Combining High-Pressure Processing and Supercritical Carbon Dioxide for Inactivation of Listeria innocua
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Samples
2.3. Bacterial Growth Conditions and Inoculation of the Fish Soup
2.4. Carbon Dioxide Packaging (CO2)
2.5. Pressure Treatment
2.6. Storage and Sampling
2.7. Headspace Gas Composition
2.8. pH Analysis
2.9. Microbial Analysis
2.10. Statistical Analyses
3. Results and Discussion
3.1. Reduction of L. innocua in Fish Soup after HPP
3.2. Regrowth of L. innocua during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, K.; Hunt, K.; Lourenco, A.; Pennone, V. Listeria monocytogenes in the Food Processing Environment. Curr. Clin. Microbiol. Rep. 2018, 5, 106–119. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Lakicevic, B.; Nastasijevic, I. Listeria monocytogenes in retail establishments: Contamination routes and control strategies. Food Rev. Int. 2017, 33, 247–269. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.A.; Xie, H.M.; Yang, Y.; Lin, C.M. Inactivation of Staphylococcus aureus and Escherichia coli by the synergistic action of high hydrostatic pressure and dissolved CO2. Int. J. Food Microbiol. 2010, 144, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, H.W.; Emire, S.A. High Pressure Processing of Foods for Microbial and Mycotoxins Control: Current trends and future prospects. Cogent Food Agric. 2019, 5, 1622184. [Google Scholar] [CrossRef]
- Al-Nehlawi, A.; Saldo, J.; Vega, L.F.; Guri, S. Effect of high carbon dioxide atmosphere packaging and soluble gas stabilization pre-treatment on the shelf-life and quality of chicken drumsticks. Meat Sci. 2013, 94, 1–8. [Google Scholar] [CrossRef]
- Torres, J.A.; Velazquez, G. Commercial opportunities and research challenges in the high pressure processing of foods. J. Food Eng. 2005, 67, 95–112. [Google Scholar] [CrossRef]
- Shigehisa, T.; Ohmori, T.; Saito, A.; Taji, S.; Hayashi, R. Effects of high hydrostatic-pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat and meat-products. Int. J. Food Microbiol. 1991, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. Lwt-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Amanatidou, A.; Schlüter, O.; Lemkau, K.; Gorris, L.G.M.; Smid, E.J.; Knorr, D. Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic salmon. Innov. Food Sci. Emerg. Technol. 2000, 1, 87–98. [Google Scholar] [CrossRef]
- Daniels, J.A.; Krishnamurthi, R.; Rizvi, S.S.H. A Review of Effects of Carbon-Dioxide on Microbial-Growth and Food Quality. J. Food Prot. 1985, 48, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Devlieghere, F.; Debevere, J. Influence of dissolved carbon dioxide on the growth of spoilage bacteria. Lebensm. -Wiss. Und-Technol. -Food Sci. Technol. 2000, 33, 531–537. [Google Scholar] [CrossRef]
- Farber, J.M. Microbiological Aspects of Modified-Atmosphere Packaging Technology—A Review. J. Food Prot. 1991, 54, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Hovda, M.B.; Rotabakk, B.T. Favourable effects of soluble gas stabilisation and modified atmosphere for supressing regrowth of high pressure treated Listeria innocua. Food Control 2015, 51, 108–113. [Google Scholar] [CrossRef]
- Corwin, H.; Shellhammer, T.H. Combined carbon dioxide and high pressure inactivation of pectin methylesterase, polyphenol oxidase, Lactobacillus plantarum and Escherichia coli. J. Food Sci. 2002, 67, 697–701. [Google Scholar] [CrossRef]
- Zhang, J.; Davis, T.A.; Matthews, M.A.; Drews, M.J.; LaBerge, M.; An, Y.H.H. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids 2006, 38, 354–372. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: A review. J. Food Sci. Technol. 2022, 59, 417–428. [Google Scholar] [CrossRef]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; Gonzalez, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 520–540. [Google Scholar] [CrossRef]
- Lorentzen, G.; Ytterstad, E.; Olsen, R.L.; Skjerdal, T. Thermal inactivation and growth potential of Listeria innocua in rehydrated salt-cured cod prepared for ready-to-eat products. Food Control 2010, 21, 1121–1126. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; George, J.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. High-pressure and thermal-assisted pasteurization of habituated, wild-type, and pressure-stressed Listeria monocytogenes, Listeria innocua, and Staphylococcus aureus. LWT 2021, 137, 110445. [Google Scholar] [CrossRef]
- Thomas, C.; Prior, O.; O’Beirne, D. Survival and growth of Listeria species in a model ready-to-use vegetable product containing raw and cooked ingredients as affected by storage temperature and acidification. Int. J. Food Sci. Technol. 1999, 34, 317–324. [Google Scholar] [CrossRef]
- Hugas, M.; Pages, F.; Garriga, M.; Monfort, J.M. Application of the bacteriocinogenic Lactobacilus sakei CTC494 to prevent growth of Listeria in fresh and cooked meat products packed with different atmospheres. Food Microbiol. 1998, 15, 639–650. [Google Scholar] [CrossRef]
- Smiddy, M.; O’Gorman, L.; Sleator, R.D.; Kerry, J.P.; Patterson, M.F.; Kelly, A.L.; Hill, C. Greater high-pressure resistance of bacteria in oysters than in buffer. Innov. Food Sci. Emerg. Technol. 2005, 6, 83–90. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Yuste, J.; Mor-Mur, M.; Capellas, M.; Pla, R. Listeria innocua and aerobic mesophiles during chill storage of inoculated mechanically recovered poultry meat treated with high hydrostatic pressure. Meat Sci. 1999, 53, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Basaran-Akgul, N.; Mousavi-Hesary, M.; Basaran, P.; Shin, J.H.; Swanson, B.G.; Rasco, B.A. High pressure processing inactivation of Listeria innocua in minced trout (Oncorhynchus mykiss). J. Food Process. Preserv. 2010, 34, 191–206. [Google Scholar] [CrossRef]
- Wei, C.I.; Balaban, M.O.; Fernando, S.Y.; Peplow, A.J. Bacterial Effect of High Pressure CO2 Treatment on Foods Spiked with Listeria or Salmonella. J. Food Prot. 1991, 54, 189–193. [Google Scholar] [CrossRef]
- Jung, W.Y.; Choi, Y.M.; Rhee, M.S. Potential use of supercritical carbon dioxide to decontaminate Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella typhimurium in alfalfa sprouted seeds. Int. J. Food Microbiol. 2009, 136, 66–70. [Google Scholar] [CrossRef]
- Li, P.; Mei, J.; Xie, J. The regulation of carbon dioxide on food microorganisms: A review. Food Res. Int. 2023, 172, 113170. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery products—Significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Gallo, L.I.; Pilosof, A.M.R.; Jagus, R.J. Effective control of Listeria innocua by combination of nisin, pH and low temperature in liquid cheese whey. Food Control 2007, 18, 1086–1092. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-Pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef] [PubMed]
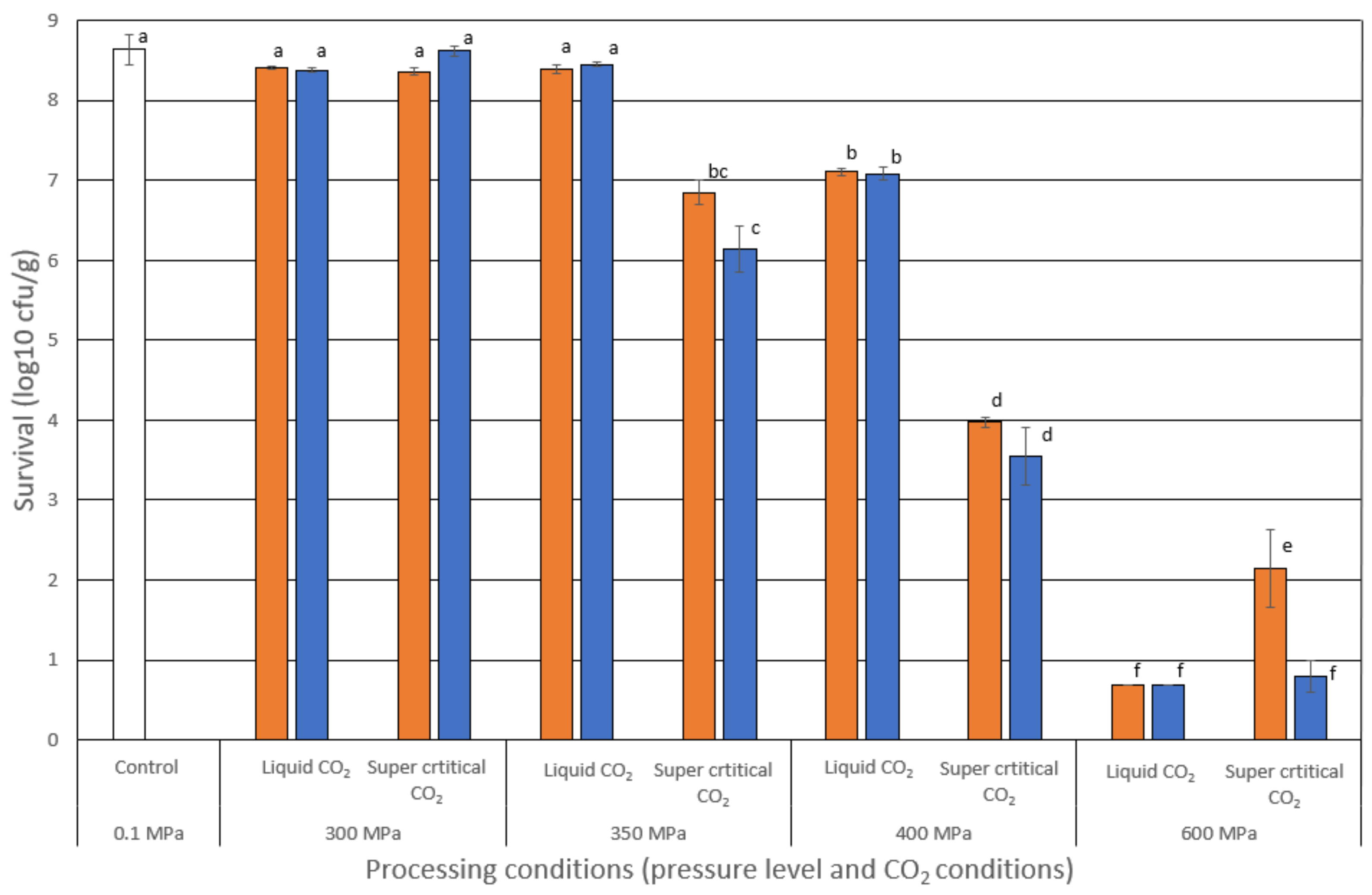
 ) or supercritical CO2 (- - - -) and stored in a modified atmosphere with 50% CO2 (♦) or 95% CO2 (○) in the initial gas mix for up to 53 days. The soup was stored at 4.0 ± 0.5 °C. Bacterial counts (log10 CFU/g) were measured in TSA-YE plates, and means ± std dev are shown.
) or supercritical CO2 (- - - -) and stored in a modified atmosphere with 50% CO2 (♦) or 95% CO2 (○) in the initial gas mix for up to 53 days. The soup was stored at 4.0 ± 0.5 °C. Bacterial counts (log10 CFU/g) were measured in TSA-YE plates, and means ± std dev are shown.
 ) or supercritical CO2 (- - - -) and stored in a modified atmosphere with 50% CO2 (♦) or 95% CO2 (○) in the initial gas mix for up to 53 days. The soup was stored at 4.0 ± 0.5 °C. Bacterial counts (log10 CFU/g) were measured in TSA-YE plates, and means ± std dev are shown.
) or supercritical CO2 (- - - -) and stored in a modified atmosphere with 50% CO2 (♦) or 95% CO2 (○) in the initial gas mix for up to 53 days. The soup was stored at 4.0 ± 0.5 °C. Bacterial counts (log10 CFU/g) were measured in TSA-YE plates, and means ± std dev are shown.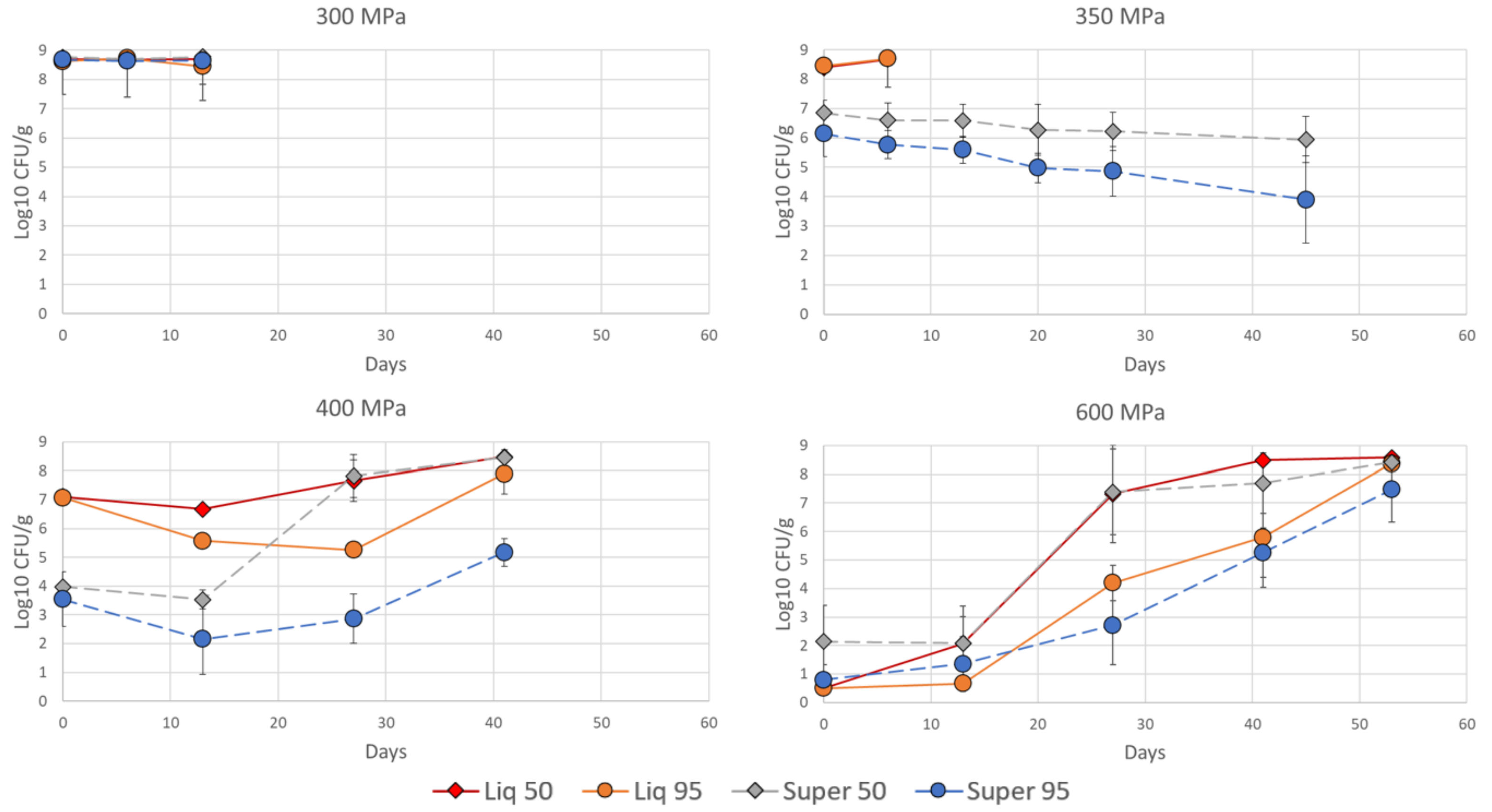
| Design Variable | Levels | ||||
|---|---|---|---|---|---|
| Pressure (MPa) | 0.1 | 300 | 350 | 400 | 600 |
| State of CO2 | Liquid | Supercritical 1 | |||
| Level of CO2 in packaging gas (%) | 50 | 95 | |||
| Pressure (MPa) | pH |
|---|---|
| 0.1 | 5.32 ± 0.19 a |
| 300 | 5.42 ± 0.14 a |
| 350 | 5.74 ± 0.15 b |
| 400 | 5.89 ± 0.24 c |
| 600 | 5.92 ± 0.23 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotabakk, B.T.; Rode, T.M. Combining High-Pressure Processing and Supercritical Carbon Dioxide for Inactivation of Listeria innocua. Foods 2023, 12, 3563. https://doi.org/10.3390/foods12193563
Rotabakk BT, Rode TM. Combining High-Pressure Processing and Supercritical Carbon Dioxide for Inactivation of Listeria innocua. Foods. 2023; 12(19):3563. https://doi.org/10.3390/foods12193563
Chicago/Turabian StyleRotabakk, Bjørn Tore, and Tone Mari Rode. 2023. "Combining High-Pressure Processing and Supercritical Carbon Dioxide for Inactivation of Listeria innocua" Foods 12, no. 19: 3563. https://doi.org/10.3390/foods12193563






