Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Postbiotic
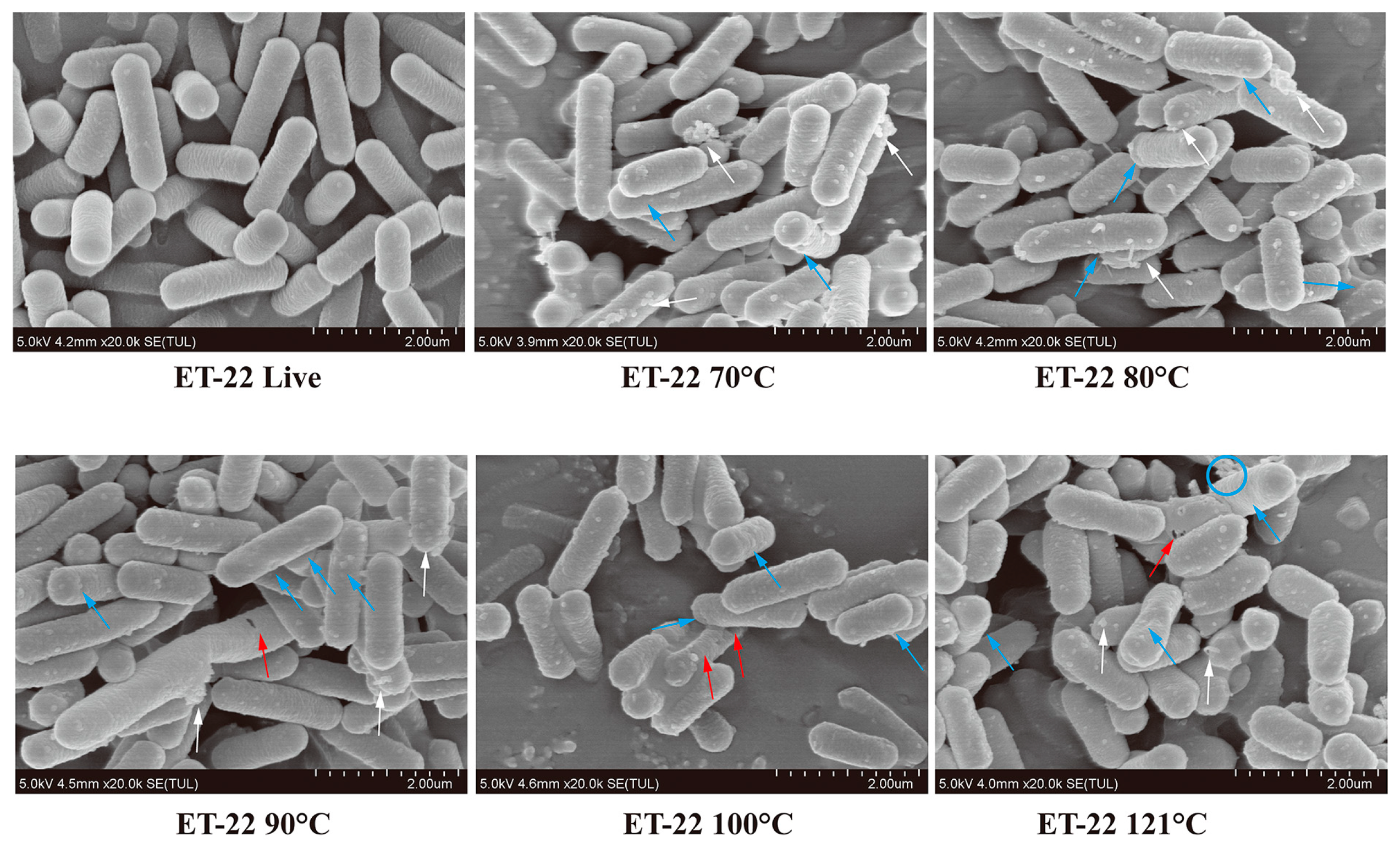
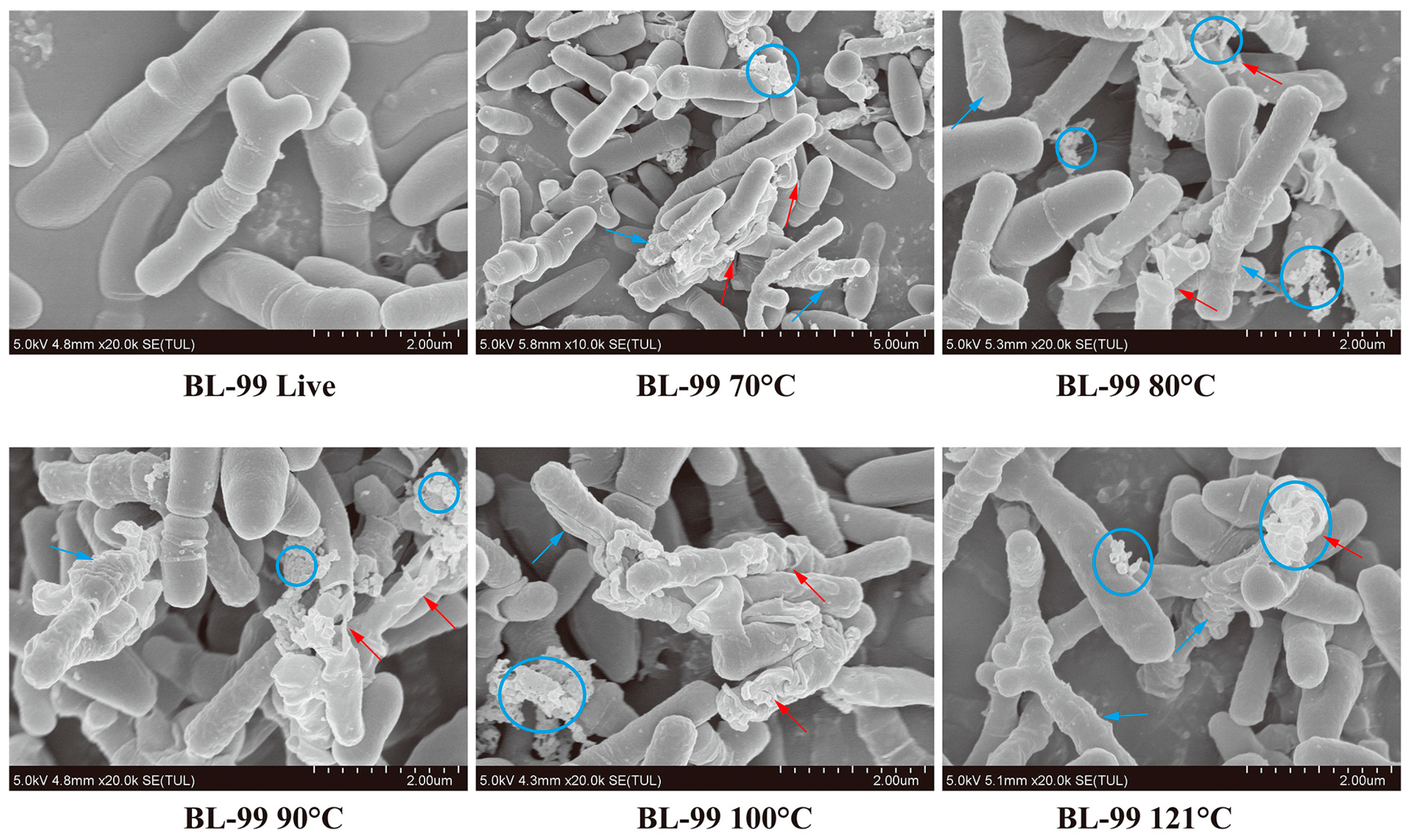
2.3. Morphological Changes of Postbiotic by Scanning Electron Microscopy (SEM)
2.4. Antioxidant Activity Assay of Postbiotic
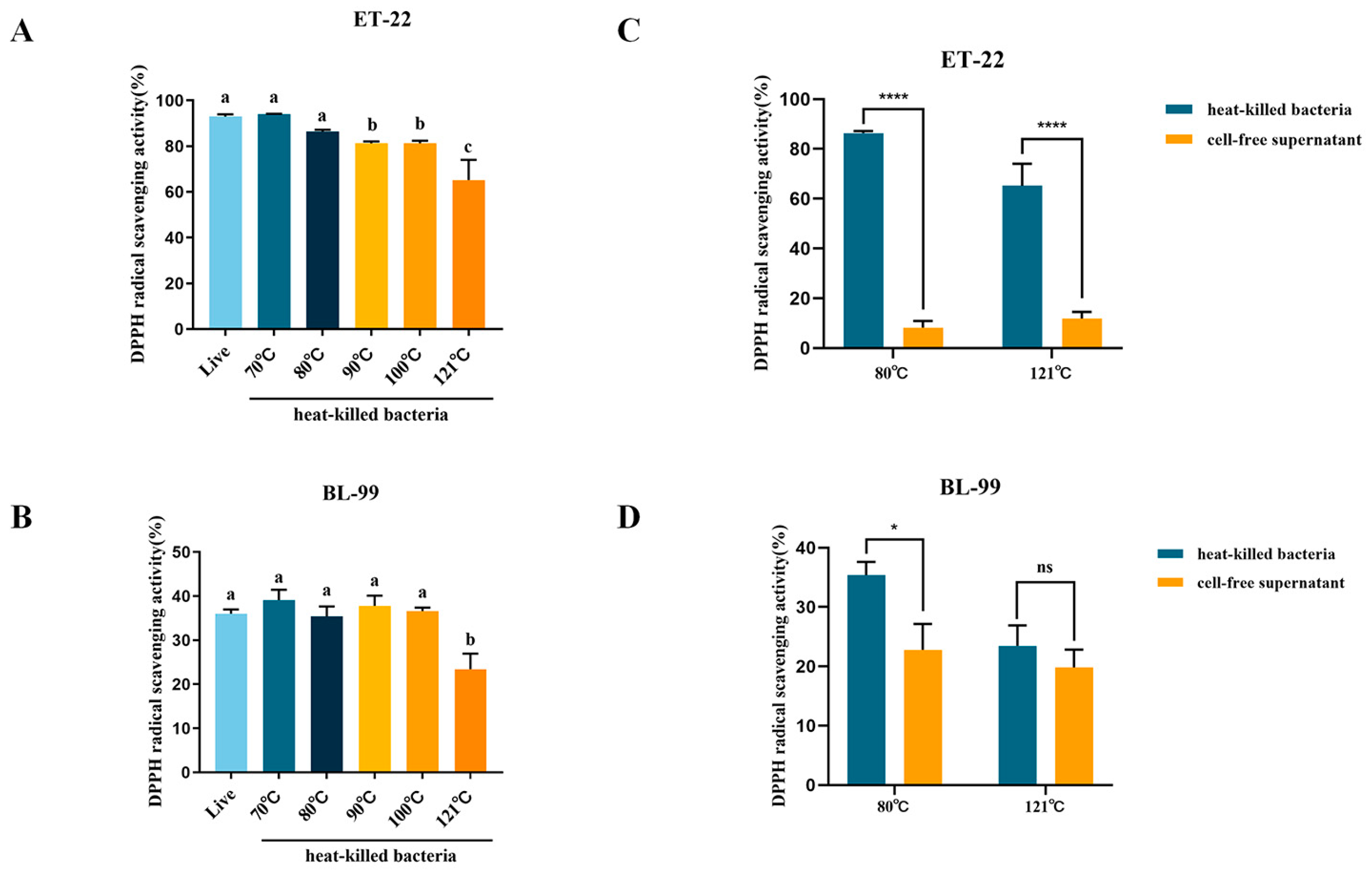
2.4.1. 2,2-Diphenyl-1-Picryl-Hydrazyl (DPPH) Free Radical Scavenging
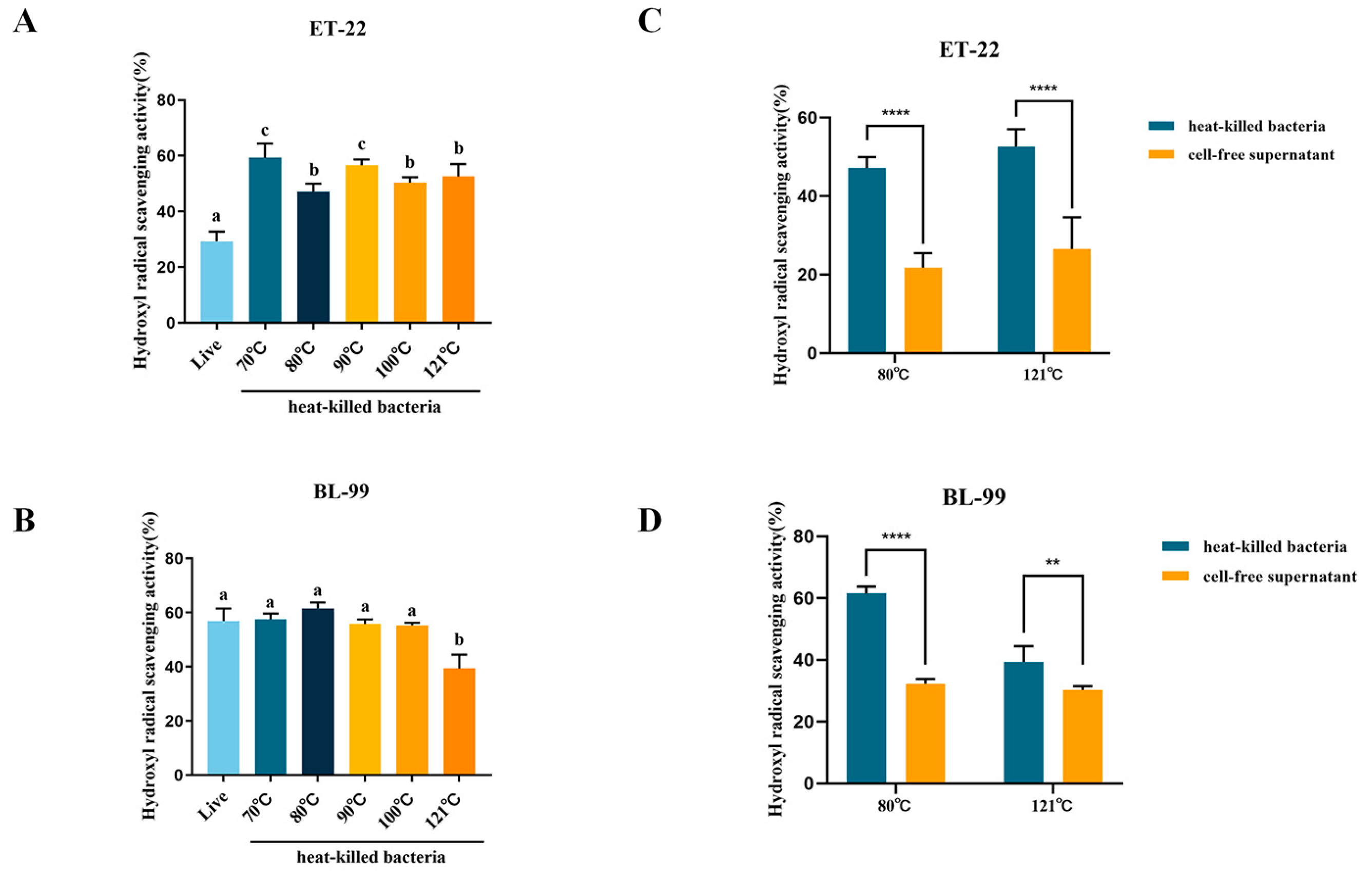
2.4.2. Hydroxyl Radical Scavenging
2.5. Anti-Inflammatory Activity Assay of Postbiotic
2.6. Chemical Profiles Assay by Non-Targeted Metabonomics
2.7. Statistical Analysis
3. Results
3.1. Effects of Inactivation Treatments on Cell Morphology by SEM
3.2. Effects of Inactivation Treatments on Antioxidant Activity
3.2.1. DPPH Radical Scavenging Capacity
3.2.2. Hydroxyl Radical Scavenging Capacity
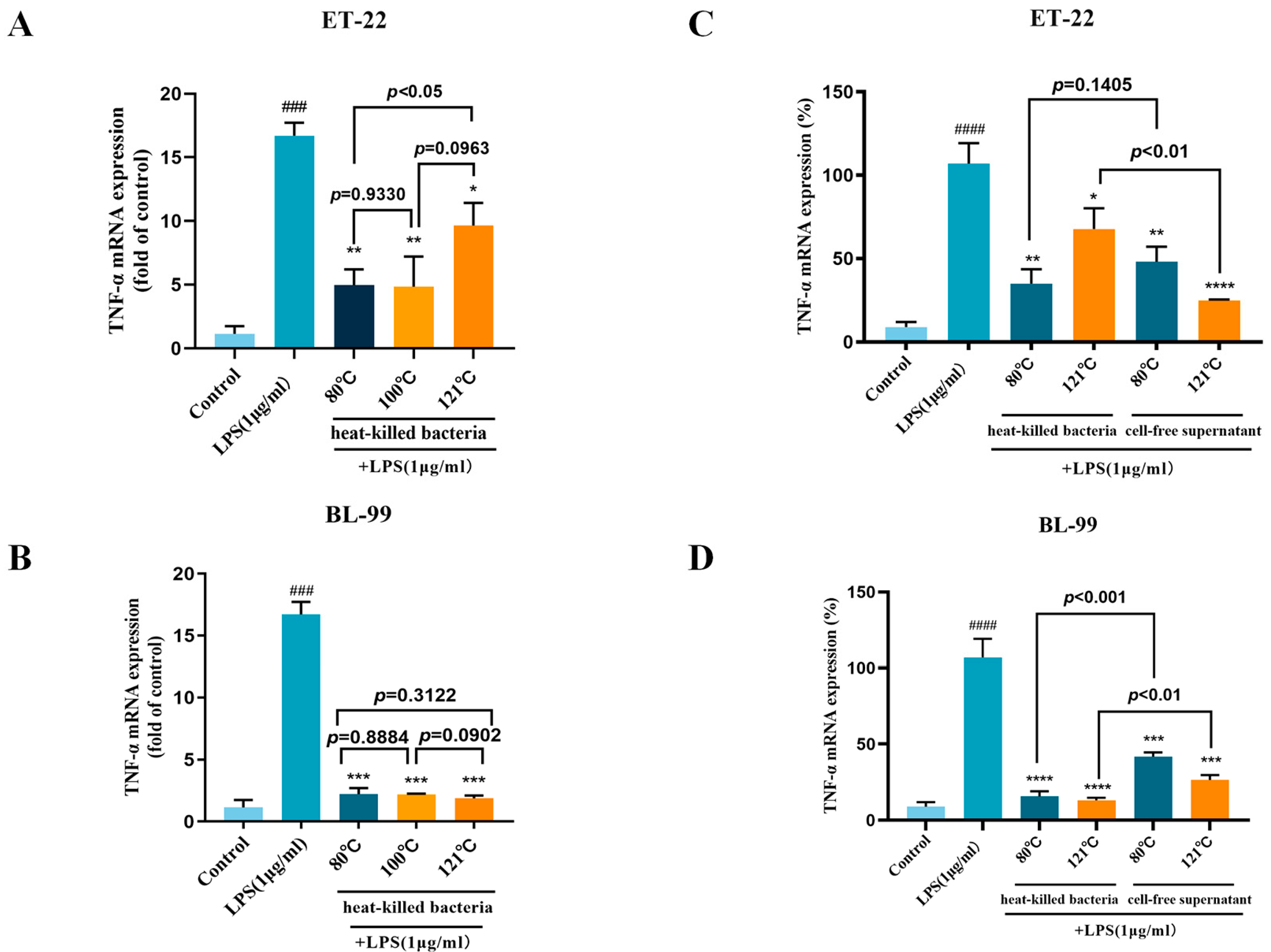
3.3. Effects of Inactivation Treatments on Anti-Inflammatory Activity
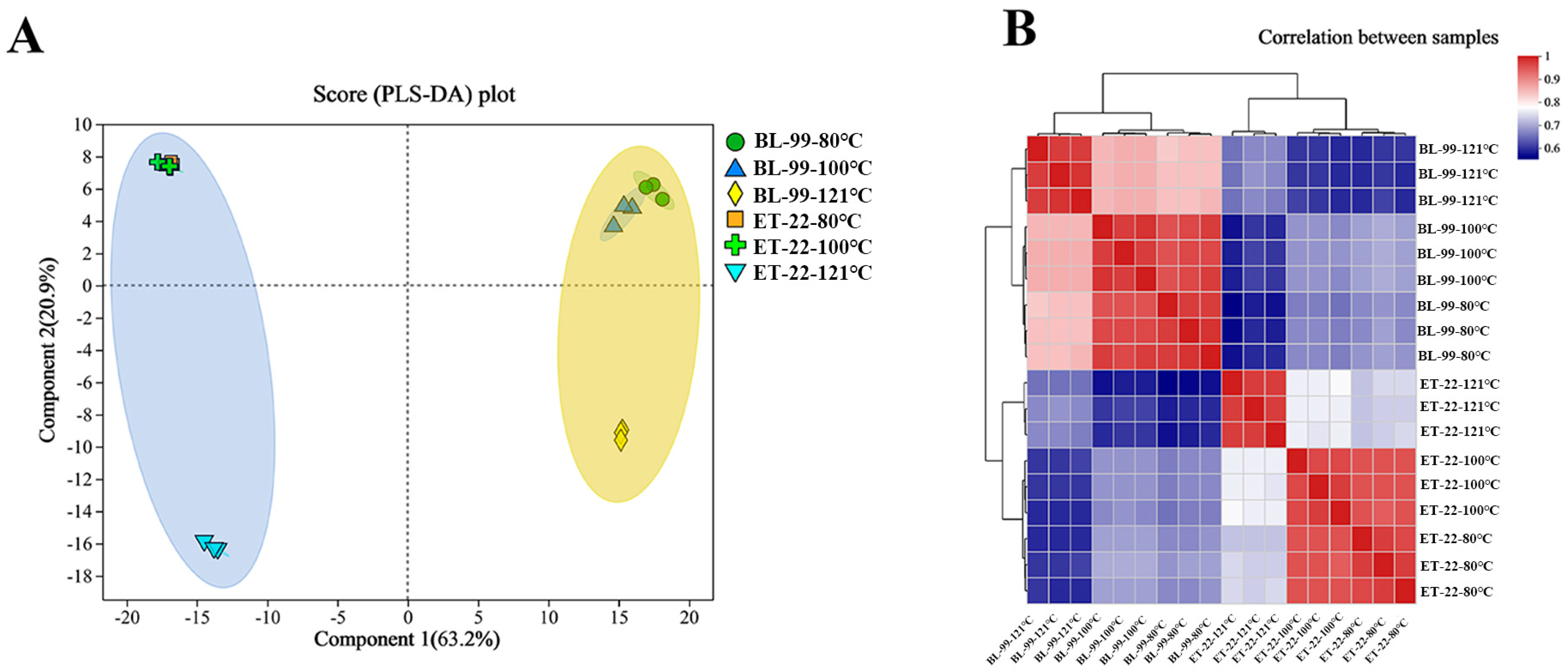
3.4. Non-Targeted Metabolomics Analysis of the Postbiotic Chemical Profiles
3.5. Differential Metabolites Analysis of Different Cell-Free Supernatants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastro Hepat. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Canani, R.B.; Chatel, J.M.; D’Auria, E.; de Freitas, M.N.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastro Hepat. 2021, 18, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Gonzalez, P.F.; Liceaga, A.M.; Aguilar-Toala, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Cassilly, C.D.; Liu, X.X.; Park, S.M.; Tusi, B.K.; Chen, X.J.; Kwon, J.; Filipcik, P.; Bolze, A.S.; Liu, Z.H.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Martin, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Abbasi, A.; Ghasempour, Z.; Sabahi, S.; Kafil, H.S.; Hasannezhad, P.; Saadat, Y.R.; Shahbazi, N. The biological activities of postbiotics in gastrointestinal disorders. Crit. Rev. Food Sci. 2022, 62, 5983–6004. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Érix, C.N.; Bonatto, M.S.; Pradella, F.; dos Santos, P.; Abud, Y.K.D.; Farias, A.S.; Martínez, J.; Sant’Anna Filho, C.B.; Lollo, P.C.; et al. Obtaining paraprobiotics from Lactobacilus acidophilus, Lacticaseibacillus casei and Bifidobacterium animalis using six inactivation methods: Impacts on the cultivability, integrity, physiology, and morphology. J. Funct. Foods 2021, 87, 104826. [Google Scholar] [CrossRef]
- Barros, C.P.; Pires, R.P.S.; Guimarães, J.T.; Abud, Y.K.D.; Almada, C.N.; Pimentel, T.C.; Sant’Anna, C.; De-Melo, L.D.B.; Duarte, M.; Silva, M.C.; et al. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Res. Int. 2021, 140, 110061. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; da Costa, W.K.; da Silva Rocha, R.; de Souza Pedrosa, G.T.; dos Santos Rocha, C.; Alves, J.M.; Alvarenga, V.O.; Sant’Ana, A.S.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. Tech. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.M.; Sun, Z.; Fan, J.B.; Liu, F.D.; Zhao, W.; Liu, W.H.; Zhang, M.; Hung, W.L. Postbiotics derived from L. paracasei ET-22 inhibit the formation of S. mutans biofilms and bioactive substances: An analysis. Molecules 2023, 28, 1236. [Google Scholar] [CrossRef] [PubMed]
- Wuri, G.; Liu, F.D.; Sun, Z.; Fang, B.; Zhao, W.; Hung, W.L.; Liu, W.H.; Zhang, X.X.; Wang, R.; Wu, F.; et al. Lactobacillus paracasei ET-22 and derived postbiotics reduce halitosis and modulate oral microbiome dysregulation—A randomized, double-blind placebo-controlled clinical trial. Food Funct. 2023, 14, 7335–7346. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Zhao, Y.; Duan, S.; Liu, W.H.; Zhang, C.; Sun, S.; Wang, T.; Wang, X.; Hung, W.L.; et al. In vitro study of Bifidobacterium lactis BL-99 with fructooligosaccharide synbiotics effected on the intestinal microbiota. Front. Nutr. 2022, 9, 890316. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Hotchkiss, A.T.; Yam, K.L. Gut microbiota: Impact of probiotics, prebiotics, synbiotics, pharmabiotics, and postbiotics on human health. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 515–523. [Google Scholar] [CrossRef]
- Song, M.W.; Jang, H.J.; Kim, K.T.; Paik, H.D. Probiotic and antioxidant properties of novel Lactobacillus brevis KCCM 12203P isolated from Kimchi and evaluation of immune-stimulating activities of its heat-killed cells in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic Lactiplantibacillus plantarum strains isolated from Malaysian Foods. Front. Vet. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Forestier, C.; Kolling, Y.; Salva, S.; Villena, J.; Alvarez, S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS ONE 2018, 13, e0194034. [Google Scholar] [CrossRef]
- Kim, E.; Lee, H.G.; Han, S.; Seo, K.-H.; Kim, H. Effect of surface layer proteins derived from paraprobiotic Kefir lactic acid bacteria on inflammation and high-fat diet-induced obesity. J. Agric. Food Chem. 2021, 69, 15157–15164. [Google Scholar] [CrossRef]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938—A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Chen, X.-M.; Ma, H.-Q.; Li, K.; Wang, Y.-L.; Li, Z.-J. Akkermansia muciniphila cell-free supernatant improves glucose and lipid metabolisms in Caenorhabditis elegans. Nutrients 2023, 15, 1725. [Google Scholar] [CrossRef] [PubMed]
- Jastrzab, R.; Graczyk, D.; Siedlecki, P. Molecular and cellular mechanisms influenced by postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Song, M.W.; Chung, Y.; Kim, K.-T.; Hong, W.S.; Chang, H.J.; Paik, H.-D. Probiotic characteristics of Lactobacillus brevis B13-2 isolated from kimchi and investigation of antioxidant and immune-modulating abilities of its heat-killed cells. Lwt 2020, 128, 109452. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Dia, V.P.; Shi, B. Probiotic activity of ropy Lactiplantibacillus plantarum NA isolated from Chinese northeast sauerkraut and comparative evaluation of its live and heat-killed cells on antioxidant activity and RAW 264.7 macrophage stimulation. Food Funct. 2023, 14, 2481–2495. [Google Scholar] [CrossRef]
- Yu, H.-S.; Lee, N.-K.; Choi, A.-J.; Choe, J.-S.; Bae, C.H.; Paik, H.-D. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 Isolated from Kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. J. Microbiol. Biotechnol. 2019, 29, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-B.; Shih, H.-Y.; Huang, C.-H.; Li, L.-T.; Chen, C.-C.; Fang, H.-W. Live and heat-killed Lactobacillus rhamnosus GG upregulate gene expression of pro-inflammatory cytokines in 5-fluorouracil-pretreated Caco-2 cells. Support. Care Cancer 2014, 22, 1647–1654. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; De Simone, N.; Capozzi, V.; Spano, G.; Fiocco, D. Immunomodulatory activity on human macrophages by cell-free supernatants to explore the probiotic and postbiotic potential of Lactiplantibacillus plantarum strains of plant origin. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef]
- Ramos, L.P.; Almeida, M.E.D.; Freire, H.P.S.; Pessoa, W.F.B.; Rezende, R.P.; Romano, C.C. Antagonistic activity of Lactiplantibacillus plantarum 6.2 extracted from cocoa fermentation and its supernatant on Gardnerella vaginalis. An. Da Acad. Bras. De. Ciências 2022, 94, e20210731. [Google Scholar] [CrossRef]
- Mayorgas, A.; Dotti, I.; Salas, A. Microbial metabolites, postbiotics, and intestinal epithelial function. Mol. Nutr. Food Res. 2021, 65, 2000188. [Google Scholar] [CrossRef]
- Xu, J.; Hu, F.L.; Wang, W.; Wan, X.C.; Bao, G.H. Investigation on biochemical compositional changes during the microbial fermentation process of Fu brick tea by LC-MS based metabolomics. Food Chem. 2015, 186, 176–184. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Yao, X.; Chen, Y.; Ho, C.T.; He, C.; Li, Z.; Wang, Y. Metabolite profiling, antioxidant and α-glucosidase inhibitory activities of buckwheat processed by solid-state fermentation with Eurotium cristatum YL-1. Food Res. Int. 2021, 143, 110262. [Google Scholar] [CrossRef]
- Honoré, A.H.; Aunsbjerg, S.D.; Ebrahimi, P.; Thorsen, M.; Benfeldt, C.; Knøchel, S.; Skov, T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 2016, 408, 83–96. [Google Scholar] [CrossRef]
- Sebag, S.C.; Qian, Q.; Upara, C.; Ding, Q.; Cao, H.; Hong, L.; Yang, L. A medium chain ratty acid, 6-hydroxyhexanoic acid (6-HHA), protects against obesity and insulin resistance. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kim, J.H. Some important metabolites produced by lactic acid bacteria originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Wang, K.; Li, T.; Yang, M.; Wang, R.; Chen, Y.; Cao, M.; Hu, R. Flumethasone enhances the efficacy of chemotherapeutic drugs in lung cancer by inhibiting Nrf2 signaling pathway. Cancer Lett. 2020, 474, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.U.; Yosaatmadja, Y.; Teague, R.M.; Chai, M.Z.; Turnbull, A.P.; Squire, C.J. Crystal structures of three classes of non-steroidal anti-inflammatory drugs in complex with aldo-keto reductase 1C3. PLoS ONE 2012, 7, e43965. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, J.H.; Choi, E.J.; Yun, Y.R.; Lee, K.W.; Chang, J.Y. UPLC-QTOF-MS/MS and GC-MS characterization of phytochemicals in vegetable juice fermented using lactic aacid bacteria from Kimchi and their antioxidant potential. Antioxidants 2021, 10, 1761. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhao, Z.; Fang, B.; Hung, W.; Gao, H.; Zhao, W.; Lan, H.; Liu, M.; Zhao, L.; Zhang, M. Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics. Foods 2023, 12, 3579. https://doi.org/10.3390/foods12193579
Sun Z, Zhao Z, Fang B, Hung W, Gao H, Zhao W, Lan H, Liu M, Zhao L, Zhang M. Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics. Foods. 2023; 12(19):3579. https://doi.org/10.3390/foods12193579
Chicago/Turabian StyleSun, Zhe, Zhi Zhao, Bing Fang, Weilian Hung, Haina Gao, Wen Zhao, Hanglian Lan, Mingkun Liu, Liang Zhao, and Ming Zhang. 2023. "Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics" Foods 12, no. 19: 3579. https://doi.org/10.3390/foods12193579






