Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Condition of Strains
2.2. Minimum Inhibitory Concentration Assays
2.3. Biofilm Formation Ability
2.4. Biofilm Inhibition and Removal Assay
2.5. Microscopic Investigation
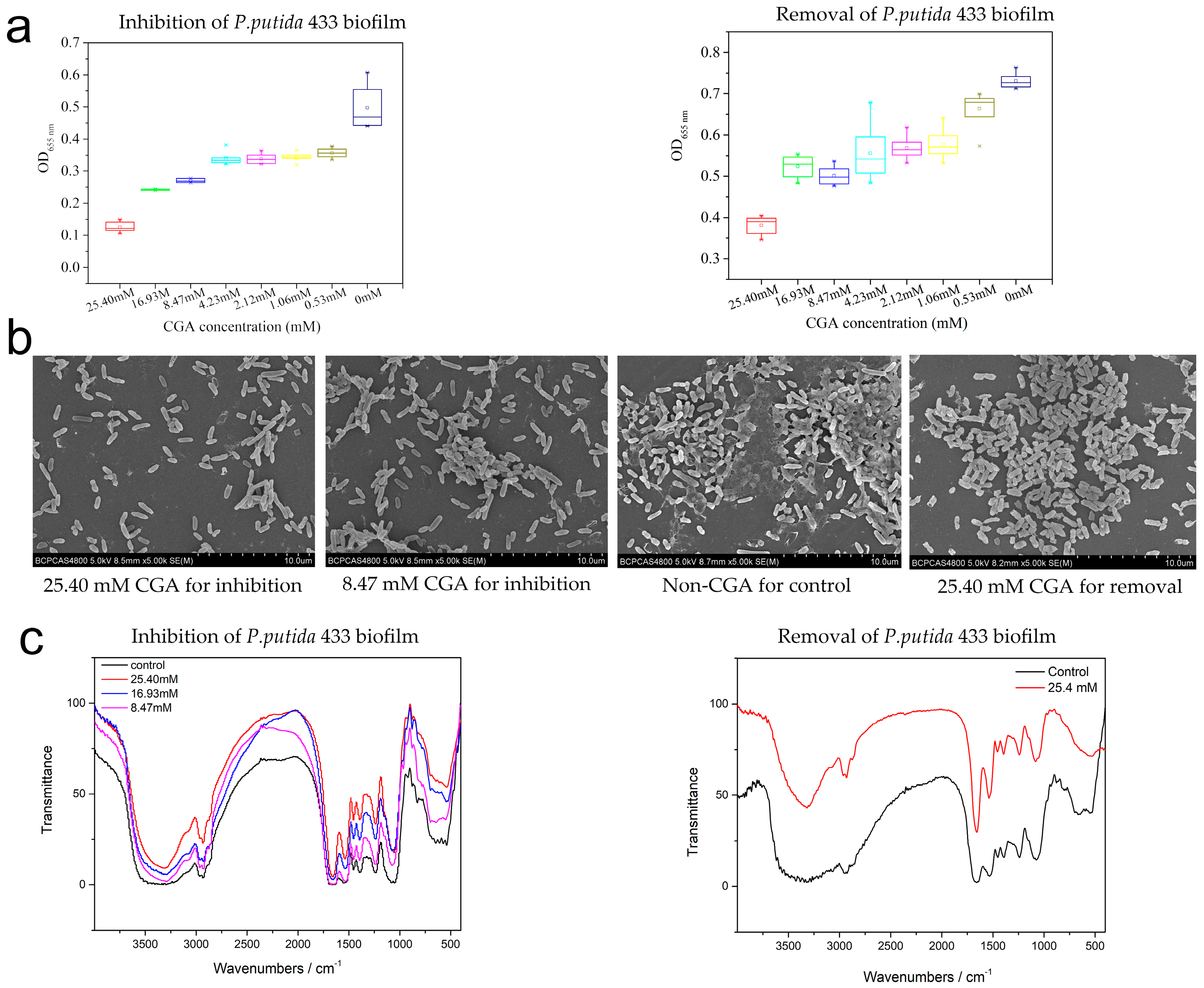
2.6. FTIR Analysis
2.7. Screening of CGA
2.8. Metabolite Extraction and GC-MS Analysis
2.9. RNA Isolation and RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. Pseudomonas Strains Biofilm Formation Ability
3.2. Antibacterial Activity of CGA against P. putida
3.3. Effects of CGA on the Biofilm Formation of P. putida
3.4. The Effect of CGA on Biofilm Microstructure
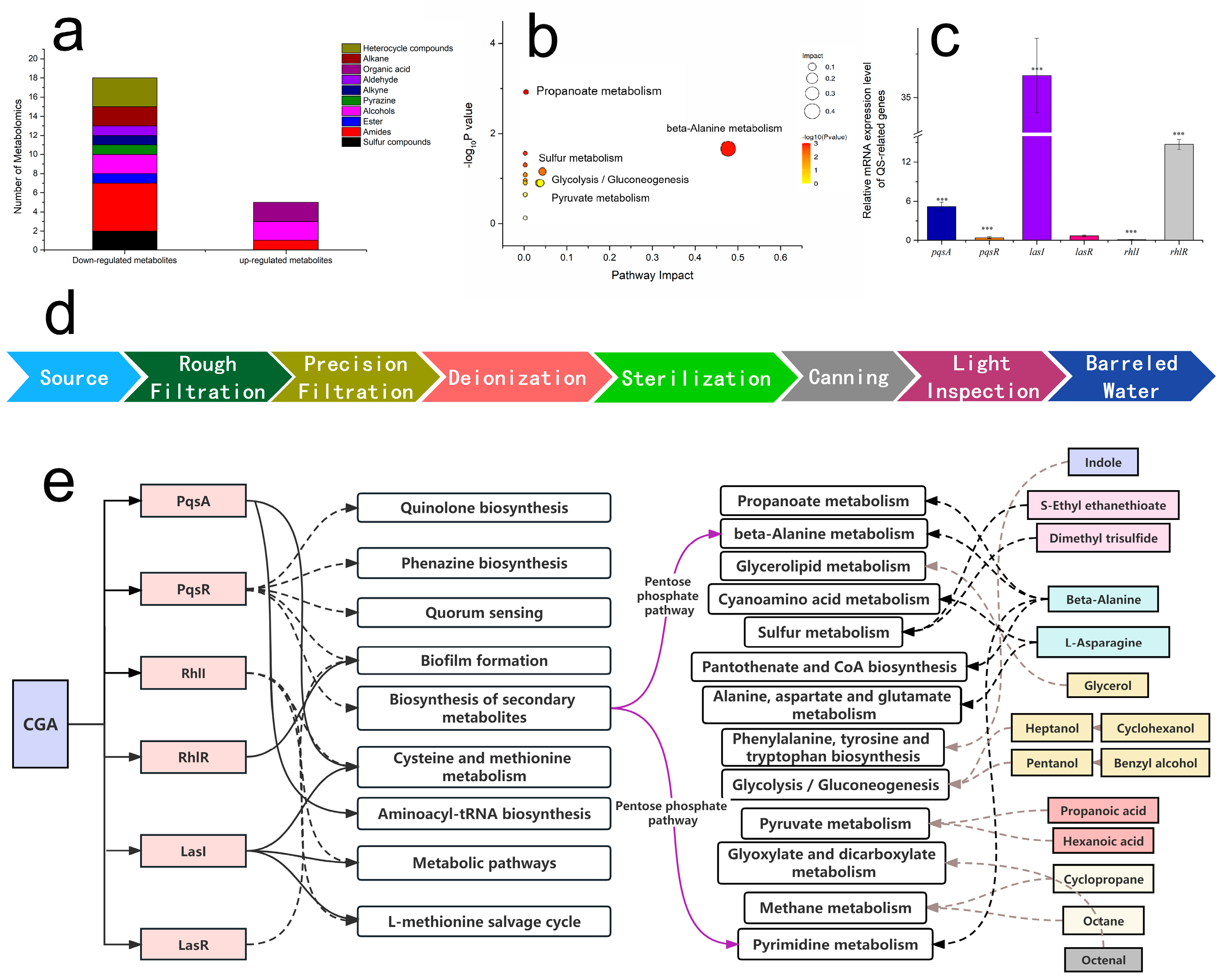
3.5. Changes in Metabolites of P. putida Biofilms Caused by CGA
3.6. CGA-Regulated QS-Related Genes
4. Discussion
4.1. Risk Analysis of Pseudomonas Contamination
4.2. Anti-Biofilm Effect of CGA on P. putida
4.3. Beta-Alanine and Pyrimidine Metabolism Were Affected by CGA through QS System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, X.; Luo, Z.; Zhou, Z. Laws and Regulations of Packaged Drinking Water from Both Domestic and Abroad. Beverage Ind. 2019, 22, 62–67. [Google Scholar]
- Liao, Z.; Cao, D.; Zhang, H.; Zhao, X. Current situation and existing problems of packaged drinking water industry in China. J. Food Saf. Qual. 2017, 8, 737–741. [Google Scholar]
- Pu, J.; Fukushi, K. Bacterial water quality and risk evaluation of bottled drinking water in China. Int. J. Food Sci. Nutr. 2016, 6, 1. [Google Scholar] [CrossRef]
- Hubbard, A.T.M.; Newire, E.; Botelho, J.; Reiné, J.; Wright, E.; Murphy, E.A.; Hutton, W.; Roberts, A.P. Isolation of an antimicrobial-resistant, biofilm-forming, Klebsiella grimontii isolate from a reusable water bottle. Microbiologyopen 2020, 9, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Golden, J.; Bilinski, A.; Beckman, A.L.; McDaniel, K.; Harding, A.S.; France, A.; Tobar, H.N.; Vecitis, C. Bacterial contamination of reusable bottled drinking water in Ecuador. J. Water Sanit. Hyg. Dev. 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, J.; Guo, K.; Lou, Y.; Wang, H.; Zhou, R.; Tang, J.; Hou, P. Spatiotemporal distribution of opportunistic pathogens and microbial community in centralized rural drinking water: One year survey in China. Environ. Res. 2023, 218, 115045. [Google Scholar] [CrossRef]
- Aloraini, S.; Alum, A.; Abbaszadegan, M. Impact of Pipe Material and Temperature on Drinking Water Microbiome and Prevalence of Legionella, Mycobacterium, and Pseudomonas Species. Microorganisms 2023, 11, 352. [Google Scholar] [CrossRef]
- Miranzadeh, M.B.; Mhsanifar, E.; Iranshahi, L. Evaluation of Bacterial Quality and Trace Elements Concentrations in 25 Brands of Iranian Bottled Drinking Water. J. Agric. Environ. Sci. 2011, 11, 341–345. [Google Scholar]
- Wang, X.; Wang, J.; Liu, S.; Guo, J.; Fang, F.; Chen, Y.; Yan, P. Mechanisms of survival mediated by the stringent response in Pseudomonas aeruginosa under environmental stress in drinking water systems: Nitrogen deficiency and bacterial competition. J. Hazard. Mater. 2023, 448, 130941. [Google Scholar] [CrossRef]
- Maes, S.; Vackier, T.; Huu, S.N.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Borjac, J.; Zeino, W.; Matar, A.; Khawaja, S.; Merheb, M.; Matar, R. Prevalence of Antibiotic-Resistant Bacteria in Domestic Water Storage Tanks in Sidon, Lebanon. Water. 2023, 15, 335. [Google Scholar] [CrossRef]
- Tripathi, S.; Purchase, D.; Govarthanan, M.; Chandra, R.; Yadav, S. Regulatory and innovative mechanisms of bacterial quorum sensing-mediated pathogenicity: A review. Environ. Monit. Assess 2023, 195, 75. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Wilder, C.N.; Diggle, S.P.; Schuster, M. Cooperation and cheating in Pseudomonas aeruginosa: The roles of the las, rhl and pqs quorum-sensing systems. ISME J. 2011, 5, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Wade, D.S.; Pesci, E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 2004, 230, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Kunpeng, G.; Juan, H.E.; Haiyun, Z.; Han, G.; Tao, Y.; Sheng, S.; Di, L. Analysis of Contamination of Pseudomonas aeruginosa in Packaged Drinking Water. China Food Saf. Mag. 2022, 50–52. [Google Scholar] [CrossRef]
- Tisler, S.; Christensen, J.H. Non-target screening for the identification of migrating compounds from reusable plastic bottles into drinking water. J. Hazard. Mater. 2022, 429, 128331. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Valcarcel, M.; Roura-Ferrer, M.; Kortazar, D.; Salado, C.; Lezama, L.; Rojo, T.; González-Baró, A.C.; Williams, P.A.M.; Ferrer, E.G. Promising antioxidant and anticancer (human breast cancer) oxidovanadium(IV) complex of chlorogenic acid. Synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem. 2014, 135, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.K.; Ramesh, S.; Satish, A.S.; Lee, J. Antibiofilm and Anti-Lactamase Activities of Burdock Root Extract and Chlorogenic Acid against Klebsiella pneumoniae. J. Microbiol. Biotechnol. 2017, 27, 542–551. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z. Chlorogenic acid attenuates virulence factors and pathogenicity of Pseudomonas aeruginosa by regulating quorum sensing. Appl. Microbiol. Biotechnol. 2019, 103, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Shazly, A.B.; Fouad, M.T.; Elaaser, M.; Sayed, R.S.; Abd El Aziz, M. Probiotic coffee ice cream as an innovative functional dairy food. J. Food Process Preserv. 2022, 46, e17253. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Progress in Antifungal Susceptibility Testing of Candida spp. by Use of Clinical and Laboratory Standards Institute Broth Microdilution Methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Wang, M.; Zhang, F.; Wang, C.; Zhang, K.; Wang, H.; Xu, S.; Hu, W.; Gu, L. DexA70, the Truncated Form of a Self-Produced Dextranase, Effectively Disrupts Streptococcus mutans Biofilm. Front. Microbiol. 2021, 12, 737458. [Google Scholar] [CrossRef]
- Chang, A.; He, Q.; Li, L.; Yu, X.; Sun, S.; Zhu, H. Exploring the quorum sensing inhibition of isolated chrysin from Penicillium chrysogenum DXY-1. Bioorg. Chem. 2021, 111, 104894. [Google Scholar] [CrossRef]
- Guo, R.; Lu, H. Targeted metabolomics revealed the regulatory role of manganese on small-molecule metabolism of biofilm formation in escherichia coli. J Anal Test. 2020, 4, 226–237. [Google Scholar] [CrossRef]
- Guo, R.; Luo, X.; Liu, J.; Lu, H. Mass spectrometry based targeted metabolomics precisely characterized new functional metabolites that regulate biofilm formation in Escherichia coli. Anal. Chim. Acta 2021, 1145, 26–36. [Google Scholar] [CrossRef]
- Kuhnert, W.L.; Quivey, R.G. Genetic and Biochemical Characterization of the F-ATPase Operon from Streptococcus sanguis 10904. J. Bacteriol. 2003, 185, 1525–1533. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Wang, L.; Zeng, W.; Sun, Y.; Zhou, C.; Zhou, T.; Shen, M. Effect of chlorogenic acid on the quorum-sensing system of clinically isolated multidrug-resistant Pseudomonas aeruginosa. J. Appl. Microbiol. 2022, 132, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real Time Quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- Majumdar, S.; Pal, S. Quorum sensing: A quantum perspective. J. Cell Commun. Signal. 2016, 10, 173–175. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Chiarelli, L.R.; Barbieri, G.; Buroni, S. Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. Int. J. Mol. Sci. 2019, 20, 1838. [Google Scholar] [CrossRef]
- Huang, H.; Shao, X.; Xie, Y.; Wang, T.; Zhang, Y.; Wang, X.; Deng, X. An integrated genomic regulatory network of virulence-related transcriptional factors in Pseudomonas aeruginosa. Nat. Commun. 2019, 10, 2931. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Nilsson, M.; Gjermansen, M.; Givskov, M.; Tolker-Nielsen, T. Pyoverdine and PQS mediated subpopulation interactions involved in Pseudomonas aeruginosa biofilm formation. Mol. Microbiol. 2009, 74, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Danshiitsoodol, N.; Noda, M.; Inoue, Y.; Sugiyama, M. 3-Phenyllactic acid generated in medicinal plant extracts fermented with plant-derived lactic acid bacteria inhibits the biofilm synthesis of Aggregatibacter actinomycetemcomitans. Front. Microbiol. 2022, 13, 991144. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Synergistic Antibiofilm Effects of Ultrasound and Phenyllactic Acid against Staphylococcus aureus and Salmonella enteritidis. Foods 2021, 10, 2171. [Google Scholar] [CrossRef]
- Giese, A.; Kerpen, J.; Weber, F.; Prediger, J. A Preliminary Study of Microplastic Abrasion from the Screw Cap System of Reusable Plastic Bottles by Raman Microspectroscopy. ACS Es&T Water 2021, 1, 1363–1368. [Google Scholar]
- Nakaya, S.; Yamamoto, A.; Kawanishi, T.; Toya, N.; Miyakawa, H.; Takeuchi, K.; Endo, M. Detection of dynamic biofouling from adenosine triphosphate measurements in water concentrated from reverse osmosis desalination of seawater. Desalination. 2021, 518, 115286. [Google Scholar] [CrossRef]
- Keerthana Devi, M.; Karmegam, N.; Manikandan, S.; Subbaiya, R.; Song, H.; Kwon, E.E.; Sarkar, B.; Bolan, N.; Kim, W.; Rinklebe, J.; et al. Removal of nanoplastics in water treatment processes: A review. Sci. Total Environ. 2022, 845, 157168. [Google Scholar] [CrossRef] [PubMed]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Pan, C.; Chen, J.; He, L.; Ying, G. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: From drinking water source to tap water. Sci. Total Environ. 2018, 616–617, 453–461. [Google Scholar] [CrossRef]
- Sala-Comorera, L.; Blanch, A.R.; Vilaró, C.; Galofré, B.; García-Aljaro, C. Pseudomonas-related populations associated with reverse osmosis in drinking water treatment. J. Environ. Manag. 2016, 182, 335–341. [Google Scholar] [CrossRef]
- Almonacid Garrido, M.C.; Jiménez Navarro, P.; Peinador Asensio, J.; Villanueva Suárez, M.J.; Tenorio Sanz, M.D. Tap Water Lead Levels in Madrid (Spain): Degree of Compliance and Health Risk Assessment. Expo. Health 2021, 13, 207–218. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Thapa, K.; Shrestha, S.M.; Rawal, D.S.; Pant, B.R. Quality of drinking water in Kathmandu valley, Nepal. Sustain. Water Resour. Manag. 2019, 5, 1995–2000. [Google Scholar]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Kocot, A.M.; Olszewska, M.A. Biofilm formation and microscopic analysis of biofilms formed by Listeria monocytogenes in a food processing context. LWT 2017, 84, 47–57. [Google Scholar] [CrossRef]
- Sudagidan, M.; Ozalp, V.C.; Öztürk, O.; Yurt, M.N.Z.; Yavuz, O.; Tasbasi, B.B.; Ucak, S.; Mavili, Z.S.; Coban, A.; Aydin, A. Bacterial surface, biofilm and virulence properties of Listeria monocytogenes strains isolated from smoked salmon and fish food contact surfaces. Food Biosci. 2021, 41, 101021. [Google Scholar]
- Lu, L.; Zhao, Y.; Yi, G.; Li, M.; Liao, L.; Yang, C.; Cho, C.; Zhang, B.; Zhu, J.; Zou, K.; et al. Quinic acid: A potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. 2021, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes-Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.X.; Liu, G.R. Scavenging Effect of Honeysuckle and Dandelion Extracts on the Biofilm of Pseudomonas Stains from Meat. Sci. Tech. Food Ind. 2020, 41, 106–111. [Google Scholar]
- Sun, J.Y.; Huang, L.H.; Sun, Z.L.; Wang, D.B.; Liu, F.; Du, L.H.; Wang, D.Y. Combination of ultrasound and chlorogenic acid for inactivation of planktonic and biofilm cells of Pseudomonas fluorescens. Food Res. Int. 2022, 155, 111009. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Angusamy, A.; Balasubramanian, V.; Arunmurugan, B.; Arunachalam, K.; Abraham, S.V.P.I.; Murugesan, S.; Krishnasamy, B.; Sundaram, J.; Arumugam, V.R. Anti-infective potential of plant-derived quorum sensing inhibitors against multi-drug resistant human and aquatic bacterial pathogens. World J. Microbiol. Biotechnol. 2023, 39, 147. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; Sun, Y.; Zhou, T.; Shen, M. Effects of chlorogenic acid on antimicrobial, antivirulence, and anti-quorum sensing of carbapenem-resistant Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 997310. [Google Scholar] [CrossRef]
- Okuyama, K.; Hamamoto, T.; Noguchi, T.; Midorikawa, Y. Molecular cloning and expression of the pyrimidine nucleoside phosphorylase gene from Bacillus stearothermophilus TH 6-2. Biosci. Biotechnol. Biochem. 1996, 60, 1655–1659. [Google Scholar] [CrossRef]
- Kao, C.H.; Hsu, W.H. A gene cluster involved in pyrimidine reductive catabolism from Brevibacillus agri NCHU1002. Biochem. Biophys. Res. Commun. 2003, 303, 848–854. [Google Scholar] [CrossRef]
- Nie, M.; Li, K.; Li, Z. β-Alanine Metabolism Leads to Increased Extracellular pH during the Heterotrophic Ammonia Oxidation of Pseudomonas putida Y-9. Microorganisms 2023, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Hidese, R.; Mihara, H.; Kurihara, T.; Esaki, N. Escherichia coli Dihydropyrimidine Dehydrogenase Is a Novel NAD-Dependent Heterotetramer Essential for the Production of 5,6-Dihydrouracil. J. Bacteriol. 2011, 193, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Lee, D.E.; Kim, H.S. Functional Expression and Characterization of the Two Cyclic Amidohydrolase Enzymes, Allantoinase and a Novel Phenylhydantoinase, from Escherichia coli. J. Bacteriol. 2000, 182, 7021–7028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wei, Y.; Yin, J.; Liu, D.; Ang, E.L.; Zhao, H.; Zhang, Y. A Pathway for Degradation of Uracil to Acetyl Coenzyme A in Bacillus megaterium. Appl. Environ. Microbiol. 2020, 86, e02837-19. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cao, X.; Pei, H.; Liu, P.; Song, Y.; Wu, Y. Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System. Foods 2023, 12, 3601. https://doi.org/10.3390/foods12193601
Wang L, Cao X, Pei H, Liu P, Song Y, Wu Y. Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System. Foods. 2023; 12(19):3601. https://doi.org/10.3390/foods12193601
Chicago/Turabian StyleWang, Lin, Xueli Cao, Hairun Pei, Ping Liu, Ya Song, and Yulun Wu. 2023. "Anti-Biofilm Activity of Chlorogenic Acid against Pseudomonas Using Quorum Sensing System" Foods 12, no. 19: 3601. https://doi.org/10.3390/foods12193601




