Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit
Abstract
:1. Introduction
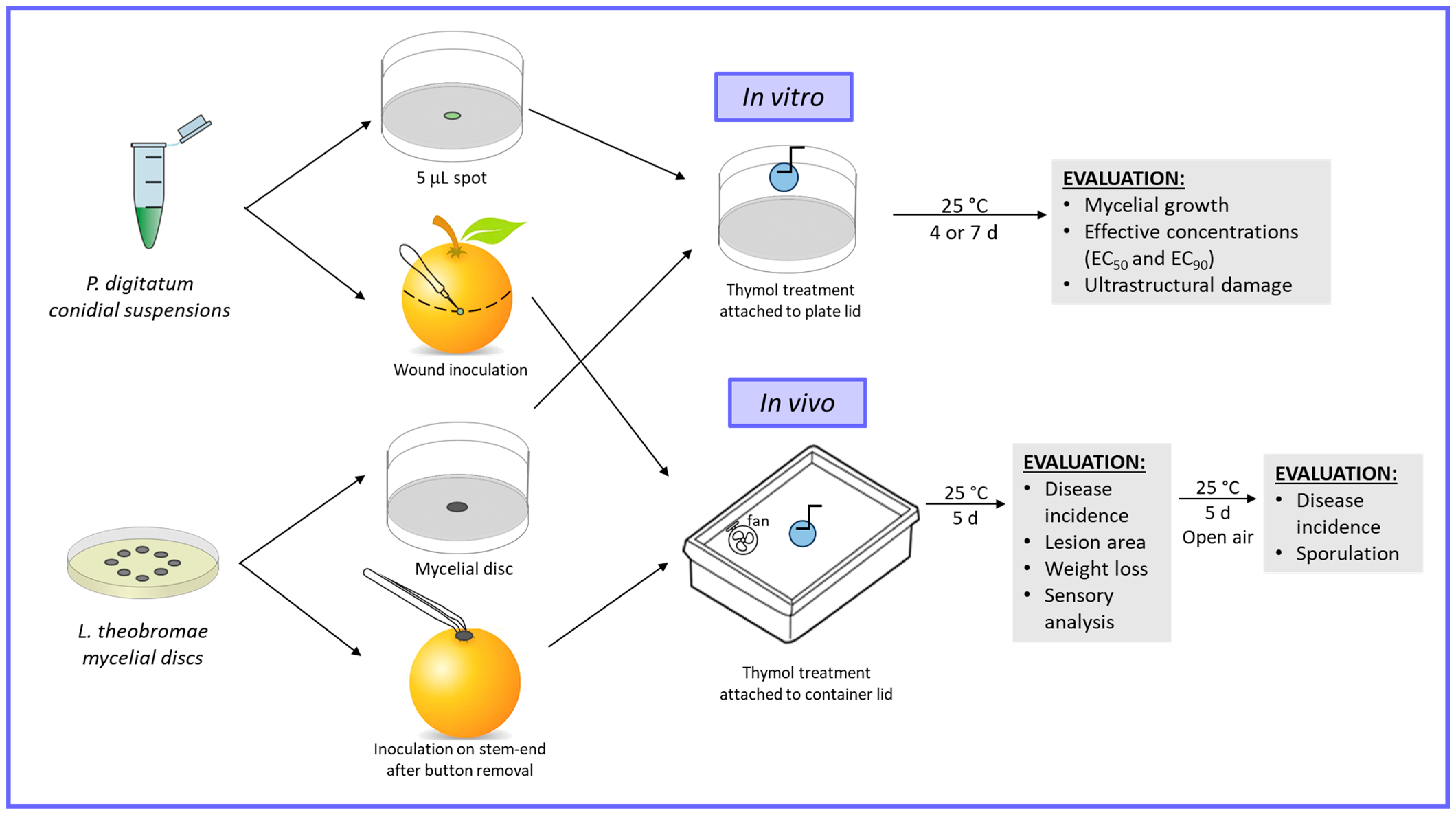
2. Materials and Methods
2.1. Fungal Pathogens and Fruit
2.2. Chemical Compounds
2.3. Effective Inhibitory Concentration Determination
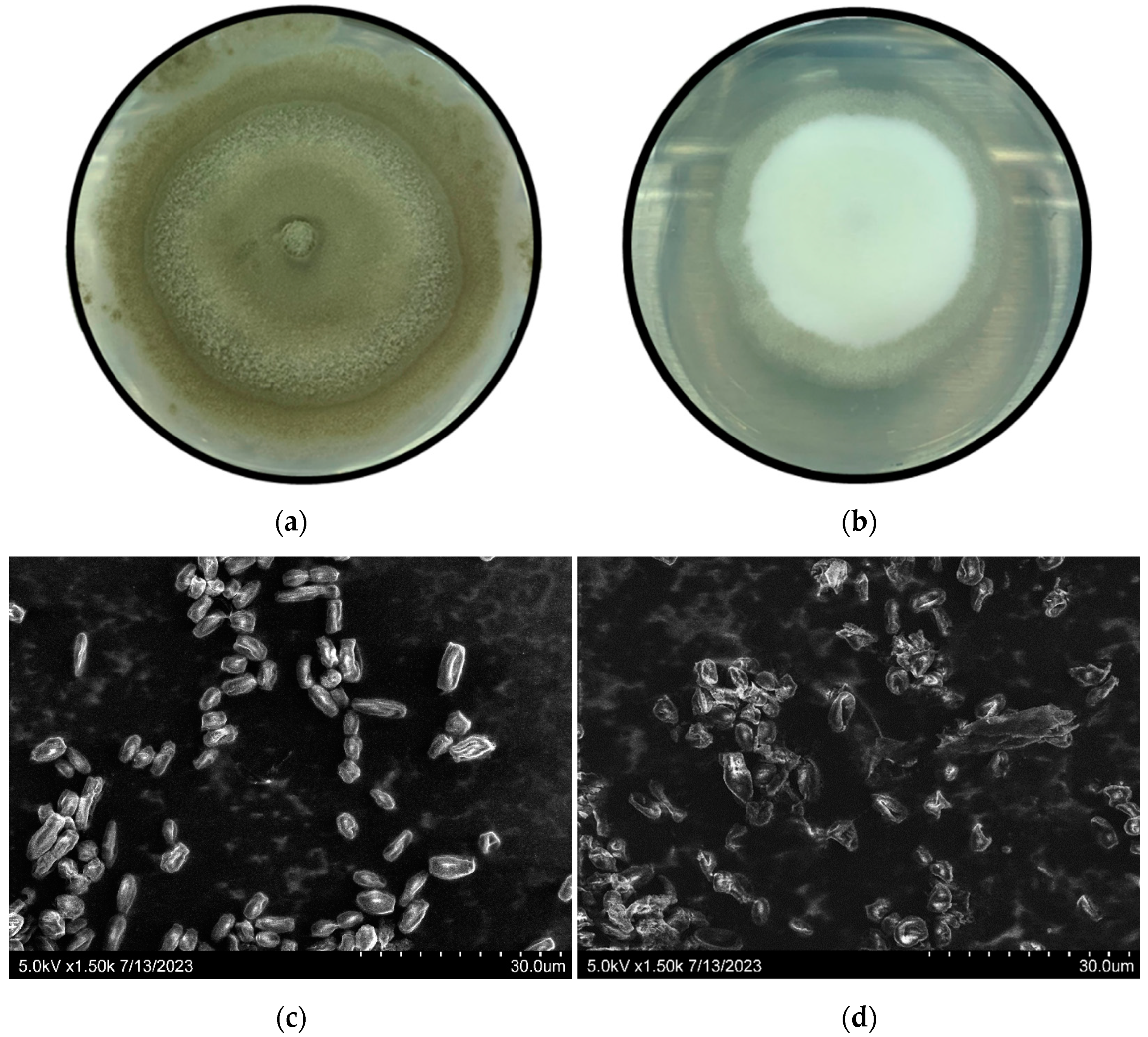
2.4. Scanning Electronic Microscopy (SEM) Observation
2.5. In Vivo Assays in Airtight Conditions
2.6. Weight Loss and Sensory Evaluation
2.7. Statistical Analysis
3. Results
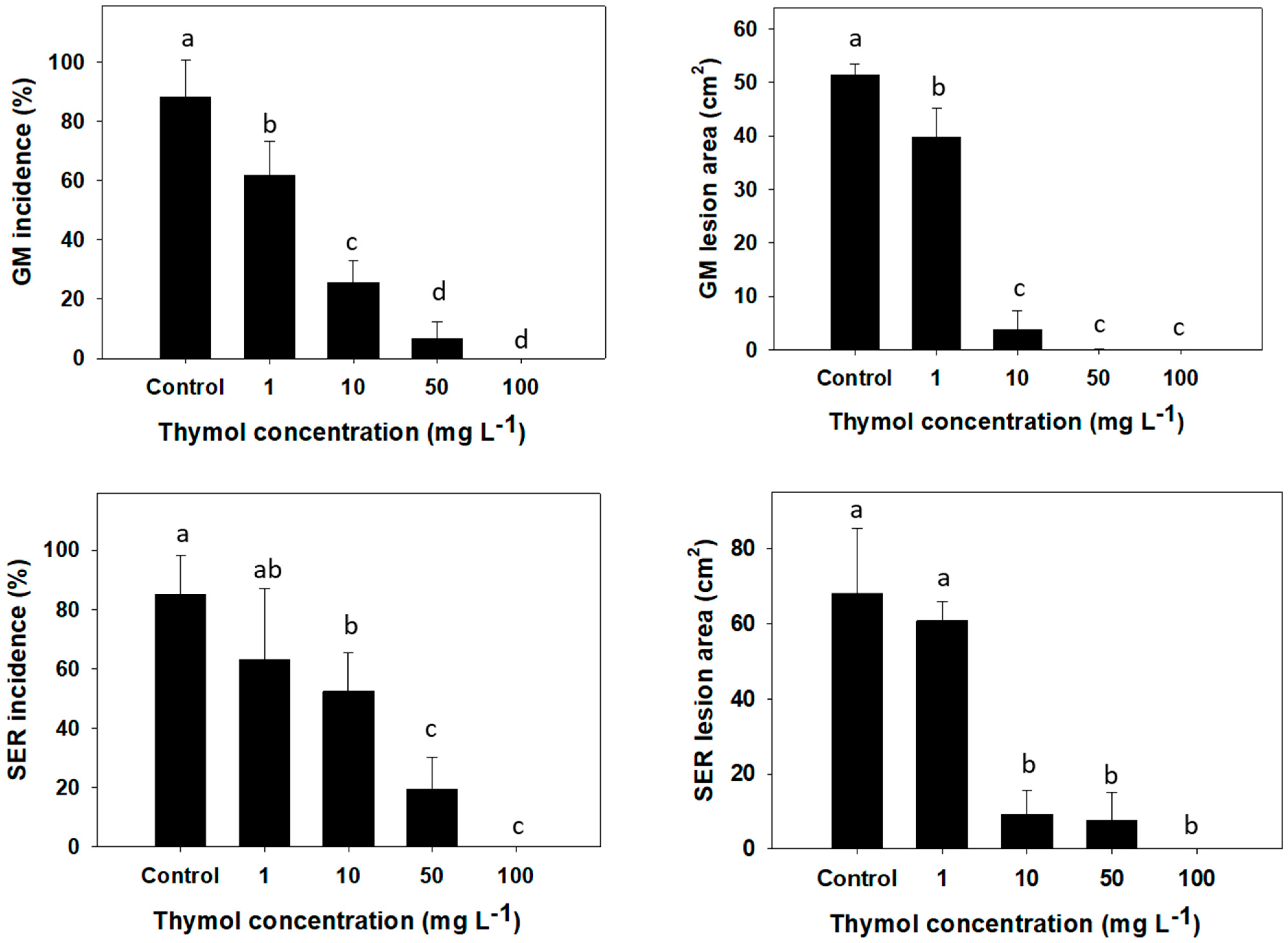
3.1. Penicillium digitatum Conidia Germination and Lasiodiplodia theobromae Mycelial Growth Inhibition by Thymol Vapors In Vitro
3.2. Postharvest Disease Control by Thymol Vapor on Inoculated Grapefruit
3.3. Fruit Weight Loss and Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Barraj, L.M.; Rampersaud, G.C. Consumption of grapefruit is associated with higher nutrient intakes and diet quality among adults, and more favorable anthropometrics in women, NHANES 2003–2008. Food Nutr. Res. 2014, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.J.; Ellington, D.R.; Zhu, Y.; Browne, K.F. The safety of grapefruit juice in patients taking atorvastatin. Circulation 2007, 115, E265. [Google Scholar]
- Zhang, J. Chapter 10—Lasiodiplodia theobromae in Citrus Fruit (Diplodia Stem-End Rot). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 309–335. [Google Scholar]
- Shahzad, F.; Vashisth, T.; Ritenour, M.A.; Brecht, J.K. Huanglongbing disease symptoms and the postharvest quality of ‘LB8-9’ (Sugar Belle®) and ‘Tango’ mandarins as affected by ethylene treatment. Sci. Hort. 2023, 310, 111766. [Google Scholar] [CrossRef]
- USDA-NASS. Florida Citrus Statistics 2021–2022. Available online: http://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/cit/2013-14/cit0514.pdf (accessed on 20 July 2023).
- Zhao, W.; Bai, J.; McCollum, G.; Baldwin, E. High incidence of preharvest colonization of huanglongbing-symptomatic citrus sinensis fruit by Lasiodiplodia theobromae (Diplodia natalensis) and exacerbation of postharvest fruit decay by that fungus. Appl. Env. Microbiol. 2015, 81, 364–372. [Google Scholar] [CrossRef]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef]
- Zhao, W.; Gottwald, T.; Bai, J.; McCollum, G.; Irey, M.; Plotto, A.; Baldwin, E. Correlation of Diplodia (Lasiodiplodia theobromae) infection, huanglongbing, ethylene production, fruit removal force and pre-harvest fruit drop. Sci. Hort. 2016, 212, 162–170. [Google Scholar] [CrossRef]
- Holmes, G.J.; Eckert, J.W. Sensitivity of Penicillium digitatum and P. italicum to Postharvest Citrus Fungicides in California. Phytopathology 1999, 89, 716–721. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. A review. Flavour. Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Khorram, F.; Ramezanian, A. Cinnamon essential oil incorporated in shellac, a novel bio-product to maintain quality of ‘Thomson navel’ orange fruit. J. Food Sci. Technol. 2021, 58, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Bika, R.; Subedi, S.; Pandey, S. Essential oils amended coatings in citrus postharvest management. J. Agric. Food Res. 2022, 10, 100375. [Google Scholar] [CrossRef]
- Jhalegar, M.D.J.; Sharma, R.R.; Singh, D. In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J. Food Sci. Technol. 2015, 52, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. Edible coatings enriched with essential oils for extending the shelf-life of ‘Bravo de Esmolfe’ fresh-cut apples. Int. J. Food Sci. Technol. 2016, 51, 87–95. [Google Scholar] [CrossRef]
- Santos, S.M.d.; Malpass, G.R.P.; Okura, M.H.; Granato, A.C. Edible active coatings incorporated with Cinnamomum cassia and Myristica fragrans essential oils to improve shelf-life of minimally processed apples. Ciência Rural 2018, 48. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Garner, C.M. A review of essential oils as antimicrobials in foods with special emphasis on fresh produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef]
- Kyoui, D.; Saito, Y.; Takahashi, A.; Tanaka, G.; Yoshida, R.; Maegaki, Y.; Kawarai, T.; Ogihara, H.; Suzuki, C. Antibacterial Activity of Hexanol Vapor In Vitro and on the Surface of Vegetables. Foods 2023, 12, 3097. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Antolinos, V.; Buendía-Moreno, L.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; Soto-Jover, S. Fresh culinary herbs decontamination with essential oil vapours applied under vacuum conditions. Postharvest Biol. Technol. 2019, 156, 110942. [Google Scholar] [CrossRef]
- Zhang, J. The potential of a new fungicide fludioxonil for stem-end rot and green mold control on Florida citrus fruit. Postharvest Biol. Technol. 2007, 46, 262–270. [Google Scholar] [CrossRef]
- Koch, R. Verber Bakteriologische Forschung. In Verhandlungen des X. Internationalen Medicinischen Congresses; A. Hirschwald: Berlin, Germany, 1890; Volume 1, pp. 35–47. [Google Scholar]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A green approach to develop zeolite-thymol antimicrobial composites: Analytical characterization and antimicrobial activity evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef] [PubMed]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. The transcription factor PdSte12 contributes to Penicillium digitatum virulence during citrus fruit infection. Postharvest Biol. Technol. 2017, 125, 129–139. [Google Scholar] [CrossRef]
- Russell, P.E. Sensitivity Baselines in Fungicide Resistance Research and Management; Crop Life International: Brussels, Belgium, 2002. [Google Scholar]
- Ali, R.; El-Boubbou, K.; Boudjelal, M. An easy, fast and inexpensive method of preparing a biological specimen for scanning electron microscopy (SEM). MethodsX 2021, 8, 101521. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, G.M.; Cerioni, L.; Sepulveda, M.; Ramallo, J.; Rapisarda, V.A.; Volentini, S.I. Polyhexamethylene guanidine as a fungicide, disinfectant and wound protector in lemons challenged with Penicillium digitatum. Food Microbiol. 2018, 76, 128–134. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Plotto, A.; Zhong, T.; Ference, C.M.; Bai, J. The Effect of Controlled-Release Carvacrol on Safety and Quality of Blueberries Stored in Perforated Packaging. Foods 2021, 10, 1487. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 448. [Google Scholar]
- Sánchez-Torres, P. Molecular Mechanisms Underlying Fungicide Resistance in Citrus Postharvest Green Mold. J. Fungi 2021, 7, 783. [Google Scholar] [CrossRef]
- Chen, D.; Förster, H.; Adaskaveg, J.E. Baseline Sensitivities of Major Citrus, Pome, and Stone Fruits Postharvest Pathogens to Natamycin and Estimation of the Resistance Potential in Penicillium digitatum. Plant Dis. 2021, 105, 2114–2121. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Zhang, Y.; Hu, X.; Li, H.; Liu, Y.; Chen, L.; Liu, Y.; Qin, W. Effects of thymol concentration on postharvest diseases and quality of blueberry fruit. Food Chem. 2023, 402, 134227. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, J.; Hu, C.; Deng, L.; Ritenour, M.A. Use of carvacrol and thymol in shellac coating to control stem-end rot on ‘Ruby Red’ grapefruit and maintain fruit quality during simulated storage and marketing. Sci. Hort. 2020, 272, 109606. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Inouye, S.; Uchida, K.; Abe, S. Vapor activity of 72 essential oils against a Trichophyton mentagrophytes. J. Infect. Chemother. 2006, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Potential Control of Postharvest Fungal Decay of Citrus Fruits by Crude or Photochemically Changed Essential Oils—A Review. Food Rev. Int. 2023, 1–18. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Hlebová, M.; Foltinová, D.; Vešelényiová, D.; Medo, J.; Šramková, Z.; Tančinová, D.; Mrkvová, M.; Hleba, L. The Vapor Phase of Selected Essential Oils and Their Antifungal Activity In Vitro and In Situ against Penicillium commune, a Common Contaminant of Cheese. Foods 2022, 11, 3517. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božik, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapour phase against bread spoilage toxicogenic aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Navarro-Martínez, A.; Barón, M.; Navarro-Segura, L.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Martínez-Hernández, G.B. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Hlebová, M.; Hleba, L.; Medo, J.; Uzsakova, V.; Kloucek, P.; Bozik, M.; Haščík, P.; Čuboň, J. Antifungal and antitoxigenic effects of selected essential oils in vapors on green coffee beans with impact on consumer acceptability. Foods 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Farrag, E.S. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Food Nahr. 2003, 47, 117–121. [Google Scholar] [CrossRef]
- Ghanei Ghooshkhaneh, N.; Golzarian, M.R.; Mamarabadi, M. Detection and classification of citrus green mold caused by Penicillium digitatum using multispectral imaging. J. Sci. Food Agric. 2018, 98, 3542–3550. [Google Scholar] [CrossRef]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; Leite, A.d.L.; de Pontes, L.G.; dos Santos, L.D.; et al. Proteomic analysis and antibacterial resistance mechanisms of Salmonella Enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteom. 2020, 214, 103625. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Rani, P.; Nidhin, P.T.; Katoch, M. A polymer-based gel fumigator, having a combination of Monarda citriodora essential oil (MEO) and hexanal: A solution for the postharvest rot and phytotoxicity of apple during storage. Postharvest Biol. Technol. 2023, 200, 112248. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Nazer, A.I.; Kobilinsky, A.; Tholozan, J.L.; Dubois-Brissonnet, F. Combinations of food antimicrobials at low levels to inhibit the growth of Salmonella sv. Typhimurium: A synergistic effect? Food Microbiol. 2005, 22, 391–398. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of modified atmosphere packaging combined with essential oils for prolonging post-harvest shelf life of mango (cv. Banganapalli and cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo, G.M.; Zhang, J.; Zhao, W.; Mattia, M.; Rosskopf, E.N.; Ritenour, M.; Plotto, A.; Bai, J. Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit. Foods 2023, 12, 3637. https://doi.org/10.3390/foods12193637
Olmedo GM, Zhang J, Zhao W, Mattia M, Rosskopf EN, Ritenour M, Plotto A, Bai J. Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit. Foods. 2023; 12(19):3637. https://doi.org/10.3390/foods12193637
Chicago/Turabian StyleOlmedo, Gabriela M., Jiuxu Zhang, Wei Zhao, Matthew Mattia, Erin N. Rosskopf, Mark Ritenour, Anne Plotto, and Jinhe Bai. 2023. "Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit" Foods 12, no. 19: 3637. https://doi.org/10.3390/foods12193637
APA StyleOlmedo, G. M., Zhang, J., Zhao, W., Mattia, M., Rosskopf, E. N., Ritenour, M., Plotto, A., & Bai, J. (2023). Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit. Foods, 12(19), 3637. https://doi.org/10.3390/foods12193637





