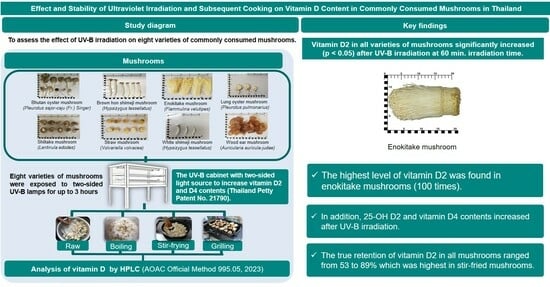
Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Vitamin D Content after Exposure to UV Irradiation
2.2.1. Mushroom Color Determination
2.2.2. Vitamin D Content Determination
2.3. Effect of Cooking on High-Vitamin-D Mushrooms
2.3.1. Yield Factor Determination
2.3.2. Weight Loss Determination
2.3.3. True Retention of Vitamin D
2.3.4. Moisture Determination
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of Raw Mushroom Samples
3.2. Effect of Ultraviolet (UV) Irradiation on Vitamin D Contents in Mushrooms
3.2.1. Vitamin D2 Content
3.2.2. Ergosterol Content
3.2.3. The 25-Hydroxyl Vitamin D Content
3.2.4. Vitamin D4 Content
3.2.5. The Correlation between Vitamin D2 and Vitamin D4 Contents in Mushrooms after Exposure to UV-B Irradiation
3.3. Effect of Cooking in Selected Mushrooms
3.3.1. Edible Portion
3.3.2. Yield Factor
3.3.3. Moisture Content
3.3.4. Effect of Different Cooking Methods on Vitamin D Content in Mushrooms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ball, G.F. Vitamins in Foods: Analysis, Bioavailability, and Stability; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of mushrooms as a Potential Source of Dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Lin, C.P.; Tsai, S.Y. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Tirakomonpong, N.; Judprasong, K.; Sridonpai, P.; Saetang, P.; Puwastien, P.; Rojroongwasinkul, N.; Ongphiphadhanakul, B. Vitamin D in commonly consumed freshwater and marine fish. J. Nutr. Assoc. Thail. 2019, 54, 55–67. [Google Scholar]
- Sridonpai, P.; Suthipibul, P.; Boonyingsathit, K.; Chimkerd, C.; Jittinandana, S.; Judprasong, K. Vitamin D Content in Commonly Consumed Mushrooms in Thailand and Its True Retention after Household Cooking. Foods 2023, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Siwamogsatham, O.; Ongphiphadhanakul, B.; Tangpricha, V. Vitamin D deficiency in Thailand. J. Clin. Transl. Endocrinol. 2015, 2, 48. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Ultraviolet irradiation: The generator of vitamin D2 in edible mushrooms. Food Chem. 2006, 95, 638–643. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.C.; Song, C.H.; Cho, K.Y.; Pang, G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.R.; Phillips, K.M.; Horst, R.L.; Munro, I.C. Vitamin D mushrooms: Comparison of the composition of button mushrooms (Agaricus bisporus) treated post-harvest with UVB light or sunlight. J. Agric. Food Chem. 2011, 59, 8724–8732. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Jakobsen, J. Dose–response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J. Agric. Food Chem. 2015, 63, 8156–8161. [Google Scholar] [CrossRef] [PubMed]
- Wittig, M.; Krings, U.; Berger, R.G. Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J. Food Compos. Anal. 2013, 31, 266–274. [Google Scholar] [CrossRef]
- Mau, J.L.; Chen, P.R.; Yang, J.H. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J. Agric. Food Chem. 1998, 46, 5269–5272. [Google Scholar] [CrossRef]
- National Bureau of Agricultural Commodity and Food Standards. Food Consumption Data of Thailand; Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2016; 355p. [Google Scholar]
- Judprasong, K.; Sridonpai, P.; Chheng, S.; Chimkerd, C.; Jittinandana, S. The UV-B Cabinet with Two-Sided Light Source to Increase Vitamin D2 and D4 Contents (Thailand Petty Patent No. 21790). Department of Intellectual Property. 2023. Available online: https://patentsearch.ipthailand.go.th/DIPSearch/Tools/GetPDF.aspx?appno=2203000310 (accessed on 6 June 2023).
- Suthipibul, P.; Judprasong, K.; Jittinandana, S.; Rojroongwasinkul, N.; Ongphiphadhanakul, B.; Sridonpai, P.; Chimkerd, C. Simultaneous determination of different types of vitamin D in foods by liquid chromatography tandem mass spectrometry (LC-MS-MS). In Proceedings of the 22nd Food Innovation Asia Conference 2020 (FIAC 2020) on Future Food Innovation for Better Health and Wellness, Bangkok, Thailand, 18–20 June 2020. [Google Scholar]
- Murphy, E.W.; Criner, P.E.; Gray, B.C. Comparisons of methods for calculating retentions of nutrients in cooked foods. J. Agric. Food Chem. 1975, 23, 1153–1157. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Mattila, P.H.; Piironen, V.I.; Uusi-Rauva, E.J.; Koivistoinen, P.E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT-Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Keegan, R.J.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Derm.-Endocrinol. 2013, 5, 165–176. [Google Scholar] [CrossRef]
- Hu, D.; Chen, W.; Li, X.; Yue, T.; Zhang, Z.; Feng, Z.; Li, C.; Bu, X.; Li, Q.X.; Hu, C.Y.; et al. Ultraviolet irradiation increased the concentration of vitamin D2 and decreased the concentration of ergosterol in shiitake mushroom (lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension. ACS Omega 2020, 5, 7361–7368. [Google Scholar] [CrossRef]
- Perera, C.O.; Jasinghe, V.J.; Ng, F.L.; Mujumdar, A.S. The effect of moisture content on the conversion of ergosterol to vitamin D in shiitake mushrooms. Dry. Technol. 2003, 21, 1091–1099. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M.; Seamans, K.M.; Urbain, P. Effect of ultraviolet light–exposed mushrooms on vitamin D status: Liquid chromatography–tandem mass spectrometry reanalysis of biobanked sera from a randomized controlled trial and a systematic review plus meta-analysis. J. Nutr. 2016, 146, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Berger, R.G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem. 2014, 149, 10–14. [Google Scholar] [CrossRef]
- Urbain, P.; Valverde, J.; Jakobsen, J. Impact on vitamin D2, vitamin D4 and agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods Hum. Nutr. 2016, 71, 314–321. [Google Scholar] [CrossRef]
- Ložnjak, P.; Jakobsen, J. Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after house-hold cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef]
- United States Department of Agriculture, USDA Food Composition Database. FDC Published: 28 October 2021. Available online: https://fdc.nal.usda.gov/ (accessed on 1 August 2023).
- Kumar, A.; Singh, M.; Singh, G. Effect of different pretreatments on the quality of mushrooms during solar drying. J. Food Sci. Technol. 2013, 50, 165–170. [Google Scholar] [CrossRef]
- Roncero, R.I.; Mendiola, L.M.; Pérez, C.M.; Delgado, A.C. Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef]


| English Name | Thai Name | Scientific Name |
|---|---|---|
| Bhutan oyster mushroom | Het-Bhutan | Pleurotus sajor-caju (Fr.) Singer |
| Brown hon shimeji mushroom | Het-Lin-Dam | Hypsizygus tessellatus |
| Enokitake mushroom | Het-Khem-Thong | Flammulina velutipes |
| Lung oyster mushroom | Het-Nang-Fa | Pleurotus pulmonarius |
| Shiitake mushroom | Het-Hom | Lentinula edodes |
| Straw mushroom | Het-Fang | Volvariella volvacea |
| White shimeji mushroom | Het-Lin-Khao | Hypsizygus tessellatus |
| Wood ear mushroom | Het-Hu-Nu | Auricularia auricula-judae |
| Mushroom | Cooking Time (min) | ||
|---|---|---|---|
| Boiling | Stir-Frying | Grilling | |
| (85–100 °C) | (110–150 °C) | (150–200 °C) | |
| Bhutan oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | 10 | 4 | 6 |
| Brown hon shimeji mushroom (Hypsizygus tessellatus) | 5 | 4 | 6 |
| Enokitake mushroom (Flammulina velutipes) | 4 | 3 | 5 |
| Lung oyster mushroom (Pleurotus pulmonarius) | 10 | 4 | 6 |
| Type | Appearance | Cap Size Average (cm) (Range) | Stalk Size Average (cm) (Range) | Height Average (cm) (Range) |
|---|---|---|---|---|
| Bhutan oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) |  | 7.27.2 (2.4–12) | 5 (4–6) | 8.8 (4.7–12.9) |
| Brown hon shimeji mushroom (Hypsizygus tessellatus) |  | 2.0 (1–3) | 12 (9–14) | 12 (10–14) |
| Enokitake mushroom (Flammulina velutipes) |  | 0.4 (0.2–0.6) | 13 (12–14) | 12 (10–14) |
| Lung oyster mushroom (Pleurotus pulmonarius) |  | 7.5 (4–11) | 6 (4–8) | 11.2 (7–15.5) |
| Shiitake mushroom (Lentinula edodes) |  | 5.2 (4.5–5.8) | 3 (3–4) | 4.4 (3.8–5) |
| Type | Appearance | Cap Size Average (cm) (Range) | Stalk Size Average (cm) (Range) | Height Average (cm) (Range) |
|---|---|---|---|---|
| Straw mushroom (Volvariella volvacea) |  | 3.5 (2.3–4.7) | N/A | 4.1 (2.5–5.7) |
| White shimeji mushroom (Hypsizygus tessellatus) |  | 2 (0.5–3) | 11 (9–12) | 11 (10–12) |
| Wood ear mushroom (Auricularia auricula-judae) |  | 5.8 (3–8.9) | 1.5 (1–2) | 7 (3.2–10.7) |
| Mushrooms | UV Time (Hour) | Vitamin D2 (µg/100 g DW) | 25-OH D2 (µg/100 g DW) | Ergosterol (mg/100 g DW) | Vitamin D4 (µg/100 g DW) | Color (Munsell Code) |
|---|---|---|---|---|---|---|
| Bhutan oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | 0 | 22.95 ± 0.30 f | 1925.52 ± 210.89 a | 114.92 ± 4.91 a | 240.46 ± 20.43 e | C: 10YR 4-6/2 S: 10YR 8-9/2 |
| 0.25 | 291.31 ± 19.62 e | 1514.86 ± 37.12 a | 125.08 ± 8.96 a | 706.59 ± 7.52 d | C: 10YR 4-6/2 S: 10YR 8-9/2 | |
| 0.5 | 530.23 ± 19.96 d | 2040.66 ± 240.66 a | 122.81 ± 4.00 a | 960.66 ± 6.24 c | C: 10YR 4-6/2 S: 10YR 8-9/2 | |
| 1 | 920.19 ± 8.71 c | 1869.49 ± 4.59 a | 99.25 ± 4.06 a | 1640.11 ± 67.36 b | C: 10YR 4-6/2 S: 10YR 8-9/2 | |
| 2 | 1052.16 ± 72.65 b | 1550.32 ± 231.30 a | 96.60 ± 4.84 a | 1587.08 ± 10.21 b | C: 10YR 4-6/2 S: 10YR 7-9/2 | |
| 3 | 1468.83 ± 34.96 a | 1574.76 ± 11.69 a | 99.22 ± 11.40 a | 2550.12 ± 106.25 a | C: 10YR 4-5/2 S: 10YR 8/2 | |
| Brown hon shimeji mushroom (Hypsizygus tessellatus) | 0 | 9.00 ± 1.58 f | 2392.79 ± 41.73 a | 138.12 ± 6.82 a | 261.23 ± 10.60 f | C: 10YR: 6-7/2 S: 5YR: 9/1 |
| 0.25 | 149.94 ± 2.82 e | 3020.79 ± 398.41 a | 127.14 ± 15.10 a | 478.54 ± 12.42 e | C: 10YR: 6-7/2 S: 5YR: 9/1 | |
| 0.5 | 301.80 ± 5.76 d | 2368.39 ± 69.06 a | 148.83 ± 3.64 a | 742.83 ± 11.18 d | C: 10YR: 6-7/2 S: 5YR: 9/1 | |
| 1 | 624.83 ± 30.14 c | 2240.45 ± 363.57 a | 124.26 ± 13.36 a | 1029.53 ± 65.52 c | C: 10YR: 6-7/2-4 S: 5YR: 9/1 | |
| 2 | 935.40 ± 13.99 b | 2035.30 ± 327.08 a | 122.70 ± 7.76 a | 1431.03 ± 55.06 b | C: 10YR: 6-7/2 S: 5YR: 9/1 | |
| 3 | 1030.29 ± 19.58 a | 2476.84 ± 218.03 a | 102.40 ± 21.02 a | 1702.47 ± 0.93 a | C: 10YR: 6-7/2-4 S: 5YR: 9/1 |
| Mushrooms | UV Time (Hour) | Vitamin D2 (µg/100 g DW) | 25-OH D2 (µg/100 g DW) | Ergosterol (mg/100 g DW) | Vitamin D4 (µg/100 g DW) | Color (Munsell Code) |
|---|---|---|---|---|---|---|
| Enokitake mushroom (Flammulina velutipes) | 0 | 6.68 ± 1.42 d | 44.96 ± 10.23 bc | 140.58 ± 3.97 abc | 282.59 ± 0.34 f | C: 5Y 9/2 S: 5Y 9/2 |
| 0.25 | 414.01 ± 20.37 cd | 69.44 ± 7.72 bc | 130.66 ± 24.28 bc | 1710.67 ± 109.76 e | C: 5Y 9/2 S: 5Y 9/2 | |
| 0.5 | 615.18 ± 22.55 c | 30.13 ± 1.05 c | 110.35 ± 18.12 c | 2777.47 ± 162.21 d | C: 5Y 9/2 S: 5Y 9/2 | |
| 1 | 1226.59 ± 100.69 b | 40.67 ± 6.81 bc | 114.29 ± 11.92 bc | 4282.19 ± 3.16 c | C: 5Y 9/2-3 S: 5Y 9/2-3 | |
| 2 | 1349.06 ± 59.34 b | 73.61 ± 22.81 ab | 152.63 ± 4.59 ab | 4973.55 ± 6.05 b | C: 5Y 9/3 S: 5Y 9/3 | |
| 3 | 1880.22 ± 197.76 a | 93.98 ± 2.99 a | 151.93 ± 10.80 a | 6504.61 ± 21.74 a | C: 5Y 9/3 S: 5Y 9/3 | |
| Lung oyster mushroom (Pleurotus pulmonarius) | 0 | 72.40 ± 0.20 e | 108.11 ± 4.32 a | 229.10 ± 53.57 a | 509.62 ± 34.12 d | C: 5Y 8.5-9/2 S: 5Y 8.5-9/2 |
| 0.25 | 258.95 ± 22.88 de | 85.06 ± 22.24 a | 222.12 ± 20.49 a | 860.09 ± 21.42 d | C: 5Y 8.5-9/2 S: 5Y 8.5-9/2 | |
| 0.5 | 457.47 ± 29.44 d | 124.89 ± 16.95 a | 237.97 ± 9.99 a | 1350.72 ± 33.33 c | C: 5Y 8-9/2 S: 5Y 8-9/2 | |
| 1 | 704.73 ± 4.48 c | 122.93 ± 21.86 a | 232.49 ± 18.22 a | 2143.33 ± 160.58 b | C: 5Y 8-9/2 S: 5Y 8-9/2 | |
| 2 | 1063.54 ± 101.83 b | 103.93 ± 1.70 a | 210.74 ± 17.95 a | 2200.19 ± 151.90 b | C: 5Y 8-8.5/2 S: 5Y 8-8.5/2 | |
| 3 | 1519.13 ±114.79 a | 104.28 ± 38.79 a | 167.33 ± 6.29 a | 2731.37 ± 140.46 a | C: 5Y 8-8.5/2 S: 5Y 8-8.5/2 |
| Mushrooms | UV Time (Hour) | Vitamin D2 (µg/100 g DW) | 25-OH D2 (µg/100 g DW) | Ergosterol (mg/100 g DW) | Vitamin D4 (µg/100 g DW) | Color (Munsell Code) |
|---|---|---|---|---|---|---|
| Shiitake mushroom (Lentinula edodes) | 0 | 5.93 ± 2.29 f | 522.23 ± 11.05 a | 211.48 ± 9.91 a | 381.83 ± 5.49 a | C: 10YR 6-7/4 S: 10YR 9/1-2 |
| 0.25 | 46.99 ± 3.72 e | 609.21 ± 42.16 a | 178.25 ± 13.98 abc | 378.13 ± 55.02 a | C: 10YR 5-6/4 S: 10YR 9/1-2 | |
| 0.5 | 91.06 ± 6.71 d | 623.52 ± 105.09 a | 181.79 ± 8.77 abc | 399.71 ± 34.17 a | C: 10YR 4-6/4 S: 10YR 9/1-2 | |
| 1 | 142.42 ± 3.10 c | 641.77 ± 39.43 a | 199.55 ± 1.28 bc | 463.18 ± 17.51 a | C: 10YR 4-6/4 S: 10YR 8-9/2 | |
| 2 | 193.14 ± 11.87 b | 624.69 ± 35.53 a | 145.52 ± 7.68 c | 364.20 ± 1.34 a | C: 10YR 4-6/4 S: 10YR 8-9/1-2 | |
| 3 | 270.44 ± 2.77 a | 533.65 ± 114.36 a | 137.16 ± 18.00 bc | 413.64 ± 31.08 a | C: 10YR 3-5/4 S: 10YR 8-9/1-2 | |
| Straw mushroom (Volvariella volvacea) | 0 | 0.00 f | 128.20 ± 5.27 b | 2.59 ± 0.21 b | 0.00 e | C: 10YR 9/2-4 S: 10YR 9/1-2 |
| 0.25 | 31.28 ± 1.54 e | 86.97 ± 3.82 b | 2.19 ± 0.12 b | 20.43 ± 2.20 de | C: 10YR 9/2-4 S: 10YR 9/1-2 | |
| 0.5 | 47.08 ± 3.87 d | 95.37 ± 18.30 b | 1.28 ± 0.12 c | 33.25 ± 10.45 cd | C: 10YR 8-9/2-4 S: 10YR 9/1-2 | |
| 1 | 93.01 ± 7.42 c | 152.37 ± 24.70 b | 1.29 ± 0.10 c | 33.86 ± 2.32 bc | C: 2.5Y 8.5/4 S: 2.5Y 9/2 | |
| 2 | 152.63 ± 6.39 b | 207.34 ± 6.57 b | 2.29 ± 0.12 b | 67.65 ± 4.89 ab | C: 2.5Y 8.5-9/2-4 S: 2.5Y 8.5-9/2 | |
| 3 | 198.32 ± 4.40 a | 372.75 ± 111.51 a | 2.85 ± 0.17 a | 73.12 ± 4.42 a | C: 2.5Y 8-8.5/4-6 S: 2.5Y 8.5-9/2-4 |
| Mushrooms | UV Time (Hour) | Vitamin D2 (µg/100 g DW) | 25-OH D2 (µg/100 g DW) | Ergosterol (mg/100 g DW) | Vitamin D4 (µg/100 g DW) | Color (Munsell Code) |
|---|---|---|---|---|---|---|
| White shimeji mushroom (Hypsizygus tessellatus) | 0 | 83.73 ± 12.79 b | 4145.52 ± 694.53 ab | 96.98 ± 26.38 a | 196.10 ± 62.65 c | C: 5Y 9/1 S: 5Y 9/1 |
| 0.25 | 130.59 ± 11.42 b | 5941.70 ± 991.57 a | 100.66 ± 29.03 a | 370.72 ± 76.45 c | C: 5Y 9/1 S: 5Y 9/1 | |
| 0.5 | 135.48 ± 20.20 b | 5081.30 ± 871.89 ab | 104.21 ± 1.44 a | 459.87 ± 45.43 bc | C: 5Y 9/1 S: 5Y 9/1 | |
| 1 | 314.38 ± 18.42 a | 5192.53 ± 245.30 ab | 124.20 ± 12.20 a | 793.99 ± 86.52 ab | C: 5Y 9/1 S: 5Y 9/1 | |
| 2 | 414.06 ± 80.25 a | 3797.78 ± 654.09 ab | 106.66 ± 25.26 a | 943.18 ± 223.35 a | C: 5Y 9/1-2 S: 5Y 9/1-2 | |
| 3 | 358.77 ± 53.19 a | 2485.51 ± 435.16 b | 67.94 ± 5.53 a | 726.15 ± 53.69 ab | C: 5Y 9/1-2 S: 5Y 9/1-2 | |
| Wood ear mushroom (Auricularia auricula-judae) | 0 | 0.00 e | 515.14 ± 7.26 bc | 1.58 ± 0.33 b | 0.00 d | C: 2.5YR 3-5/4 |
| 0.25 | 114.11 ± 1.73 d | 707.03 ± 63.74 bc | 2.12 ± 0.04 ab | 57.81 ± 28.42 c | C: 2.5YR 3-5/4 | |
| 0.5 | 177.21 ± 9.57 d | 422.94 ± 0.73 c | 2.13 ± 0.48 ab | 52.28 ± 8.61 c | C: 2.5YR 3-6/4 | |
| 1 | 393.45 ± 39.29 c | 757.77 ± 58.73 ab | 2.29 ± 0.19 ab | 76.60 ± 2.83 c | C: 2.5YR 3-6/4 | |
| 2 | 478.47 ± 7.64 b | 571.37 ± 101.76 ab | 2.04 ± 0.31 ab | 107.03 ± 1.71 b | C: 2.5YR 2-6/4 | |
| 3 | 802.75 ± 23.19 a | 666.29 ± 92.98 a | 2.12 ± 0.12 a | 186.34 ± 13.26 a | C: 2.5YR 2-6/4 |
| Mushroom | Type of Sample | % Edible Portion | % Yield Factor | % Moisture |
|---|---|---|---|---|
| Bhutan oyster Mushroom (Pleurotus sajor-caju (Fr.) Singer) | Raw (without UV) * | 95 ± 2 | - | 92 ± 1 |
| Raw * | 90 ± 1 | - | 91 ± 0 | |
| Boiled | 88 ± 0 | 84 ± 4 | 93 ± 0 | |
| Stir-fried | 88 ± 1 | 69 ± 3 | 81 ± 1 | |
| Grilled | 86 ± 1 | 64 ± 2 | 89 ± 1 | |
| Brown hon shimeji mushroom (Hypsizygus tessellatus) | Raw (without UV) | 95 ± 2 | - | 89 ± 0 |
| Raw | 91 ± 1 | - | 89 ± 0 | |
| Boiled | 90 ± 1 | 66 ± 2 | 93 ± 1 | |
| Stir-fried | 91 ± 1 | 80 ± 3 | 85 ± 2 | |
| Grilled | 89 ± 3 | 60 ± 1 | 88 ± 1 | |
| Enokitake mushroom (Flammulina velutipes) | Raw (without UV) | 87 ± 2 | - | 88 ± 2 |
| Raw | 85 ± 2 | - | 88 ± 0 | |
| Boiled | 84 ± 2 | 79 ± 1 | 94 ± 1 | |
| Stir-fried | 85 ± 1 | 83 ± 4 | 80 ± 1 | |
| Grilled | 83 ± 1 | 61 ± 2 | 86 ± 1 | |
| Lung oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | Raw (without UV) | 93 ± 2 | - | 90 ± 0 |
| Raw | 85 ± 0 | - | 89 ± 0 | |
| Boiled | 84 ± 1 | 88 ± 7 | 94 ± 0 | |
| Stir-fried | 84 ± 1 | 72 ± 2 | 82 ± 2 | |
| Grilled | 85 ± 1 | 61 ± 5 | 88 ± 1 |
| Mushroom | Type of Cooking | True Retention of Vitamin D2 (%) | True Retention of 25-OH D2 (%) | True Retention of Vitamin D4 (%) | Weight Loss (%) |
|---|---|---|---|---|---|
| Bhutan oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | Boiling | 88.8 ± 9.7 c | 66.1 ± 9.2 i | 80.5 ± 20.3 p | 16 ± 13 |
| Stir-frying | 87.4 ± 14.6 c | 63.7 ± 4.6 i | 93.8 ± 8.0 p | 31 ± 3 | |
| Grilling | 87.2 ± 19.9 c | 100.0 ± 0.0 j | 74.9 ± 23.6 p | 36 ± 2 | |
| Brown hon shimeji mushroom (Hypsizygus tessellatus) | Boiling | 55.5 ± 16.8 b | 50.2 ± 5.5 g | 52.1 ± 13.4 o | 34 ± 2 |
| Stir-frying | 63.0 ± 32.1 b | 45.3 ± 12.2 g | 80.3 ± 17.1 o | 20 ± 3 | |
| Grilling | 53.4 ± 3.9 b | 99.8 ± 0.4 h | 61.8 ± 11.2 o | 40 ± 1 | |
| Enokitake mushroom (Flammulina velutipes) | Boiling | 73.2 ± 6.7 a | 85.2 ± 25.6 f | 67.2 ± 6.3 m | 21 ± 1 |
| Stir-frying | 89.3 ± 9.8 a | 84.6 ± 26.1 f | 88.6 ± 12.2 n | 17 ± 4 | |
| Grilling | 68.2 ± 13.4 a | 93.9 ± 8.2 f | 64.9 ± 8.7 m | 39 ± 2 | |
| Lung oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | Boiling | 69.4 ± 2.7 d | 50.3 ± 12.3 k | 66.9 ± 6.3 q | 12 ± 7 |
| Stir-frying | 75.7 ± 9.6 e | 46.8 ± 15.4 k | 80.2 ± 17.2 q | 28 ± 2 | |
| Grilling | 57.0 ± 8.2 de | 94.9 ± 8.9 l | 58.8 ± 6.1 q | 39 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judprasong, K.; Chheng, S.; Chimkerd, C.; Jittinandana, S.; Tangsuphoom, N.; Sridonpai, P. Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand. Foods 2023, 12, 3632. https://doi.org/10.3390/foods12193632
Judprasong K, Chheng S, Chimkerd C, Jittinandana S, Tangsuphoom N, Sridonpai P. Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand. Foods. 2023; 12(19):3632. https://doi.org/10.3390/foods12193632
Chicago/Turabian StyleJudprasong, Kunchit, Sochannet Chheng, Chanika Chimkerd, Sitima Jittinandana, Nattapol Tangsuphoom, and Piyanut Sridonpai. 2023. "Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand" Foods 12, no. 19: 3632. https://doi.org/10.3390/foods12193632
APA StyleJudprasong, K., Chheng, S., Chimkerd, C., Jittinandana, S., Tangsuphoom, N., & Sridonpai, P. (2023). Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand. Foods, 12(19), 3632. https://doi.org/10.3390/foods12193632