Dried Chokeberry Fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of Phenolic Compounds in Different Anatomical Pear (Pyrus communis L.) Parts by Ultra-Performance Liquid Chromatography Photodiode Detector-Quadrupole/Time of Flight-Mass Spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Ramadan, M.F. Enzymes in Fruit Juice Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132807. [Google Scholar]
- Guerrini, L.; Corti, F.; Angeloni, G.; Masella, P.; Spadi, A.; Calamai, L.; Parenti, A. The Effects of Destemming/Crushing and Pressing Conditions in Rosé Wine Production. Aust. J. Grape Wine Res. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Bendaali, Y.; Vaquero, C.; González, C.; Morata, A. Elaboration of an Organic Beverage Based on Grape Juice with Positive Nutritional Properties. Food Sci. Nutr. 2022, 10, 1768–1779. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of Pectinase Treatment on Extraction of Antioxidant Phenols from Pomace, for the Production of Puree-Enriched Cloudy Apple Juices. Food Chem. 2011, 127, 623–631. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, ISBN 0470010355. [Google Scholar]
- Guler, A. Effects of Different Maceration Techniques on the Colour, Polyphenols and Antioxidant Capacity of Grape Juice. Food Chem. 2023, 404, 134603. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic Compounds and Antioxidant Activity of Spanish Commercial Grape Juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, I.M.; Cruz, F.A.; Alves, T.D.L.; De Gois, J.S.; Borges, D.L.G.; Cunha, H.P.; Da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive Potential of Vitis labrusca L. Grape Juices from the Southern Region of Brazil: Phenolic and Elemental Composition and Effect on Lipid Peroxidation in Healthy Subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef]
| Cultivars | Parents | Skin Color | Pulp Color |
|---|---|---|---|
| Johanniter | Riesling and Seyve-Villard × Ruländer × Gutedel | Yellowish green | White |
| Muscaris | Muskateller × Solaris | Green | White |
| Aurora | Seibel 788 × Seibel 29 | Green | White |
| Golubok | Severnyy × pollen from different varieties: 40 Let Okyabrya, Odesskiy Ranniy and No. 1-17-54 (Alicante Bouschet and Cabernet Sauvignon) | Dark | Dark |
| Regent | Diana × Chambourcin | Dark | White |
| Sample | JD | JDE | JI | JIE | MD | MDE | MI | MIE | AD | ADE | AI | AIE | GD | GDE | GI | GIE | RD | RDE | RI | RIE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety | Johanniter | Muscaris | Aurora | GoIubok | Regent | |||||||||||||||
| Grape Type | White | White | White | Red | Red | |||||||||||||||
| Destemmed | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | No |
| Enzyme | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Sample | Dry Mass | TA | TSS | Turbidity | Viscosity |
|---|---|---|---|---|---|
| JD | 19.63 ± 0.01 efg | 0.73 ± 0.05 a | 18.92 ± 0.32 c | 297 ± 31 abcd | 5.95 ± 0.44 gh |
| JDE | 19.22 ± 0.15 cdef | 0.87 ± 0.07 a | 18.60 ± 0.25 bc | 366 ± 49 cd | 3.90 ± 0.31 cde |
| JI | 19.71 ± 0.00 fg | 0.65 ± 0.05 a | 19.05 ± 0.30 c | 197 ± 14 ab | 5.24 ± 0.36 efg |
| JIE | 21.32 ± 0.06 h | 0.79 ± 0.07 a | 20.21 ± 0.33 d | 214 ± 12 abc | 3.11 ± 0.28 abcd |
| MD | 19.10 ± 0.02 cde | 0.58 ± 0.04 a | 18.53 ± 0.22 bc | 179 ± 11 ab | 5.97 ± 0.54 gh |
| MDE | 17.86 ± 0.08 a | 0.75 ± 0.07 a | 17.45 ± 0.21 a | 233 ± 28 abcd | 4.21 ± 0.21 de |
| MI | 19.71 ± 0.24 fg | 0.53 ± 0.04 a | 19.07 ± 0.28 c | 190 ± 15 ab | 5.65 ± 0.39 gh |
| MIE | 19.37 ± 0.05 cdef | 0.69 ± 0.05 a | 18.74 ± 0.30 c | 231 ± 16 abcd | 3.04 ± 0.24 abcd |
| AD | 21.58 ± 0.14 hi | 0.63 ± 0.05 a | 20.82 ± 0.33 de | 855 ± 63 f | 6.30 ± 1.10 gh |
| ADE | 21.18 ± 0.15 h | 0.63 ± 0.05 a | 20.35 ± 0.30 de | 658 ± 73 e | 3.34 ± 0.15 bcd |
| AI | 21.47 ± 0.13 h | 0.49 ± 0.04 a | 20.78 ± 0.28 de | 317 ± 28 bcd | 1.96 ± 0.22 ab |
| AIE | 21.95 ± 0.01 i | 0.63 ± 0.05 a | 21.14 ± 0.30 e | 393 ± 30 g | 3.96 ± 0.26 cde |
| GD | 19.03 ± 0.04 cd | 0.73 ± 0.07 a | 18.72 ± 0.24 bc | 905 ± 85 f | 1.93 ± 0.30 a |
| GDE | 18.92 ± 0.05 bc | 0.69 ± 0.06 a | 18.74 ± 0.30 c | 915 ± 73 f | 6.90 ± 0.47 i |
| GI | 18.92 ± 0.00 bc | 0.71 ± 0.05 a | 18.48 ± 0.26 bc | 874 ± 68 f | 2.97 ± 0.44 abcd |
| GIE | 19.46 ± 0.02 cdef | 0.65 ± 0.05 a | 19.09 ± 0.27 c | 992 ± 11 f | 4.32 ± 0.10 def |
| RD | 18.47 ± 0.10 b | 0.69 ± 0.06 a | 17.83 ± 0.22 ab | 152 ± 16 a | 5.83 ± 0.92 gh |
| RDE | 19.50 ± 0.23 def | 0.67 ± 0.05 a | 19.00 ± 0.26 c | 896 ± 89 f | 5.10 ± 0.41 efg |
| RI | 20.08 ± 0.37 g | 0.71 ± 0.06 a | 19.15 ± 0.30 c | 221 ± 33 abc | 2.75 ± 0.22 abc |
| RIE | 19.24 ± 0.07 cdef | 0.71 ± 0.07 a | 18.68 ± 0.27 bc | 865 ± 11 f | 2.55 ± 0.35 abc |
| Sample | TPC (g GAE/100 mL) | TEAC ABTS (mmol TxE/L) | FRAP (mmol TxE/L) |
|---|---|---|---|
| JD | 13.17 ± 1.04 efg | 12.30 ± 1.52 ef | 4.63 ± 0.33 defg |
| JDE | 11.38 ± 0.98 cdef | 10.08 ± 1.26 cde | 2.59 ± 0.33 bc |
| JI | 15.32 ± 0.95 g | 17.42 ± 1.30 hi | 4.95 ± 0.39 efg |
| JIE | 11.53 ± 0.75 cdef | 10.76 ± 1.28 def | 3.83 ± 0.53 cdef |
| MD | 6.95 ± 0.48 ab | 6.18 ± 0.68 abc | 2.57 ± 0.32 bc |
| MDE | 8.67 ± 1.17 abcd | 5.47 ± 0.40 ab | 3.79 ± 0.27 cde |
| MI | 8.15 ± 0.85 abc | 5.72 ± 0.51 ab | 2.45 ± 0.17 ab |
| MIE | 6.68 ± 0.44 ab | 8.08 ± 1.10 bcd | 2.40 ± 0.15 ab |
| AD | 5.84 ± 0.61 a | 4.05 ± 0.30 a | 1.29 ± 0.09 a |
| ADE | 12.93 ± 1.88 efg | 11.12 ± 0.63 def | 3.52 ± 0.25 cde |
| AI | 9.24 ± 1.29 abcd | 12.73 ± 0.89 efg | 4.11 ± 0.28 defg |
| AIE | 12.81 ± 1.07 efg | 11.18 ± 1.43 def | 4.31 ± 0.37 defg |
| GD | 11.70 ± 1.45 def | 14.12 ± 1.93 fgh | 4.79 ± 0.65 defg |
| GDE | 15.33 ± 1.02 g | 12.89 ± 1.82 efg | 4.82 ± 0.59 efg |
| GI | 16.12 ± 1.00 g | 12.72 ± 1.65 eg | 4.26 ± 0.37 defg |
| GIE | 14.47 ± 1.66 fg | 11.38 ± 1.19 def | 5.08 ± 0.32 fgh |
| RD | 10.15 ± 1.30 bcde | 12.80 ± 1.19 efg | 3.82 ± 0.34 cdef |
| RDE | 13.21 ± 0.92 efg | 12.21 ± 0.88 ef | 5.42 ± 0.39 h |
| RI | 13.64 ± 1.63 efg | 16.43 ± 2.37 gh | 4.95 ± 0.58 efg |
| RIE | 13.11 ± 1.05 efg | 20.84 ± 1.45 i | 5.30 ± 0.80 g |
| Tentative Identification | Rt (min) | UV Max (nm) | [M-H]- (m/z) | MS/MS (m/z) | Group |
|---|---|---|---|---|---|
| Caffeic acid | 0.94 | 327/241 | 179.0506 | - | Phenolic acid |
| Caftaric acid | 3.26 | 327/241 | 311.0339 | 179.0277 | Phenolic acid |
| Coutaric acid | 4.15 | 326/218 | 295.0368 | 163.0318 | Phenolic acid |
| Fertaric acid | 4.62 | 326/241 | 325.0469 | 193.0431/149.0014 | Phenolic acid |
| Kaempferol-3-O-rutinoside | 2.99 | 343/240 | 593.1319 | 285.0628 | Flavonols |
| Quercetin-3-rutinoside | 6.66 | 350/251 | 609.1573 | 301.0521 | Flavonols |
| Kaempferol 3-O-galactoside | 6.79 | 351/255 | 447.2055 | 285.1445 | Flavonols |
| Quercetin-3-glucuronide | 6.90 | 350/241 | 477.065 | 301.0311 | Flavonols |
| Quercetin-3-galactoside | 7.04 | 352/255 | 463.0869 | 301.0279 | Flavonols |
| Quercetin-3-glucoside | 7.35 | 348/252 | 463.2199 | 301.0455 | Flavonols |
| Quercetin-3-rhamnoside | 7.64 | 342/241 | 447.0904 | 301.0325 | Flavonols |
| Kaempferol-3-O-glucoside | 7.71 | 347/241 | 447.0914 | 285.0387 | Flavonols |
| Isorhamnetin 3-glucoside | 8.12 | 352 | 477.1172 | 315.1055 | Flavonols |
| Dihydroquercetin-3,5-rhamnoside | 8.32 | 346/242 | 449.1120 | 303.0239 | Flavonols |
| Dihydrokaempferol-3-glucoside | 8.75 | 346/242 | 285.0774 | 257.2076 | Flavonols |
| Procyanidin trimer | 2.90 | 278 | 865.2130 | 575.1028/289.1090 | Flavan-3-ols |
| Procyanidin B2 | 4.00 | 279 | 577.1296 | 289.0646 | Flavan-3-ols |
| Procyanidin tetramer | 4.56 | 280 | 1153.2663 | 865.2170/577.1296/289.0458 | Flavan-3-ols |
| (−)-epicatechin | 5.21 | 280 | 289.0646 | - | Flavan-3-ols |
| (−)-epicatechin 3-gallate | 5.93 | 280 | 441.1541 | 289.0646 | Flavan-3-ols |
| Petunidyn-3-O-glucoside | 4.00 | 525/245 | 479.0999 | 317.0625 | Anthocyanins |
| Malvidyn-3-O-glucoside | 4.48 | 521/277 | 493.1141 | 331.0829 | Anthocyanins |
| Delphinidyn-3-O-(6′-acetyl)-glucoside | 5.17 | 525/277 | 507.1306 | 303.0517 | Anthocyanins |
| Petunidyn-3-O-(6″-p-coumaroyl)-glucoside | 7.83 | 529/280 | 625.1394 | 317.0465 | Anthocyanins |
| Malvidyn-3-O-(6″-caffeoyl)-glucoside | 8.40 | 531/283 | 655.1709 | 331.0641 | Anthocyanins |
| Sample | Anthocyanins | ||||
|---|---|---|---|---|---|
| D3O6aGlu | M3O6cGlu | M3OGlu | P3O6cGlu | P3OGlu | |
| GD | 50.52 ± 6.26 a | nd | nd | 6.36 ± 0.81 b | 112.00 ± 0.77 b |
| GDE | 94.75 ± 7.55 d | nd | 2.49 ± 0.20 a | 13.24 ± 2.19 c | 191.20 ± 10.69 c |
| GI | 69.46 ± 5.39 c | nd | nd | 8.66 ± 0.54 b | 131.22 ± 11.54 b |
| GIE | 183.01 ± 11.78 e | 24.04 ± 1.70 b | 2.47 ± 0.37 a | 13.10 ± 0.96 c | 269.24 ± 16.84 d |
| RD | 18.23 ± 2.87 b | nd | nd | nd | nd |
| RDE | 37.64 ± 4.18 a | nd | 2.15 ± 0.30 a | 1.19 ± 0.07 a | 41.48 ± 4.92 a |
| RI | 18.12 ± 3.11 b | nd | nd | nd | nd |
| RIE | 36.99 ± 5.42 a | 11.21 ± 0.24 a | 2.30 ± 0.33 a | 0.56 ± 0.05 a | 43.40 ± 6.06 a |
| Sample | Phenolic Acids | Flavan-3-ols | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CafA | CftA | CotA | FrtA | Ep | Ep3Gll | PB2 | PTt | PTm | |
| JD | 5.24 ± 0.05 b | nd | 7.93 ± 0.76 b | 3.71 ± 0.29 c | 14.87 ± 2.17 ab | nd | 4.43 ± 0.64 ab | 19.05 ± 1.38 c | 3.73 ± 0.08 ab |
| JDE | 6.80 ± 0.26 cd | nd | 8.02 ± 1.36 b | 10.32 ± 0.93 a | 10.05 ± 0.38 a | nd | 10.19 ± 1.48 bc | 42.97 ± 5.97 d | 11.27 ± 0.21 bcd |
| JI | 6.00 ± 0.82 bc | nd | 16.83 ± 0.13 c | 3.78 ± 0.28 c | 21.10 ± 1.80 bc | nd | 3.85 ± 0.34 ab | 11.05 ± 1.40 abc | 4.24 ± 0.57 abc |
| JIE | 5.83 ± 0.35 bc | nd | 15.29 ± 1.21 c | 10.26 ± 1.60 a | 11.71 ± 0.86 a | nd | 9.26 ± 0.86 abc | 44.44 ± 7.16 d | 13.05 ± 1.16 cde |
| MD | nd | 1.12 ± 0.09 a | 29.41 ± 1.57 d | nd | nd | 0.80 ± 0.07 a | 12.83 ± 0.19 cde | 3.95 ± 0.16 a | 3.17 ± 0.05 ab |
| MDE | nd | 1.95 ± 0.1 a | 25.68 ± 3.82 d | nd | nd | 1.27 ± 0.1 a | 12.93 ± 0.90 dce | 4.54 ± 0.29 a | 5.22 ± 0.42 ab |
| MI | nd | 0.92 ± 0.01 a | 7.81 ± 0.57 b | nd | nd | 0.53 ± 0.06 a | 14.41 ± 1.31 cde | 3.25 ± 0.21 a | 3.26 ± 0.34 ab |
| MIE | nd | 1.35 ± 0.12 a | 12.23 ± 1.34 bc | nd | nd | 0.97 ± 0.01 a | 29.54 ± 2.57 i | 4.79 ± 0.50 a | 8.41 ± 0.54 abc |
| AD | nd | 0.94 ± 0.02 a | 17.71 ± 1.82 c | nd | nd | nd | 20.37 ± 1.51 fgh | 2.84 ± 0.21 a | 3.96 ± 0.37 ab |
| ADE | nd | 0.81 ± 0.13 a | 16.6 ± 2.55 c | 12.97 ± 2.25 ab | nd | 1.08 ± 0.07 a | 17.74 ± 1.1 efg | 7.14 ± 0.59 ab | 5.09 ± 0.70 abc |
| AI | nd | 0.55 ± 0.03 a | 7.57 ± 0.12 b | nd | nd | nd | 3.02 ± 0.23 a | 4.84 ± 0.34 a | 1.92 ± 0.10 a |
| AIE | nd | 0.89 ± 0.06 a | 6.42 ± 0.50 ab | 12.83 ± 1.07 ab | 28.71 ± 2.82 cd | nd | 8.62 ± 1.07 abc | 7.78 ± 1.17 abc | 5.46 ± 0.4 abc |
| GD | 8.27 ± 0.55 a | 28.6 ± 2.77 d | nd | nd | 31.11 ± 3.38 d | 39.36 ± 3.47 b | 25.58 ± 4.16 hi | 12.62 ± 1.09 abc | 35.44 ± 3.23 f |
| GDE | 8.89 ± 0.56 a | 47.93 ± 2.8 e | nd | 16.64 ± 1.95 b | 13.57 ± 2.00 ab | 40.29 ± 6.80 b | 15.11 ± 0.32 def | 70.13 ± 6.45 e | 66.48 ± 2.89 g |
| GI | 7.72 ± 0.61 ad | 18.05 ± 1.4 c | nd | nd | 28.90 ± 5.21 cd | 31.51 ± 2.10 b | 26.03 ± 3.92 hi | 13.06 ± 1.72 abc | 31.28 ± 2.43 f |
| GIE | 8.81 ± 0.50 a | 46.69 ± 3.17 e | nd | 44.8 ± 3.44 d | 14.11 ± 2.24 ab | 39.25 ± 6.73 b | 12.93 ± 1.98 abc | 82.78 ± 11.32 b | 99.64 ± 11.74 h |
| RD | nd | 2.91 ± 0.21 a | 5.09 ± 0.06 a | nd | 32.08 ± 2.36 d | nd | 24.87 ± 2.11 hi | 7.16 ± 0.57 ab | 4.14 ± 0.31 ab |
| RDE | nd | 15.14 ± 1.4 bc | 4.87 ± 0.59 a | nd | 17.81 ± 3.41 ab | nd | 25.87 ± 4.37 hi | 18.24 ± 1.68 bc | 18.59 ± 2.23 e |
| RI | nd | 2.19 ± 0.17 a | 3.08 ± 0.15 a | nd | 36.02 ± 4.45 d | nd | 23.19 ± 1.75ɡhi | 1.70 ± 0.15 a | 0.81 ± 0.01 a |
| RIE | nd | 11.96 ± 0.87 b | 4.91 ± 0.65 a | nd | 33.56 ± 5.05 d | nd | 27.29 ± 2.76 i | 9.16 ± 0.67 ab | 16.21 ± 0.18 de |
| Sample | Flavonols | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| dHK3Glu | IR3Glu | K3OGal | K3OGlu | K3Orut | Q3Gal | Q3Glu | Q3rut | Q3Glcr | Q3rha | dHQ35rha | |
| JD | 2.13 ± 0.15 fg | 2.92 ± 0.27 a | nd | nd | nd | nd | nd | nd | nd | nd | 1.84 ± 0.27 a |
| JDE | 2.78 ± 0.25 h | 2.13 ± 0.14 a | nd | nd | nd | nd | nd | nd | nd | nd | 6.36 ± 0.40 a |
| JI | 0.45 ± 0.04 ab | 3.4 ± 0.50 a | nd | nd | nd | nd | nd | nd | nd | nd | 3.91 ± 0.31 a |
| JIE | 2.74 ± 0.39 h | 3.34 ± 0.47 a | nd | nd | nd | nd | nd | nd | nd | nd | 5.52 ± 0.44 a |
| MD | 0.22 ± 0.03 ab | 0.65 ± 0.11 a | nd | nd | 0.51 ± 0.05 a | nd | 3.08 ± 0.28 a | nd | nd | nd | 0.74 ± 0.10 a |
| MDE | 0.98 ± 0.06 cd | 0.69 ± 0.09 a | nd | nd | 2.24 ± 0.02 a | 1.11 ± 0.20 b | 4.30 ± 0.23 b | nd | nd | nd | 2.21 ± 0.23 a |
| MI | 0.32 ± 0.02 ab | 0.61 ± 0.05 a | nd | 0.05 ± 0.00 a | 0.53 ± 0.08 a | nd | 3.20 ± 0.45 a | nd | nd | 0.43 ± 0.03 a | 0.95 ± 0.07 a |
| MIE | 1.69 ± 0.13 ef | 0.7 ± 0.12 a | nd | 0.95 ± 0.05 a | 2.38 ± 0.29 a | 1.17 ± 0.02 b | 4.34 ± 0.54 b | nd | nd | 0.71 ± 0.07 a | 3.15 ± 0.39 a |
| AD | 0.38 ± 0.03 ab | 0.87 ± 0.09 a | nd | nd | 3.88 ± 0.58 a | nd | nd | nd | 1.24 ± 0.15 a | nd | 0.38 ± 0.02 a |
| ADE | 0.71 ± 0.04 bc | 2.01 ± 0.06 a | nd | nd | 24.28 ± 3.29 d | nd | nd | nd | 1.12 ± 0.14 a | nd | 1.22 ± 0.10 a |
| AI | 0.41 ± 0.07 ab | 0.71 ± 0.04 a | nd | 0.46 ± 0.06 a | 9.88 ± 1.62 b | nd | nd | 19.56 ± 2.34 a | 1.22 ± 0.03 a | nd | 1.26 ± 0.17 a |
| AIE | 0.69 ± 0.10 bc | 1.64 ± 0.16 a | nd | 1.03 ± 0.14 a | 36.85 ± 0.56 e | nd | nd | nd | 1.11 ± 0.07 a | nd | 4.33 ± 0.26 a |
| GD | 2.05 ± 0.05 fg | 12.92 ± 1.11 b | 103.62 ± 13.87 b | nd | 17.98 ± 1.02 c | nd | nd | nd | nd | nd | 1.83 ± 0.13 a |
| GDE | 2.30 ± 0.29 fh | 69.33 ± 6.77 d | 142.75 ± 4.01 c | nd | 26.45 ± 1.87 d | 70.52 ± 9.45 a | nd | nd | nd | nd | 143.40 ± 17.09 b |
| GI | 1.37 ± 0.08 de | 1.22 ± 0.08 a | 107.75 ± 1.11 b | 43.2 ± 3.52 b | 24.22 ± 3.20 d | nd | nd | 16.94 ± 1.29 a | nd | 31.22 ± 1.01 b | 3.53 ± 0.32 a |
| GIE | 1.93 ± 0.25 fg | 73.17 ± 5.73 d | 131.74 ± 8.93 c | 66.42 ± 5.20 c | 34.23 ± 2.68 e | 68.91 ± 3.90 a | nd | nd | nd | 61.56 ± 4.34 d | 194.75 ± 14.23 c |
| RD | 0.43 ± 0.06 ab | 0.33 ± 0.02 a | 13.78 ± 1.93 a | nd | 0.45 ± 0.06 a | nd | nd | nd | nd | nd | 0.30 ± 0.02 a |
| RDE | 1.39 ± 0.10 de | 26.49 ± 1.50 c | 16.74 ± 1.93 a | nd | 10.57 ± 0.80 b | 72.63 ± 9.24 a | nd | nd | nd | nd | 3.95 ± 0.26 a |
| RI | 0.14 ± 0.01 a | 0.33 ± 0.04 a | 13.80 ± 1.62 a | nd | 0.45 ± 0.02 a | nd | nd | 29.36 ± 2.95 b | nd | 6.98 ± 0.64 c | 0.29 ± 0.02 a |
| RIE | 1.89 ± 0.26 fg | 24.10 ± 3.32 c | 19.26 ± 2.57 a | nd | 18.11 ± 2.80 c | 76.20 ± 9.63 a | nd | nd | nd | 33.60 ± 2.42 b | 1.79 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubaidi, M.A.; Czaplicka, M.; Kolniak-Ostek, J.; Nawirska-Olszańska, A. Influence of Variety, Enzyme Addition and Destemming on Yield and Bioactive Compounds of Juices from Selected Hybrid Grape Varieties Cultivated in Poland. Foods 2023, 12, 3475. https://doi.org/10.3390/foods12183475
Zubaidi MA, Czaplicka M, Kolniak-Ostek J, Nawirska-Olszańska A. Influence of Variety, Enzyme Addition and Destemming on Yield and Bioactive Compounds of Juices from Selected Hybrid Grape Varieties Cultivated in Poland. Foods. 2023; 12(18):3475. https://doi.org/10.3390/foods12183475
Chicago/Turabian StyleZubaidi, Muhamad Alfiyan, Marta Czaplicka, Joanna Kolniak-Ostek, and Agnieszka Nawirska-Olszańska. 2023. "Influence of Variety, Enzyme Addition and Destemming on Yield and Bioactive Compounds of Juices from Selected Hybrid Grape Varieties Cultivated in Poland" Foods 12, no. 18: 3475. https://doi.org/10.3390/foods12183475
APA StyleZubaidi, M. A., Czaplicka, M., Kolniak-Ostek, J., & Nawirska-Olszańska, A. (2023). Influence of Variety, Enzyme Addition and Destemming on Yield and Bioactive Compounds of Juices from Selected Hybrid Grape Varieties Cultivated in Poland. Foods, 12(18), 3475. https://doi.org/10.3390/foods12183475









