Sustainable Pickering Emulsions with Nanocellulose: Innovations and Challenges
Abstract
:1. Introduction
2. Scientometrics Analysis
3. Nanocellulose for Pickering Emulsions
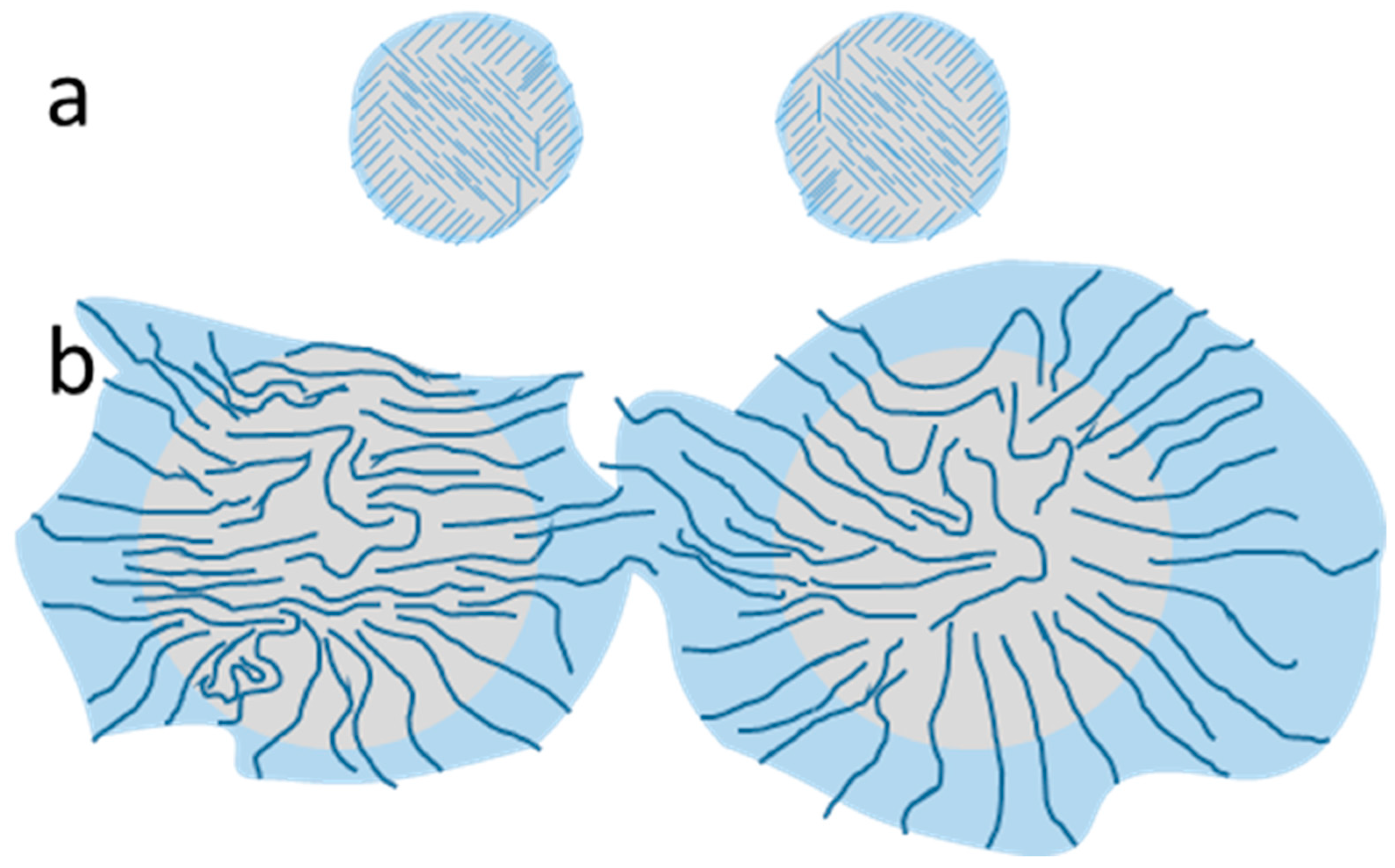
3.1. Cellulose-Based Pickering Emulsions Stability
3.2. Impact of the Phase Composition on the Stability of Pickering Emulsions
4. Applying Pickering Emulsions to Food
4.1. Foodstuffs
4.2. Food Packaging
4.3. Innovative Food Applications
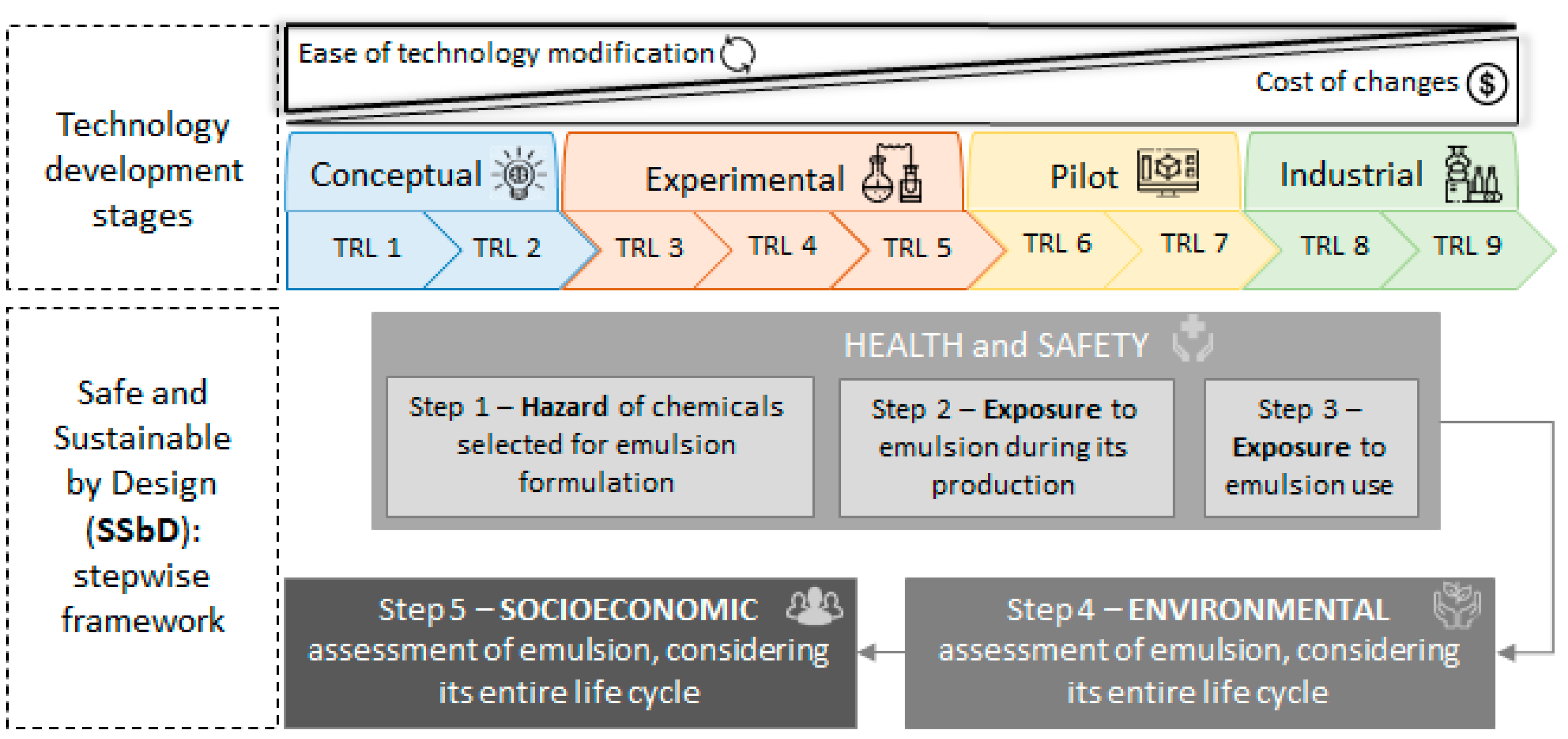
5. The Sustainability of Pickering Emulsions
- The chemical intrinsic properties (step 1);
- Occupational health and safety in the workplace where the chemical/material is produced (step 2);
- Occupational health and safety at the site where the chemical/material is used (formulation of another product, step 3).
- Life cycle environmental impact assessment of the novel chemical (step 4);
- Socioeconomic impacts of the novel chemical (step 5).
5.1. The Occupational Health and Safety of Nanocellulose Pickering Emulsions
5.2. Environmental Impact Assessment at Early Emulsion Development Stages
5.3. Environmental Impacts of Emulsions and Main Components
5.4. Socioeconomic Assessment
- Workers: child labor, fair salary, forced labor, health, and safety (focusing on accidents at the workplace since other aspects are considered in steps 2), freedom of association and collective bargaining, working hours, and equal opportunities/discrimination;
- Local community: community engagement and local employment;
- Consumers/users: health and safety (not covered in step 3) and accountable communication.
6. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, L.; Huan, S.; Zhu, Y.; Chu, G.; McClements, D.J.; Rojas, O.J. Recent Advances in Food Emulsions and Engineering Foodstuffs Using Plant-Based Nanocelluloses. Annu. Rev. Food Sci. Technol. 2021, 12, 383–406. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-Stabilized Pickering Emulsions and Their Applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef]
- Zhu, M.; Huan, S.; Liu, S.; Li, Z.; He, M.; Yang, G.; Liu, S.; McClements, D.J.; Rojas, O.J.; Bai, L. Recent Development in Food Emulsion Stabilized by Plant-Based Cellulose Nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 56, 101512. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of Nanocelluloses at Interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. Journal of the Chemical Society. Transactions 1907, 91, 2001–2021. [Google Scholar]
- Kaptay, G. On the Equation of the Maximum Capillary Pressure Induced by Solid Particles to Stabilize Emulsions and Foams and on the Emulsion Stability Diagrams. Colloids Surf. A Physicochem. Eng. Aspects 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions Stabilised Solely by Colloidal Particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Kuang, Y.; Liao, J.; Liu, K.; Li, J.; Mo, L.; He, S.; Zhu, W.; Song, J.; et al. Residual Lignin in Cellulose Nanofibrils Enhances the Interfacial Stabilization of Pickering Emulsions. Carbohydr. Polym. 2021, 253, 117223. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean Label: Why This Ingredient but Not That One? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Souza, N.F.; Almeida, J.S.; Pinheiro, J.A.; Pereira, P.H.F.; Saraiva Morais, J.P.; Figueirêdo, C.B.; Azeredo, H.M.C.; Leitão, R.C.; de Rosa, M.F. Progress in Organosolv and Steam Explosion Pretreatments of Oil Palm Fibers for Biomacromolecules Extraction. J. Nat. Fibers 2022, 19, 10708–10722. [Google Scholar] [CrossRef]
- de Figueirêdo, M.C.B.; de Rosa, M.F.; Ugaya, C.M.L.; de Souza Filho, M.d.S.M.; da Silva Braid, A.C.C.; de Melo, L.F.L. Life Cycle Assessment of Cellulose Nanowhiskers. J. Clean. Prod. 2012, 35, 130–139. [Google Scholar] [CrossRef]
- Haroni, S.; Zaki Dizaji, H.; Bahrami, H.; González Alriols, M. Sustainable Production of Cellulose Nanofiber from Sugarcane Trash: A Quality and Life Cycle Assessment. Ind. Crops Prod. 2021, 173, 114084. [Google Scholar] [CrossRef]
- do Nascimento, D.M.; Dias, A.F.; de Araújo Junior, C.P.; de Rosa, M.F.; Morais, J.P.S.; de Figueirêdo, M.C.B. A Comprehensive Approach for Obtaining Cellulose Nanocrystal from Coconut Fiber. Part II: Environmental Assessment of Technological Pathways. Ind. Crops Prod. 2016, 93, 58–65. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of Deep Eutectic Solvents in Polymer Chemistry—A Review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of Nanocellulose by Enzymatic Hydrolysis: Trends and Challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.d.S.M.; de Rosa, M.F. Bacterial Cellulose Nanocrystals Produced under Different Hydrolysis Conditions: Properties and Morphological Features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, M.; Yang, S.; Song, X.; Xu, Y. Combined Mechanical Grinding and Enzyme Post-Treatment Leading to Increased Yield and Size Uniformity of Cellulose Nanofibrils. Cellulose 2020, 27, 7447–7461. [Google Scholar] [CrossRef]
- Sungsinchai, S.; Niamnuy, C.; Seubsai, A.; Prapainainar, P.; Wattanapan, P.; Thakhiew, W.; Raghavan, V.; Devahastin, S. Comparative Evaluation of the Effect of Microfluidisation on Physicochemical Properties and Usability as Food Thickener and Pickering Emulsifier of Autoclaved and TEMPO-Oxidised Nanofibrillated Cellulose. Int. J. Food Sci. Technol. 2021, 1–18. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, M.; Xu, Q.; Zhong, C. Rheological Behaviors of Pickering Emulsions Stabilized by TEMPO-Oxidized Bacterial Cellulose. Carbohydr. Polym. 2019, 215, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Białopiotrowicz, T. Wettability of Starch Gel Films. Food Hydrocoll. 2003, 17, 141–147. [Google Scholar] [CrossRef]
- Koller, B.; Müller-Wiefel, A.S.; Rupec, R.; Korting, H.C.; Ruzicka, T. Chitin Modulates Innate Immune Responses of Keratinocytes. PLoS ONE 2011, 6, e16594. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Woodhead Publishing: Sawston, UK, 2008; pp. 517–542. [Google Scholar]
- Nayak, A.; Genot, C.; Meynier, A.; Dorlando, A.; Capron, I. Impact of Process and Physico-Chemical Conditions on the Formation of Curcumin-Whey Protein Composite Particles Capable to Stabilize Food-Compatible Oil in Water Emulsions. LWT 2022, 153, 112421. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-Ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Willhammar, T.; Daicho, K.; Johnstone, D.N.; Kobayashi, K.; Liu, Y.; Midgley, P.A.; Bergström, L.; Saito, T. Local Crystallinity in Twisted Cellulose Nanofibers. ACS Nano 2021, 15, 2730–2737. [Google Scholar] [CrossRef]
- Ni, Y.; Gu, Q.; Li, J.; Fan, L. Modulating in Vitro Gastrointestinal Digestion of Nanocellulose-Stabilized Pickering Emulsions by Altering Cellulose Lengths. Food Hydrocoll. 2021, 118, 106738. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Chen, Y.; Ma, L.; Wu, J.; Zhang, Y. Co-Stabilization and Properties Regulation of Pickering Emulsions by Cellulose Nanocrystals and Nanofibrils from Lemon Seeds. Food Hydrocoll. 2021, 120, 106884. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Gong, J.; Kuang, Y.; Mo, L.; Song, T. Cellulose Nanocrystals (CNCs) with Different Crystalline Allomorph for Oil in Water Pickering Emulsions. Carbohydr. Polym. 2018, 183, 303–310. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Feng, X.; Ma, L.; Zhang, Y.; Dai, H. Lignocellulose Nanocrystals from Pineapple Peel: Preparation, Characterization and Application as Efficient Pickering Emulsion Stabilizers. Food Res. Int. 2021, 150, 110738. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-Water Pickering Emulsions via Microfluidization with Cellulose Nanocrystals: 1. Formation and Stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of Surface Charge Density of Bacterial Cellulose Nanofibrils on the Rheology Property of O/W Pickering Emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Kuang, Y.; Guo, S.; Mo, L.; Ni, Y. Stabilization of Pickering Emulsions with Cellulose Nanofibers Derived from Oil Palm Fruit Bunch. Cellulose 2020, 27, 839–851. [Google Scholar] [CrossRef]
- Cathala, B.; Capron, I.; Bizot, H.; Bulcon, A.; Kalashnikova, I. Composition in the Form of an Emulsion, Comprising a Hydrophobic Phase Dispersed in an Aqueous Phase. European Patent Office EP2595738A1, 29 May 2013. [Google Scholar]
- Yuan, M.; Wantong, Y.; Yong, Z.; Shiru, J. A Method for Preparing Pickering Using Bacterial Cellulose. China CN102309943A, 11 January 2012. [Google Scholar]
- Xie, J.; Luo, Y.; Chen, Y.; Liu, Y.; Ma, Y.; Zheng, Q.; Yue, P.; Yang, M. Redispersible Pickering Emulsion Powder Stabilized by Nanocrystalline Cellulose Combining with Cellulosic Derivatives. Carbohydr. Polym. 2019, 213, 128–137. [Google Scholar] [CrossRef]
- Szlapak Franco, T.; Martínez Rodríguez, D.C.; Jiménez Soto, M.F.; Jiménez Amezcua, R.M.; Urquíza, M.R.; Mendizábal Mijares, E.; de Muniz, G.I.B. Production and Technological Characteristics of Avocado Oil Emulsions Stabilized with Cellulose Nanofibrils Isolated from Agroindustrial Residues. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124263. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Tonon, R.V.; McClements, D.J. Designing Healthier Foods: Reducing the Content or Digestibility of Key Nutrients. Trends Food Sci. Technol. 2021, 118, 459–470. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-Water Pickering Emulsions via Microfluidization with Cellulose Nanocrystals: 2. In vitro lipid digestion. Food Hydrocoll. 2019, 96, 709–7126. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdagi, S.I.; Yildiz, U. Pickering Emulsions Stabilized Nanocellulosic-Based Nanoparticles for Coumarin and Curcumin Nanoencapsulations: In Vitro Release, Anticancer and Antimicrobial Activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Xu, H.; Feng, X.; Wang, B.; Pukánszky, B.; Mao, Z.; Sui, X. Poly(Lactic Acid)/Cellulose Nanocrystal Composites via the Pickering Emulsion Approach: Rheological, Thermal and Mechanical Properties. Int. J. Biol. Macromol. 2019, 137, 197–204. [Google Scholar] [CrossRef]
- Li, X.; Hegyesi, N.; Zhang, Y.; Mao, Z.; Feng, X.; Wang, B.; Pukánszky, B.; Sui, X. Poly(Lactic Acid)/Lignin Blends Prepared with the Pickering Emulsion Template Method. Eur. Polym. J. 2019, 110, 378–384. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the Preparation and Antibacterial Activity of Emulsions Stabilized with Nanocellulose Particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Zhao, Y.; Powell-Palm, M.J.; Wang, J.; Bilbao-Sainz, C.; McHugh, T.; Rubinsky, B. Analysis of Global Energy Savings in the Frozen Food Industry Made Possible by Transitioning from Conventional Isobaric Freezing to Isochoric Freezing. Renew. Sustain. Energy Rev. 2021, 151, 111621. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Chen, P.; Rojas, R.; Hajian, A.; Berglund, L. Cellulose Nanofibers Enable Paraffin Encapsulation and the Formation of Stable Thermal Regulation Nanocomposites. Nano Energy 2017, 34, 541–548. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Scalable Pickering Stabilization to Design Cellulose Nanofiber-Wrapped Block Copolymer Microspheres for Thermal Energy Storage. ACS Sustain. Chem. Eng. 2020, 8, 4623–4632. [Google Scholar] [CrossRef]
- Kavousi, F.; Nikfarjam, N. Highly Interconnected Macroporous Structures Made from Starch Nanoparticle-Stabilized Medium Internal Phase Emulsion Polymerization for Use in Cell Culture. Polymer 2019, 180, 121744. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Lenart, A.; Janowicz, M. Sustainable Development in the Agri-Food Sector in Terms of the Carbon Footprint: A Review. Sustainability 2020, 12, 6463. [Google Scholar] [CrossRef]
- Pakseresht, A.; Ahmadi Kaliji, S.; Canavari, M. Review of Factors Affecting Consumer Acceptance of Cultured Meat. Appetite 2022, 170, 105829. [Google Scholar] [CrossRef]
- Rzymski, P.; Kulus, M.; Jankowski, M.; Dompe, C.; Bryl, R.; Petitte, J.N.; Kempisty, B.; Mozdziak, P. COVID-19 Pandemic Is a Call to Search for Alternative Protein Sources as Food and Feed: A Review of Possibilities. Nutrients 2021, 13, 150. [Google Scholar] [CrossRef]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future Foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-Based and Cell-Based Approaches to Meat Production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Cui, R.; Lu, S.; Hu, X.; Xu, B.; Song, Y.; Hu, X. High Internal Phase Pickering Emulsions Stabilized by Cellulose Nanocrystals for 3D Printing. Food Hydrocoll. 2022, 125, 107418. [Google Scholar] [CrossRef]
- Polverini, D. Regulating the Circular Economy within the Ecodesign Directive: Progress so Far, Methodological Challenges and Outlook. Sustain. Prod. Consum. 2021, 27, 1113–1123. [Google Scholar] [CrossRef]
- Arvidsson, R.; Tillman, A.; Sandén, B.A.; Janssen, M.; Nordelöf, A.; Kushnir, D.; Molander, S. Environmental Assessment of Emerging Technologies: Recommendations for Prospective LCA. J. Ind. Ecol. 2018, 22, 1286–1294. [Google Scholar] [CrossRef]
- European Commission. Safe and Sustainable by Design Chemicals and Materials: Framework for the Definition of Criteria and Evaluation Procedure for Chemicals and Materials; Publications Office: Luxembourg, 2022. [Google Scholar]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological Effects of Ingested Nanocellulose in in Vitro Intestinal Epithelium and in Vivo Rat Models. Environ. Sci.: Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef]
- Khare, S.; DeLoid, G.M.; Molina, R.M.; Gokulan, K.; Couvillion, S.P.; Bloodsworth, K.J.; Eder, E.K.; Wong, A.R.; Hoyt, D.W.; Bramer, L.M.; et al. Effects of Ingested Nanocellulose on Intestinal Microbiota and Homeostasis in Wistar Han Rats. NanoImpact 2020, 18, 100216. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Kim, B. Cellulose Nanomaterials: Life Cycle Risk Assessment, and Environmental Health and Safety Roadmap. Environ. Sci. Nano 2015, 2, 477–499. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In Vitro Biological Responses to Nanofibrillated Cellulose by Human Dermal, Lung and Immune Cells: Surface Chemistry Aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Meschini, S.; Pellegrini, E.; Maestri, C.A.; Condello, M.; Bettotti, P.; Condello, G.; Scarpa, M. In Vitro Toxicity Assessment of Hydrogel Patches Obtained by Cation-induced Cross-linking of Rod-like Cellulose Nanocrystals. J. Biomed. Mater. Res. 2020, 108, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial Exposure, Toxicity, and Impact on Human Health. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Khaliullin, T.O.; Shurin, M.R.; Kisin, E.R.; Yanamala, N.; Fadeel, B.; Chang, J.; Shvedova, A.A. Fibrous Nanocellulose, Crystalline Nanocellulose, Carbon Nanotubes, and Crocidolite Asbestos Elicit Disparate Immune Responses upon Pharyngeal Aspiration in Mice. J. Immunotoxicol. 2018, 15, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yanamala, N.; Farcas, M.T.; Hatfield, M.K.; Kisin, E.R.; Kagan, V.E.; Geraci, C.L.; Shvedova, A.A. In Vivo Evaluation of the Pulmonary Toxicity of Cellulose Nanocrystals: A Renewable and Sustainable Nanomaterial of the Future. ACS Sustain. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Stoudmann, N.; Schmutz, M.; Hirsch, C.; Nowack, B.; Som, C. Human Hazard Potential of Nanocellulose: Quantitative Insights from the Literature. Nanotoxicology 2020, 14, 1241–1257. [Google Scholar] [CrossRef]
- Brand, W.; van Kesteren, P.C.E.; Swart, E.; Oomen, A.G. Overview of Potential Adverse Health Effects of Oral Exposure to Nanocellulose. Nanotoxicology 2022, 16, 217–246. [Google Scholar] [CrossRef]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.T.; Louro, H.; Silva, M.J. On the Toxicity of Cellulose Nanocrystals and Nanofibrils in Animal and Cellular Models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 4 March 2022).
- U.S. Food & Drug Administration GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&sort=GRN_No&order=DESC&startrow=1&type=basic&search=cellulose (accessed on 11 May 2022).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- Da Silva, A.K.P.; Cardoso, A.; Benício de Sá Filho, E.; Monteiro Cordeiro de Azeredo, H.; Freire, F.; Casimiro Filho, F.; Brito de Figueirêdo, M.C. Integrating Life Cycle Assessment in Early Process Development Stage: The Case of Extracting Starch from Mango Kernel. J. Clean. Prod. 2021, 321, 128981. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the Environmental Impact of a Future Nanocellulose Production at Industrial Scale: Application of the Life Cycle Assessment Scale-up Framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- van der Giesen, C.; Cucurachi, S.; Guinée, J.; Kramer, G.J.; Tukker, A. A Critical View on the Current Application of LCA for New Technologies and Recommendations for Improved Practice. J. Clean. Prod. 2020, 259, 120904. [Google Scholar] [CrossRef]
- Steubing, B.; de Koning, D. Making the Use of Scenarios in LCA Easier: The Superstructure Approach. Int. J. Life Cycle Assess. 2021, 26, 2248–2262. [Google Scholar] [CrossRef]
- European Commission. Recommendation on the Use of the Environmental Footprint Methods; Publications Office: Luxembourg, 2021. [Google Scholar]
- Bondancia, T.J.; Batista, G.; de Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Leão, R.M.; Miléo, P.C.; Maia, J.M.L.L.; Luz, S.M. Environmental and Technical Feasibility of Cellulose Nanocrystal Manufacturing from Sugarcane Bagasse. Carbohydr. Polym. 2017, 175, 518–529. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, X.; Ai, Y.; Huang, R.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Greener Production of Cellulose Nanocrystals: An Optimised Design and Life Cycle Assessment. J. Clean. Prod. 2022, 345, 131073. [Google Scholar] [CrossRef]
- Berglund, L.; Breedveld, L.; Oksman, K. Toward Eco-Efficient Production of Natural Nanofibers from Industrial Residue: Eco-Design and Quality Assessment. J. Clean. Prod. 2020, 255, 120274. [Google Scholar] [CrossRef]
- Krexner, T.; Bauer, A.; Zollitsch, W.; Weiland, K.; Bismarck, A.; Mautner, A.; Medel-Jiménez, F.; Gronauer, A.; Kral, I. Environmental Life Cycle Assessment of Nano-Cellulose and Biogas Production from Manure. J. Environ. Manag. 2022, 314, 115093. [Google Scholar] [CrossRef]
- Li, Q.; McGinnis, S.; Sydnor, C.; Wong, A.; Renneckar, S. Nanocellulose Life Cycle Assessment. ACS Sustain. Chem. Eng. 2013, 1, 919–928. [Google Scholar] [CrossRef]
- Nadeem, H.; Dehghani, M.; Garnier, G.; Batchelor, W. Life Cycle Assessment of Cellulose Nanofibril Films via Spray Deposition and Vacuum Filtration Pathways for Small Scale Production. J. Clean. Prod. 2022, 342, 130890. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Life Cycle Assessment of a New Technology to Extract, Functionalize and Orient Cellulose Nanofibers from Food Waste. ACS Sustain. Chem. Eng. 2015, 3, 1047–1055. [Google Scholar] [CrossRef]
- Fernández-Lobato, L.; López-Sánchez, Y.; Baccar, R.; Fendri, M.; Vera, D. Life Cycle Assessment of the Most Representative Virgin Olive Oil Production Systems in Tunisia. Sustain. Prod. Consum. 2022, 32, 908–923. [Google Scholar] [CrossRef]
- Prado, V.; Daystar, J.; Pires, S.; Wallace, M.; Laurin, L. Comparative Life Cycle Assessment of Edible Vegetable Frying Oils. Trans. ASABE 2021, 64, 1717–1733. [Google Scholar] [CrossRef]
- Arvidsson, R.; Nguyen, D.; Svanström, M. Life Cycle Assessment of Cellulose Nanofibrils Production by Mechanical Treatment and Two Different Pretreatment Processes. Environ. Sci. Technol. 2015, 49, 6881–6890. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Eco-Efficient Process Improvement at the Early Development Stage: Identifying Environmental and Economic Process Hotspots for Synergetic Improvement Potential. Environ. Sci. Technol. 2018, 52, 5959–5967. [Google Scholar] [CrossRef]
- Bai, Y.; Zhai, Y.; Ji, C.; Zhang, T.; Chen, W.; Shen, X.; Hong, J. Environmental Sustainability Challenges of China’s Edible Vegetable Oil Industry: From Farm to Factory. Resour. Conserv. Recycl. 2021, 170, 105606. [Google Scholar] [CrossRef]
- van Paassen, M.; Braconi, N.; Kuling, L.; Durlinger, B.; Gual, P. Agri-Footprint 5.0; Agri-footprint: Gouda, The Netherlands, 2019. [Google Scholar]
- Benoît Norris, C.; Traverso, M.; Neugebauer, S.; Ekener, E.; Schaubroeck, T.; Russo Garrido, S.; Berger, M.; Valdivia, S.; Lehmann, A.; Finkbeiner, M.; et al. Guidelines for Social Life Cycle Assessment of Products and Organizations 2020; United Nations Environment Programme (UNEP): Paris, France, 2020. [Google Scholar]
- Alvarado, C.; Hallberg, K.; Nieuwenhuizenn, P.; Saling, P.; Chan, K.; Gupta, J.D.; Morris, D.; Nicole, G.; Wientjes, F.; Dierckx, A.; et al. Social Life Cycle Metrics for Chemical Products; World Business Council for Sustainable Development: Geneva, Switzerland, 2016; ISBN 978-2-940521-52-4. [Google Scholar]
- Goedkoop, M.J.; de Beer, I.M.; Harmens, R.; Saling, P.; Morris, D.; Florea, A.; Hettinger, A.L.; Indrane, D.; Visser, D.; Morao, A.; et al. Product Social Impact Assessment Handbook; Social Valuen Initiative: Amersfoot, The Netherlands, 2020. [Google Scholar]
- Desiderio, E.; García-Herrero, L.; Hall, D.; Segrè, A.; Vittuari, M. Social Sustainability Tools and Indicators for the Food Supply Chain: A Systematic Literature Review. Sustain. Prod. Consum. 2022, 30, 527–540. [Google Scholar] [CrossRef]
- Tragnone, B.M.; D’Eusanio, M.; Petti, L. The Count of What Counts in the Agri-Food Social Life Cycle Assessment. J. Clean. Prod. 2022, 354, 131624. [Google Scholar] [CrossRef]
- Traverso, M.; Mankaa, M.N.; Valdivia, S.; Roche, L.; Luthin, A.; Garrido, S.R.; Neugebauer, S. Pilot Projects on Guidelines for Social Life Cycle Assessment of Products and Organizations 2022; Life Cycle Initiative: Paris, France, 2022. [Google Scholar]
- França, W.T.; Barros, M.V.; Salvador, R.; de Francisco, A.C.; Moreira, M.T.; Piekarski, C.M. Integrating Life Cycle Assessment and Life Cycle Cost: A Review of Environmental-Economic Studies. Int. J. Life Cycle Assess. 2021, 26, 244–274. [Google Scholar] [CrossRef]
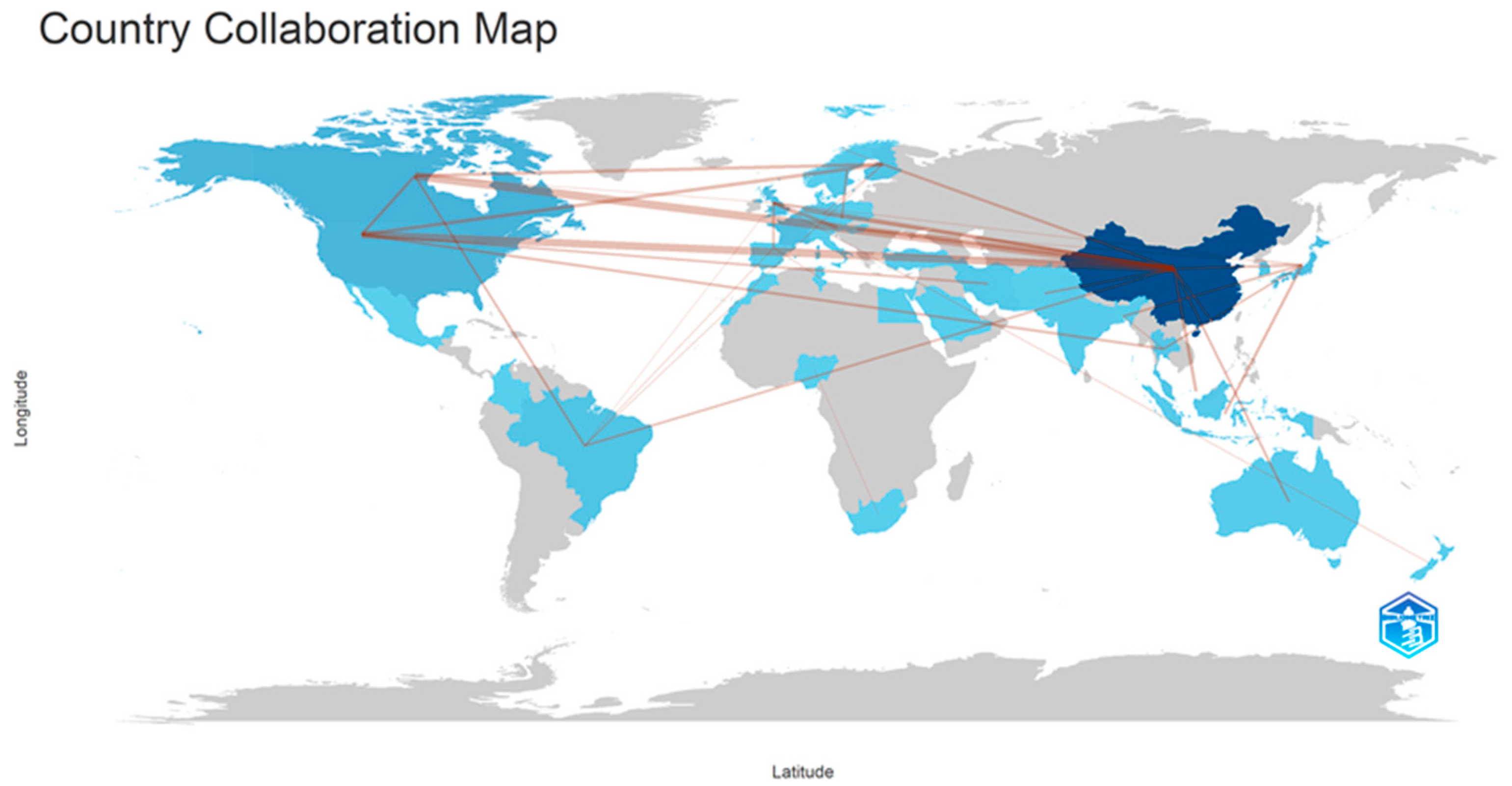
| Published Articles | Sources |
|---|---|
| 69 | Carbohydrate Polymers |
| 53 | Food Hydrocolloids |
| 40 | International Journal of Biological Macromolecules |
| 32 | Cellulose |
| 28 | Colloids and Surfaces A: Physicochemical and Engineering Aspects |
| 24 | Langmuir |
| 21 | Journal of Colloid and Interface Science |
| 18 | ACS Applied Materials and Interfaces |
| 16 | Biomacromolecules |
| 14 | Food Chemistry |
| References * | Feedstock | Processing Routes | Scale | Impact Categories | Environmental Impact Assessment Methods | Best Results for GWP ** for 100 years (kg CO2-eq/kg of Nanocellulose or Oil) |
|---|---|---|---|---|---|---|
| CNC | ||||||
| (Figueirêdo et al., 2012) [13] | Coconut fiber | EUC—Chopping, washing, beaching (NaClO2), hydrolysis (H2SO4), centrifugation, dialysis. | Lab | Global warming potential/climate change (100 years), water depletion (total blue water consumption), eutrophication, and human toxicity | Recipe | 122 for EC |
| Cotton linter | EC—Chopping, hydrolysis (H2SO4), centrifugation, dialysis. | |||||
| (Nascimento et al., 2016) [15] | Coconut fiber | CNH1: gridding, pulping, bleaching, hydrolysis with diluted acid, dialysis. | Lab | Global warming potential/climate change (100 years), acidification, freshwater eutrophication, marine eutrophication, human toxicity and water depletion (total blue water consumption). | Recipe | 207 for CNU |
| CNH2: gridding, pulping, bleaching, hydrolysis with concentrated acid, dialysis. | ||||||
| CNO: gridding, pulping, oxidation with ammonium persulfate. | ||||||
| CNU: gridding, pulping, bleaching, and high power ultrasound. | ||||||
| (Leão et al., 2017) [82] | Sugarcane bagasse | I—NaOH/NaClO2/H2SO4/60 min/4x. | Lab | Global warming potential/climate change (100 years) and water footprint (total water consumption = blue+ green + gray quantities) | CML 2010 | 13.7 |
| II—NaOH/NaClO2/H2SO4/60 min/1x. | ||||||
| III—NaClO2/NaOH/H2SO4/30 min/4x. | ||||||
| IV—NaClO2/NaOH/H2SO4/30 min/4x. | ||||||
| V—NaClO2/NaOH/H2SO4/30 min/1x. | ||||||
| VI—NaOH/NaClO2/HNO3/H2SO4/60 min/4x. | ||||||
| VII—NaOH/NaClO2/HNO3/H2SO4/60 min/1x. | ||||||
| VIII—NaClO2/NaOH/HNO3/H2SO4/30 min/4x. | ||||||
| IX—NaClO2/NaOH/HNO3/H2SO4/30 min/1x. | ||||||
| X—NaOH/H2O2/H2SO4/30 min/4x. | ||||||
| XI—NaOH/H2O2/H2SO4/30 min/1x. | ||||||
| XII—NaOH/H2O2/H2SO4/30 min/1x. | ||||||
| (Bondancia et al., 2022) [81] | Sugarcane bagasse | S-CNC: pretreatment (Hydrothermal enzymatic hydrolysis, organosolv, bleaching) and hydrolysis with sulfuric acid. | Modeled industrial scale | Global warming potential/climate change (100 years) | CML-IA Baseline v. 3.04 2000 | No real values presented but indication of S-CNC as best case (twice lower impact than other routes). |
| Cit-CNC: pretreatment (Hydrothermal enzymatic hydrolysis, organosolv, bleaching) and hydrolysis with citric acid. | ||||||
| Cit-S-CNC: pretreatment (Hydrothermal enzymatic hydrolysis, organosolv, bleaching) and hydrolysis with a combination of acids. | ||||||
| (Zhang et al., 2022) [83] | Cotton | 1—Sulfuric acid hydrolysis, followed by the separation of sulfuric acid from hydrolysate mixture using gravity settling, followed by microfiltration, tubular centrifugation, and ultrafiltration. | Pilot | Global warming potential/climate change (100 years) aquatic ecotoxicity, terrestrial ecotoxicity, aquatic acidification, aquatic eutrophication, and non-renewable energy. | IMPACT 2002+ | 30 for route 4 |
| 2—Sulfuric acid hydrolysis, followed by the separation of sulfuric acid from hydrolysate mixture using gravity settling, followed by disk stack centrifugation, microfiltration, tubular centrifugation, and ultrafiltration. | ||||||
| 3—Sulfuric acid hydrolysis, followed by the separation of sulfuric acid from hydrolysate mixture using low-speed centrifugation, followed by disk stack centrifugation, microfiltration, tubular centrifugation, and ultrafiltration. | ||||||
| 4—Sulfuric acid hydrolysis, followed by the separation of sulfuric acid from hydrolysate mixture using ceramic membrane microfiltration, followed by disk stack centrifugation, microfiltration, tubular centrifugation, and ultrafiltration. | ||||||
| CNF | ||||||
| (Li et al., 2013) [86] | Delignified Kraft pulp from wood | TOSO: TEMPO-oxidation for chemical modification; sonication for mechanical disintegration. | Lab | Global warming potential/climate change (100 years), carcinogens human health, respiratory organics, respiratory inorganics, radiation, ozone layer, ecotoxicity ecosystem quality, acidification/eutrophication, land use, mineral resources, and fossil fuels. | Eco-Indicator 99 | 190 for TOHO route |
| TOHO: TEMPO-oxidation for chemical modification; homogenization for mechanical disintegration. | ||||||
| CESO: chloroacetic acid etherification for chemical modification; sonication for mechanical disintegration. | ||||||
| CEHO: chloroacetic acid etherification for chemical modification; homogenization for mechanical disintegration. | ||||||
| (Arvidsson et al., 2015) [91] | Wood pulp | 1—Enzymatic pretreatment, followed by microfluidization. | Lab | Energy use, global warming potential, acidification, and water use. | CED for energy use and Recipe for the other categories | 0.79 for route 1 |
| 2—Carboxymethylation pretreatment, followed by microfluidization. | ||||||
| 3—No pretreatment route, followed by homogenization. | ||||||
| (Piccinno et al., 2015) [88] | carrot waste (by-product of carrot juice production) | Boiling, enzymatic depolymerization, and homogenization. | Lab | Global warming potential, fossil fuel depletion potential, freshwater ecotoxicity potential, human toxicity potential, ionizing radiation potential, marine ecotoxicity potential, ozone depletion potential, photochemical oxidant formation potential, terrestrial acidification. | ReCiPe | 107 |
| (Piccinno et al., 2018b) [92] | Pilot | Human health, ecosystem quality and resources | ReCiPe endpoint | 32 *** | ||
| (Nadeem et al., 2022) [87] | Refining | 1—Refining (milling), homogenization, and high-pressure homogenization. | Lab | Global warming potential/climate change (100 years), energy demand, and water depletion (total water consumption) | IPCC, CED and Water depletion | 20 for route 1 |
| 2—Refining (milling) and homogenization. | ||||||
| 3—High-pressure homogenization. | ||||||
| (Berglund et al., 2020) [84] | Carrot waste (by-product of carrot juice production) | Pretreatment (distilled heated water, alkali treatment with NaOH and bleaching with NaClO2), followed by gridding stone fibrillation (ultra-fine grinder and high-pressure homogenizer). | Pilot | Acidification, global warming potential/climate change (100 years), ecotoxicity freshwater, eutrophication freshwater, eutrophication marine, eutrophication terrestrial, human toxicity/cancer effects, human toxicity/non cancer effects, ionizing radiation/human health, land use, ozone depletion, particulate matter/respiratory inorganics, photochemical ozone formation, resource depletion water, and resource depletion/minerals and metals, and resource depletion/fossils, and resource depletion/renewables. | ILCD/PEF | 2.9 (high-pressure homogenizer and European energy mix) |
| (Haroni et al., 2021) [14] | Sugarcane bagasse | Two different pretreatments for removing lignin (alkaline NaOH or organosolv) and two different methods for depolymerization (TEMPO oxidation or lime juice hydrolysis). | Lab | Global warming potential/climate change (100 years). | IPCC 2013 GWP 100a | 4.45 (alkaline delignification and TEMPO oxidation) |
| (Krexner et al., 2022) [85] | Manure (maize silage and pig slurry) | Anaerobic fermentation of manure (biogas and cellulose-residue), pulping (Kraft bleaching), and grinding (high-pressure homogenizing). | Pilot (fermentation and pulping), lab for CNF extraction | Global warming potential/climate change (100 years), fossil resource scarcity, freshwater eutrophication, human toxicity, terrestrial acidification (TAP) and terrestrial ecotoxicity potential. | ReCiPe 2016 | 4.41 |
| Vegetable oil | ||||||
| (Bai et al., 2021) [93] | Soybean | Conventional irrigated crop production and oil refining in China. | Industrial | Global warming potential/climate change (100 years), ozone formation-human health, fine particulate matter formation, ozone formation, stratospheric ozone depletion, terrestrial acidification, freshwater eutrophication, marine eutrophication, freshwater ecotoxicity, marine ecotoxicity, human carcinogenic toxicity, land use, fossil resource scarcity, terrestrial ecotoxicity, human non-carcinogenic toxicity, ionizing radiation, water consumption. | ReCiPe 2016 | 5.67 |
| Rapeseed | 3.35 | |||||
| Peanut | 3.15 | |||||
| (Prado et al., 2021) [90] | Cotton | Conventional crop production and oil refining in US. | Industrial | Global warming potential/climate change (100 years), abiotic depletion, eutrophication, acidification, photochemical oxidation, fine particulate matter, ozone layer depletion, water scarcity. | IPCC (2007) for climate change, AWARE (Water scarcity), Recipe 2016 for Fine particulate matter, and CML-IA for the other impact categories. | 1.1 |
| Soybean | Conventional crop production and oil refining (global average). | 6.4 | ||||
| Canola | Conventional crop production and oil refining in US. | 3.8 | ||||
| Palm | Conventional crop production and oil refining in Indonesia and Malaysia. | 4.8 | ||||
| (Fernández-Lobato et al., 2022) [89] | Olive oil | Olive cultivation systems in Tunisia (extensive, intensive, and super-intensive) as well as the main extraction systems (press, 3-phase and 2–3 phase-combined system). | Industrial | Acidification, global warming potential/climate change (100 years), ecotoxicity freshwater, eutrophication freshwater, eutrophication marine, eutrophication terrestrial, human toxicity/cancer effects, human toxicity/non cancer effects, ionizing radiation/human health, land use, ozone depletion, particulate matter/respiratory inorganics, photochemical ozone formation, resource depletion, water and resource depletion/minerals and metals, and resource depletion/fossils, and resource depletion/renewables. | ILCD/PEF | 3.53 (most representative system: extensive crop production and refining with three phase extraction) |
| (van Paassen et al., 2019) [94] | Refined coconut oil | Industrial | Global warming potential/climate change (100 years). | Recipe 2016 | 3.33 | |
| Refined rice bran oil | 1.61 | |||||
| Refined rapeseed oil | 1.90 | |||||
| Refined maize germ oil | 0.86 | |||||
| Refined palm oil | 7.44 | |||||
| Refined palm kernel oil | 10.45 | |||||
| Refined sunflower oil | 0.79 | |||||
| Refined soybean oil | 5.13 | |||||
| Refined lupine oil | 1.46 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, J.P.S.; Rosa, M.d.F.; Brito, E.S.d.; Azeredo, H.M.C.d.; Figueirêdo, M.C.B.d. Sustainable Pickering Emulsions with Nanocellulose: Innovations and Challenges. Foods 2023, 12, 3599. https://doi.org/10.3390/foods12193599
Morais JPS, Rosa MdF, Brito ESd, Azeredo HMCd, Figueirêdo MCBd. Sustainable Pickering Emulsions with Nanocellulose: Innovations and Challenges. Foods. 2023; 12(19):3599. https://doi.org/10.3390/foods12193599
Chicago/Turabian StyleMorais, João Paulo Saraiva, Morsyleide de Freitas Rosa, Edy Sousa de Brito, Henriette Monteiro Cordeiro de Azeredo, and Maria Cléa Brito de Figueirêdo. 2023. "Sustainable Pickering Emulsions with Nanocellulose: Innovations and Challenges" Foods 12, no. 19: 3599. https://doi.org/10.3390/foods12193599







