Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film-Forming Properties of CEO–CS Coatings
2.3. In Vitro Antifungal Activity of CS Films with Different CEO Concentrations
2.4. Apple Fruit Coating Treatment and Inoculation
2.5. Extraction of PAL and Determination of PAL Activity
2.6. Determination of Total Phenolic Content of Apples
2.7. Extraction and Determination of GLU and CHI in Apples
2.8. Antimicrobial Kinetics of CS–CEO Coatings
2.9. Statistical Analysis
3. Results and Discussion
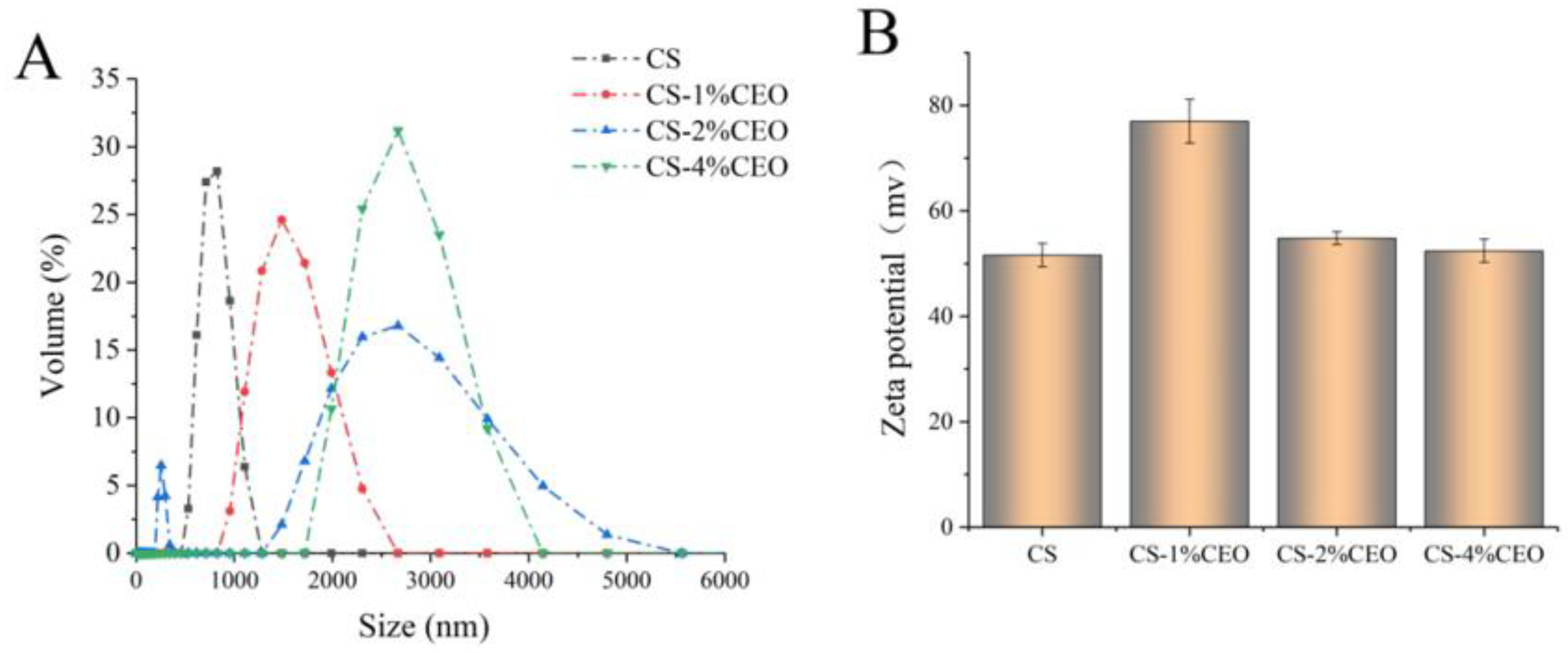
3.1. Particle Size of the CS–CEO Composite
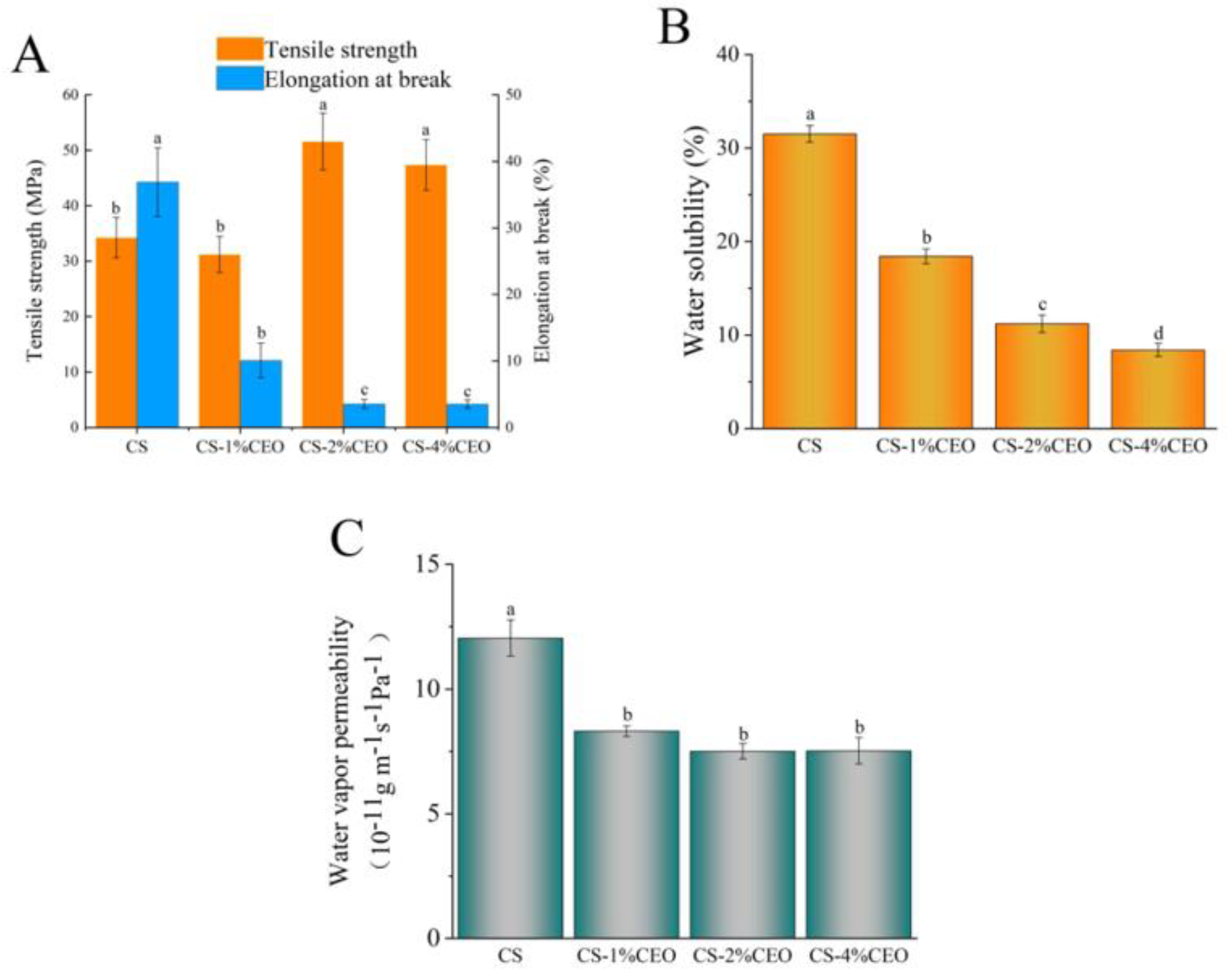
3.2. Mechanical Properties of the CS–CEO Composite
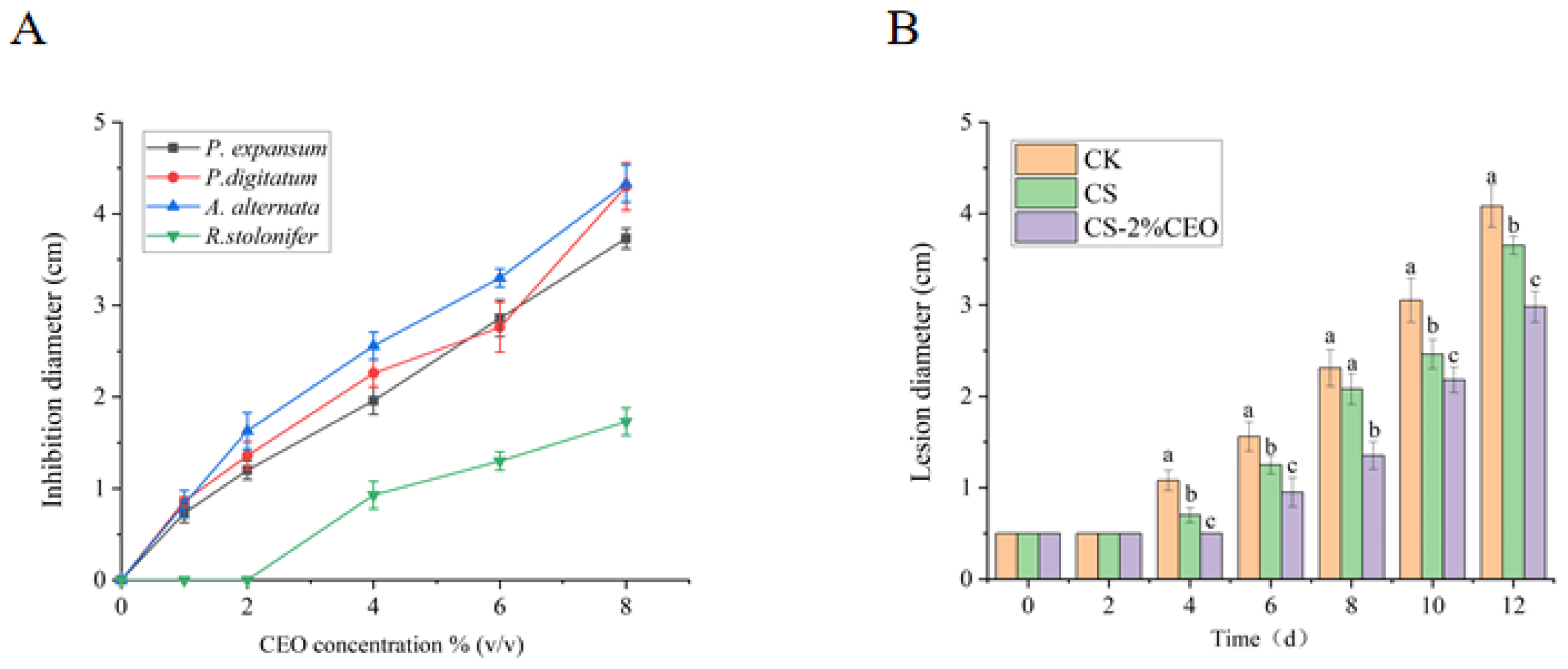
3.3. Analysis of In Vitro Antifungal Ability of CEO–CS Coatings with Different Concentrations
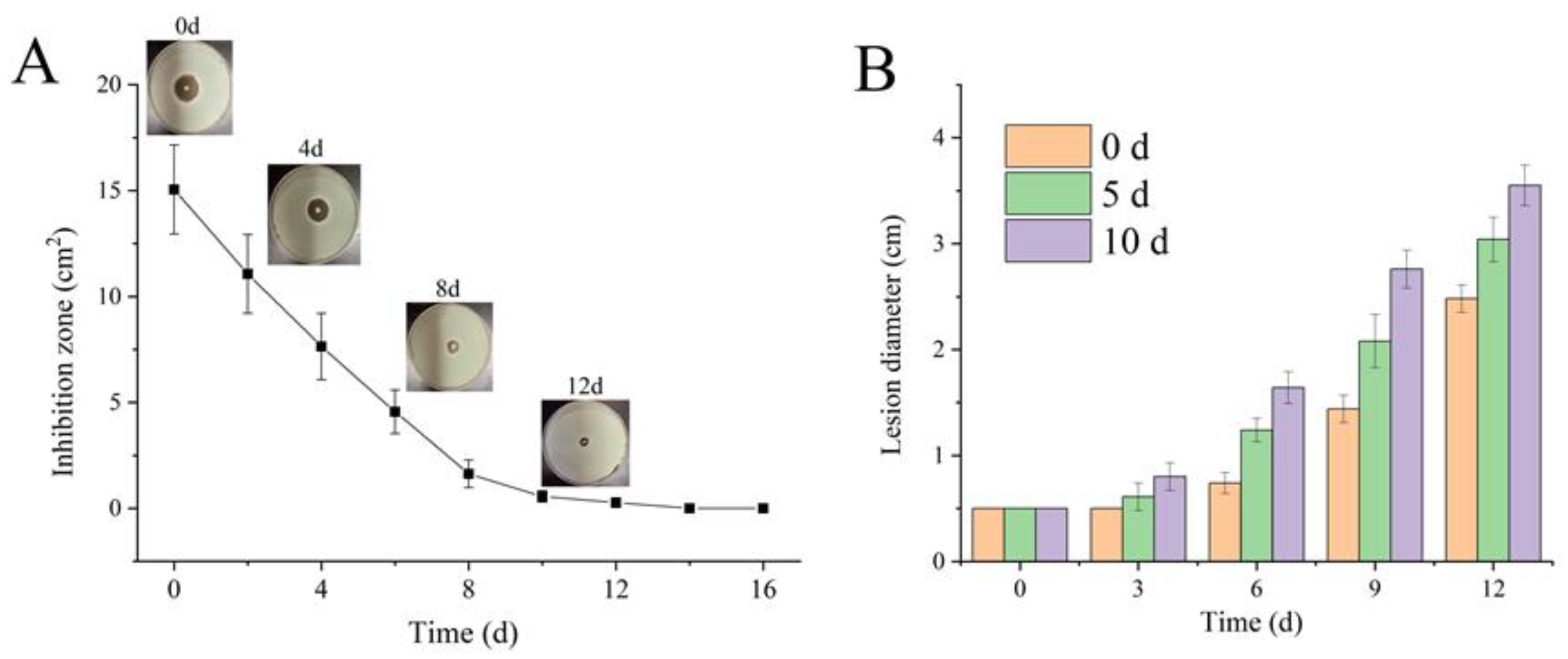
3.4. Inhibition of Apple Diseases by Coating with CS–CEO Composites
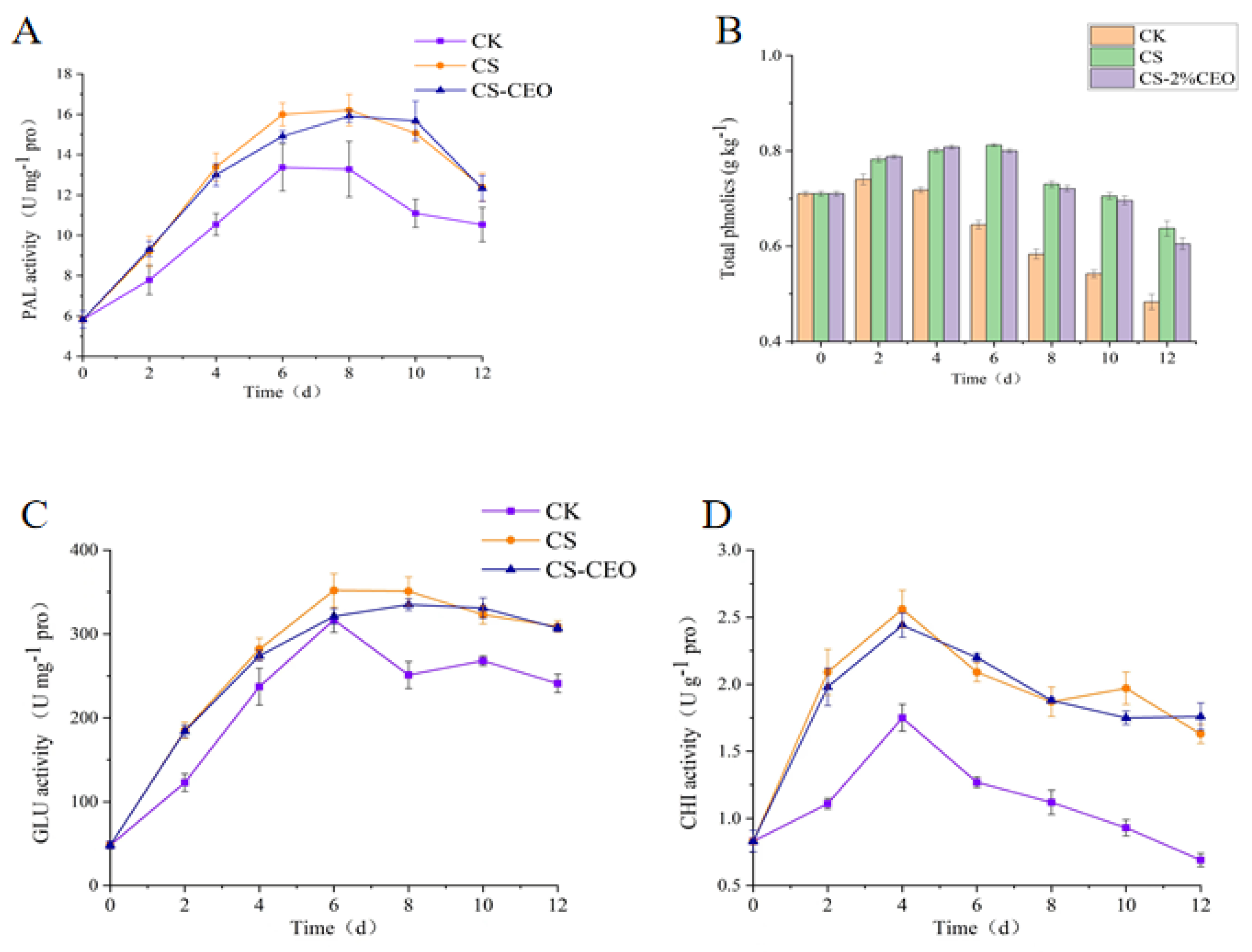
3.5. Effect of CS–CEO Coatings on PAL Activity in Apples
3.6. Effect of CS–CEO Coatings on the Total Phenolic Content of Apples
3.7. Effect of CS–CEO Coatings on the Activity of GLU and CHI in Apple
3.8. Antimicrobial Kinetics of CS–CEO Composite Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.; Zhang, W.; Xu, Y.; Zhang, Y.; Pu, Y.; Cao, J.; Jiang, W.A. Applications of plant-derived food by-products to maintain quality of postharvest fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 1105–1119. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol. Technol. 2021, 180, 111613. [Google Scholar] [CrossRef]
- Nabi, S.U.; Raja, W.H.; Kumawat, K.L.; Mir, J.I.; Sharma, O.C.; Singh, D.B.; Sheikh, M.A. Post harvest diseases of temperate fruits and their management strategies-a review. Int. J. Pure Appl. Biosci. 2017, 5, 885–898. [Google Scholar]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W.; et al. Advances in the role and mechanisms of essential oils and plant extracts as natural preservatives to extend the postharvest shelf life of edible mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Functional edible films/coatings integrated with lactoperoxidase and lysozyme and their application in food preservation. Food Control 2022, 133, 108670. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Jain, S.; Vaishnav, A.; Kumari, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Chitinolytic Bacillus-mediated induction of jasmonic acid and defense-related proteins in soybean (Glycine max L. Merrill) plant against Rhizoctonia solani and Fusarium oxysporum. J. Plant Growth Regul. 2017, 36, 200–214. [Google Scholar] [CrossRef]
- Jongsri, P.; Rojsitthisak, P.; Wangsomboondee, T.; Seraypheap, K. Influence of chitosan coating combined with spermidine on anthracnose disease and qualities of ‘Nam Dok Mai’mango after harvest. Sci. Hortic. 2017, 224, 180–187. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Grande-Tovar, C.D.; Chaves-López, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef]
- Shah, N.N.; Vishwasrao, C.; Singhal, R.S. Ananthanarayan L. n-Octenyl succinylation of pullulan: Effect on its physico-mechanical and thermal properties and application as an edible coating on fruits. Food Hydrocoll. 2016, 55, 179–188. [Google Scholar] [CrossRef]
- Han, X.; Mao, L.; Wei, X.; Lu, W. Stimulatory involvement of abscisic acid in wound suberization of postharvest kiwifruit. Sci. Hortic. 2017, 224, 244–250. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Wang, X.; Cao, J.; Jiang, W. Chlorogenic acid induces resistance against Penicillium expansum in peach fruit by activating the salicylic acid signaling pathway. Food Chem. 2018, 260, 274–282. [Google Scholar] [CrossRef]
- Ma, D.; Ji, D.; Liu, J.; Xu, Y.; Chen, T.; Tian, S. Efficacy of methyl thujate in inhibiting Penicillium expansum growth and possible mechanism involved. Postharvest Biol. Technol. 2020, 161, 111070. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Jiang, W. Effects of purple passion fruit peel extracts on characteristics of Pouteria campechiana seed starch films and the application in discernible detection of shrimp freshness. Food Hydrocoll. 2023, 138, 108477. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Effect of different cation in situ cross-linking on the properties of pectin-thymol active film. Food Hydrocoll. 2022, 128, 107594. [Google Scholar] [CrossRef]
- Peng, W.; Wang, N.; Wang, S.; Wang, J.; Bian, Z. Effect of co-treatment of microwave and exogenous l-phenylalanine on the enrichment of flavonoids in Tartary buckwheat sprouts. J. Sci. Food Agric. 2023, 103, 2014–2022. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Zhou, L.; Zou, L.; Liu, W. Effect of chitosan coatings with cinnamon essential oil on postharvest quality of mangoes. Foods 2021, 10, 3003. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest chitosan oligochitosan and salicylic acid treatments enhance phenol metabolism and maintain the postharvest quality of apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267, 109334. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Wang, J.; Zhai, H.; Xue, X. Inhibitory effect and underlying mechanism of cinnamon and clove essential oils on Botryosphaeria dothidea and Colletotrichum gloeosporioides causing rots in postharvest bagging-free apple fruits. Front. Microbiol. 2023, 14, 1109028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J.; Lin, Y. A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchii in association with ROS metabolism. Food Chem. 2018, 266, 299–308. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Filho, J.G.; De Deus, I.P.; Valadares, A.C.; Fernandes, C.C.; Estevam, E.B.; Egea, M.B. Chitosan film with citrus limonia essential oil: Physical and morphological properties and antibacterial activity. Colloids Interfaces 2020, 4, 18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Goksen, G.; Zhou, Y.; Yang, J.; Khan, M.R.; Ahmad, N.; Fei, T. Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases. Foods 2023, 12, 3518. https://doi.org/10.3390/foods12183518
Zhang W, Goksen G, Zhou Y, Yang J, Khan MR, Ahmad N, Fei T. Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases. Foods. 2023; 12(18):3518. https://doi.org/10.3390/foods12183518
Chicago/Turabian StyleZhang, Wanli, Gulden Goksen, Yuanping Zhou, Jun Yang, Mohammad Rizwan Khan, Naushad Ahmad, and Tao Fei. 2023. "Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases" Foods 12, no. 18: 3518. https://doi.org/10.3390/foods12183518
APA StyleZhang, W., Goksen, G., Zhou, Y., Yang, J., Khan, M. R., Ahmad, N., & Fei, T. (2023). Application of a Chitosan–Cinnamon Essential Oil Composite Coating in Inhibiting Postharvest Apple Diseases. Foods, 12(18), 3518. https://doi.org/10.3390/foods12183518




