Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals
Abstract
1. Introduction
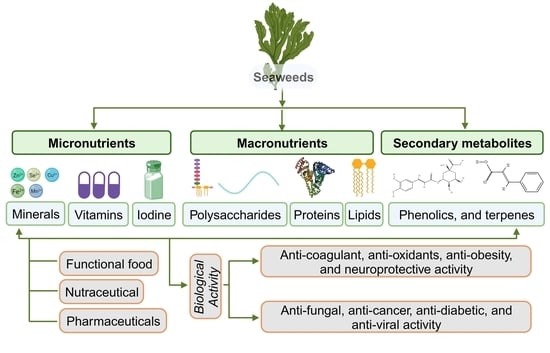
2. Seaweeds as a Source of Nutrients and Metabolites
2.1. Micronutrient Composition
2.1.1. Minerals
2.1.2. Vitamins
2.2. Macronutrient Composition
2.2.1. Seaweed Polysaccharides
Sulfated Fucose-Fucoidans
Sulfated Galactan-Carrageenans
Sulfated Heteroglycans-Ulvans
2.2.2. Seaweed Proteins and Peptides
2.2.3. Seaweed Pigments
2.2.4. Seaweed Lipid and Fatty Acid
3. Secondary Metabolic Compounds in Seaweeds
3.1. Phenolic Compounds
3.2. Terpenes
4. Biological Activity of Seaweeds
4.1. Antioxidant Activities
4.2. Anticoagulant Activity
4.3. Anticancer Activity
4.4. Neuroprotective Activity
4.5. Antiviral Activity
4.6. Antifungal Activity
4.7. Anti-Diabetic Properties
4.8. Anti-Obesity
4.9. Anti-Inflammatory Activities
5. Challenges
5.1. Efficient Green Methods for Compound Extraction and Purification
5.2. Toxicity and Antinutrients
5.3. Need for Unified Global Regulations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1O2 | Singlet oxygen |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ACE I | Angiotensin-converting enzyme |
| AD | Alzheimer’s disease |
| AI | Atherogenicity index |
| ALS | Amyotrophic Lateral Sclerosis |
| ALT | Alanine aminotransferase |
| APC | Allophycocyanin |
| APTT | Activated partial thromboplastin time |
| AST | Aspartate aminotransferase |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| CFS | Chronic fatigue syndrome |
| CHIKV | Chikungunya virus |
| COVID-19 | Coronavirus disease 2019 |
| CVDs | Cardiovascular diseases |
| DENV-2 | Dengue virus |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EFAs | Essential fatty acids |
| EPA | Eicosapentaenoic acid |
| FAs | Fatty acids |
| FCSPs | Fucose-containing sulfated polysaccharides |
| FFA | Free fatty acids |
| FRAP | Ferric-reducing ability of plasma |
| GLA | Gamma-linolenic acid |
| GPs | Glycoproteins |
| H2O2 | Hydrogen peroxide |
| HFD | High-fat diet |
| HIV | Human immunodeficiency virus |
| hMPV | Human metapneumo virus |
| HSV-1 | Herpes simplex virus type 1 |
| HSV-2 | Herpes simplex virus type 2 |
| LDL | Low-density lipoprotein |
| LNA | Linolenic acid |
| MUFAs | Monosaturated fatty acids |
| O2− | Anion radicals |
| OH | Hydroxyl radicals |
| PC | Phycocyanin |
| PE | Phycoerythrin |
| PT | Prothrombin time |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| RVFV | Rift valley fever virus |
| SA | Stearidonic acid |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SFAs | Saturated fatty acids |
| TBA | Total bile acid |
| TBHQ | Tertiary butylhydroquinone |
| TI | Thrombogenicity index |
| TT | Thrombin time |
| UA | Uric acid |
| UAE | Ultrasonic-assisted extraction |
| UI | Unsaturation index |
| UV | Ultraviolet |
| WAT | White adipose tissue |
References
- Baghel, R.S. Developments in seaweed biorefinery research: A comprehensive review. J. Chem. Eng. 2023, 454, 140177. [Google Scholar] [CrossRef]
- Samiee, S.; Ahmadzadeh, H.; Hosseini, M.; Lyon, S. Algae as a source of microcrystalline cellulose. In Advanced Bioprocessing for Alternative Fuels, Biobasedchemicals, and Bioproducts; Woodhead Publishing: Sawston, UK, 2019; pp. 331–350. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and Minor Compounds in Algae: In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 205–251. [Google Scholar] [CrossRef]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Jha, B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresour. Technol. 2014, 159, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.R.K.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae 1. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Merrill, J.E. Development of nori markets in the western world. J. Appl. Phycol. 1993, 5, 149–154. [Google Scholar] [CrossRef]
- Chapman, R.L. Algae: The world’s most important “plants”—An introduction. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef]
- Baghel, R.S.; Mantri, V.A.; Reddy, C.R.K. A new wave of research interest in marine macroalgae for chemicals and fuels: Challenges and potentials. In Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; Wiley: Hoboken, NJ, USA, 2017; pp. 43–63. [Google Scholar] [CrossRef]
- McHugh, D.J. Worldwide distribution of commercial resources of seaweeds including Gelidium. In International Workshop on Gelidium; Springer: Dordrecht, The Netherlands, 1991; pp. 19–29. [Google Scholar] [CrossRef]
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Pereira, L. Colloid producing seaweeds: Agarophytes, carrageenophytes and alginophytes biodiversity. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; Volume 1, pp. 161–326. [Google Scholar] [CrossRef]
- Cai, J. Global Status of Seaweed Production, Trade and Utilization; Food and Agriculture Organisation of United Nations: Rome, Italy, 2021; pp. 1–18. [Google Scholar]
- Choudhary, B.; Khandwal, D.; Gupta, N.K.; Patel, J.; Mishra, A. Nutrient Composition, Physicobiochemical Analyses, Oxidative Stability and Antinutritional Assessment of Abundant Tropical Seaweeds from the Arabian Sea. Plants 2023, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.; Pinto, D.C.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Jiménez Escrig, A.; Cambrodon, G. Nutritional evaluation and physiological effects of edible seaweeds. Arch. Latinoam. Nutr. 1999, 49, 114–120. [Google Scholar] [PubMed]
- Tanna, B.; Mishra, A. Metabolomics of Seaweeds: Tools and Techniques. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Alpharetta, GA, USA, 2018; pp. 37–52. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A.; Yadav, S.; Chauhan, O.P.; Elansary, H.O.; Shokralla, S.; El-Abedin, T.K.Z.; Mahmoud, E.A. Biochemical and Anti-proliferative activities of seven abundant tropical red seaweeds confirm nutraceutical potential of Grateloupia indica. Arab. J. Chem. 2022, 15, 103868. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Santoso, J.; Gunji, S.; Yoshie-Stark, Y.; Suzuki, T. Mineral contents of Indonesian seaweeds and mineral solubility affected by basic cooking. Food Sci. Technol. Res. 2006, 12, 59–66. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Robledo, D.; Freile Pelegrín, Y. Chemical and mineral composition of six potentially edible seaweed species of Yucatan. Bot. Mar. 1997, 40, 301–306. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Imchen, T. Nutritional value of seaweeds and their potential to serve as nutraceutical supplements. Phycologia 2021, 60, 534–546. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int. J. Food Sci. Nutr. 2013, 65, 299–305. [Google Scholar] [CrossRef]
- Yoganandham, S.T.; Raguraman, V.; Muniswamy, G.; Sathyamoorthy, G.; Renuka, R.R.; Chidambaram, J.; Rajendran, T.; Chandrasekaran, K.; Ravindranath, R.R.S. Mineral and trace metal concentrations in seaweeds by microwave-assisted digestion method followed by quadrupole inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res. 2019, 187, 579–585. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Benjama, O.; Masniyom, P. Nutritional composition and physicochemical properties of two green seaweeds (Ulva pertusa and U. intestinalis) from the Pattani Bay in Southern Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 575–583. [Google Scholar]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Campbell, J.D. Lifestyle, minerals and health. Med. Hypotheses. 2001, 57, 521–531. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.T.; Wang, F.H.; Wen, D.; Yang, H.; Zhao, X.L. An investigation of toxic metal levels (Pb, Cd, Cr, As, Hg) in dried porphyra and laminaria collected from coastal cities, China. Biol. Trace Elem. Res. 2021, 199, 3987–3997. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Calvo, J.J.; Mazuelos, C.; Hermosin, B.; Sáiz-Jiménez, C. Chemical composition of Spirulina and eukaryotic algae food products marketed in Spain. J. Appl. Phycol. 1993, 5, 425–435. [Google Scholar] [CrossRef]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2020, 62, 1592–1607. [Google Scholar] [CrossRef]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae, Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 171–192. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Biores. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. Cell wall polysaccharides of marine algae. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 543–590. [Google Scholar] [CrossRef]
- Chen, S.; Sathuvan, M.; Zhang, X.; Zhang, W.; Tang, S.; Liu, Y.; Cheong, K.L. Characterization of polysaccharides from different species of brown seaweed using saccharide mapping and chromatographic analysis. BMC Chem. 2021, 15, 1. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M. Ferreira da Silva, F.R., Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Fayaz, M.; Namitha, K.K.; Murthy, K.C.; Swamy, M.M.; Sarada, R.; Khanam, S.; Subbarao, P.V.; Ravishankar, G.A. Chemical composition, iron bioavailability, and antioxidant activity of Kappaphycus alvarezzi (Doty). J. Agric. Food Chem. 2005, 53, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carmona, G.; Carrillo-Domínguez, S.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Castillo-Domínguez, R.M. Monthly variation in the chemical composition of Eisenia arborea JE Areschoug. J. Appl. Phycol. 2009, 21, 607–616. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Vinceti, M. Sodium intake and risk of hypertension: A systematic review and dose–response meta-analysis of observational cohort studies. Curr. Hypertens. Rep. 2022, 24, 133–144. [Google Scholar] [CrossRef]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of hypertension: A review. Jama 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.R.; Arunkumar, K. Sodium, potassium and sulphate composition in some seaweeds occurring along the coast of Gulf of Mannar, India. Asian J. Plant Sci. 2009, 8, 500–504. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83e96. [Google Scholar] [CrossRef]
- Indergaard, M. Animal and human nutrition. In Seaweed Resources in Europe: Uses and Potential; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 21–64. [Google Scholar]
- Kasmiati, K.; Syahrul, S.; Badraeni, B.; Rahmi, M.H. Proximate and mineral compositions of the green seaweeds Caulerpa lentilifera and Caulerpa racemosa from South Sulawesi Coast, Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1119, p. 12049. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Duinker, A.; Roiha, I.S.; Amlund, H.; Dahl, L.; Lock, E.J.; Kögel, T.; Måge, A.; Lunestad, B.T. Potential Risks Posed by Macroalgae for Application as Feed and Food—A Norwegian Perspective; National Institute of Nutrition and Seafood Research: Bergen, Norway, 2016; p. 23. [Google Scholar]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef] [PubMed]
- Küpper, F.C.; Schweigert, N.; Ar Gall, E.; Legendre, J.M.; Vilter, H.; Kloareg, B. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 1998, 207, 163–171. [Google Scholar] [CrossRef]
- Nitschke, U.; Stengel, D.B. A new HPLC method for the detection of iodine applied to natural samples of edible seaweeds and commercial seaweed food products. Food Chem. 2015, 172, 326–334. [Google Scholar] [CrossRef]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed minerals as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 371–390. [Google Scholar] [CrossRef]
- Bellows, A.L.; Smith, E.R.; Muhihi, A.; Briegleb, C.; Noor, R.A.; Mshamu, S.; Sudfeld, C.; Masanja, H.; Fawzi, W.W. Micronutrient deficiencies among breastfeeding infants in Tanzania. Nutrients 2017, 9, 1258. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Elec. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Norziah, M.H.; Ching, C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Chapman, V.J.; Chapman, D.J. (Eds.) Seaweeds and Their Uses, 3rd ed.; Chapman & Hall: New York, NY, USA, 1980; pp. 25–42. [Google Scholar]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Norris, E.R.; Simeon, M.K.; Williams, H.B. The vitamin B and vitamin C content of marine algae. J. Nutr. 1937, 13, 425–433. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Hernández, J.; Paseiro-Losada, P. High-performance liquid chromatographic determination of atocopherol in macroalgae. J. Chromatogr. A 2002, 976, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.; Ruslan, F.S.; Shukor, M.I.; Nor, N.M.; Aminudin, N.I.; Taher, M.; Khotib, J. Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design. Molecules 2022, 27, 4459. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea Antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Mathew, S.; Ravishankar, C.N. Seaweeds as a Source of Micro and Macro Nutrients; ICAR-Central Institute of Fisheries Technology: Cochin, India, 2018. [Google Scholar]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed polysaccharide based products and materials: An assessment on their production from a sustainability point of view. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. In vitro antioxidant and antibacterial activity of sulfated polysaccharides isolated from Spatoglossum asperum. Carbohydr. Polym. 2017, 170, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.; Methacanon, P.; Thayanukul, P.; Hongsprabhas, P.; Zhang, W. Gut microbiome modulation and gastrointestinal digestibility in vitro of polysaccharide-enriched extracts and seaweeds from Ulva rigida and Gracilaria fisheri. J. Funct. Foods 2022, 96, 105204. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs. 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Schiehser, S.; Rosenau, T.; Böhmdorfer, S. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Stortz, C.A.; Cerezo, A.S. Novel findings in carrageenans, agaroids and “hybrid” red seaweed galactans. Chem. Inform. 2002, 33. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Ponce, N.; Stortz, C.A. Seaweed polysaccharides: Structure and applications. In Industrial Applications of Renewable Biomass Products; Springer: Cham, Switzerland, 2017; pp. 75–116. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Chin, Y.X.; Mi, Y.; Cao, W.X.; Lim, P.E.; Xue, C.H.; Tang, Q.J. A pilot study on anti-obesity mechanisms of Kappaphycus alvarezii: The role of native κ-carrageenan and the leftover sans-carrageenan fraction. Nutrients 2019, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.S.; Dolan, T.C.; Rees, D.A. Carrageenans. Part VII. Polysaccharides from Eucheuma spinosum and Eucheuma cottonii. The covalent structure of ι-carrageenan. J. Chem. Soc. Perkin Trans. 1973, 1, 2173–2176. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Cicinskas, E.; Kalitnik, A.A.; Karetin, Y.A.; Mohan Ram, M.S.G.; Achary, A.; Kravchenko, A.O. Immunomodulating properties of carrageenan from Tichocarpus crinitus. Inflammation 2020, 43, 1387–1396. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, Y.; Zhang, Y.; Xu, Y.; Zheng, L.; Zhu, B.; Yao, Z. Carrageenan oligosaccharides: A comprehensive review of preparation, isolation, purification, structure, biological activities and applications. Algal Res. 2022, 61, 102593. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr. Res. 2000, 325, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Hiroe, M.; Noda, S.; Asai, I.; Ikeda, S.; Nishinari, K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocoll. 2007, 21, 617–629. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Pimentel, F.; Alves, R.; Harnedy, P.; FitzGerald, R.; Oliveira, M. Macroalgal-Derived Protein Hydrolysates and Bioactive Peptides: Enzymatic Release and Potential Health Enhancing Properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Gordalina, M.; Pinheiro, H.M.; Mateus, M.; da Fonseca, M.M.R.; Cesário, M.T. Macroalgae as protein sources—A review on protein bioactivity, extraction, purification and characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Yoshiie, T.; Maeda, M.; Kimura, M.; Hama, Y.; Uchida, M.; Kimura, Y. Structural features of N-glycans of seaweed glycoproteins: Predominant occurrence of high-mannose type N-glycans in marine plants. Biosci. Biotechnol. Biochem. 2012, 76, 1996–1998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current Knowledge and Challenges in Extraction, Characterization and Bioactivity of Seaweed Protein and Seaweed-Derived Proteins. Adv. Bot. Res. 2020, 95, 289–326. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.; Sowder, R.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.; Buckheit, R.; McMahon, J.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-Inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef]
- Chaves, R.; Silva, S.; Neto, L.; Carneiro, R.; Silva, A.; Sampaio, A.; Sousa, B.; Cabral, M.; Videira, P.; Teixeira, E.; et al. Structural Characterization of Two Isolectins from the Marine Red Alga solieria Filiformis (Kützing) P.W. Gabrielson and Their Anticancer Effect on MCF-7 Breast Cancer Cells. Int. J. Biol. Macromol. 2018, 107, 1320–1329. [Google Scholar] [CrossRef]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A Novel High-Mannose Specific Lectin from the Green Alga Halimeda renschiit Exhibits a Potent Anti-Influenza Virus Activity through High-Affinity Binding to the Viral Hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins J. Fleurence, University of Nantes, France. Proteins Food Process. 2004, 98, 197. [Google Scholar]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Mancini Filho, J.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Vinoj Kumar, V.; Kaladharan, P. Amino acids in the seaweeds as an alternate source of protein for animal feed. J. Mar. Biol. Assoc. India 2007, 49, 35–40. [Google Scholar]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.D.S.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Cserháti, T. Liquid Chromatography of Natural Pigments and Synthetic Dyes; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780444522221. [Google Scholar]
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Mondal, S.; Seo, H.; Dae Lee, K.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fukuda, S.; Izumi, H.; Saga, N. Anti-oxidant and fucoxanthin contents of brown alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Mar. Drugs 2018, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid profiling of a red seaweed Pyropia yezoensis: Insights into biosynthetic pathways in the order Bangiales. Mar. Drugs. 2018, 16, 426. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.M.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef] [PubMed]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Ananthalakshmi, J.S.; Nair, B.B. Extraction, purification and study on antioxidant properties of fucoxanthin from brown seaweeds. J. Chem. Pharm. Res. 2013, 5, 169–175. [Google Scholar]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Doumeizel, V.; Aass, K.; McNevin, A.; Cousteau, A.; Yap, A.Y.; Cai, J.; Cottier-Cook, E.J.; Giercksky, E.; Chen, H.; Skjermo, J.; et al. Seaweed Revolution: A Manifesto for a Sustainable Future; Lloyd’s Register Foundation: London, UK, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Sangha, J.S.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Macek Jilkova, Z.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kopecky, J.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Miraliakbari, H. Omega-3 (n-3) fatty acids in health and disease: Part 1—Cardiovascular disease and cancer. J. Med. Food 2004, 7, 387–401. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Domínguez-González, R.; Alonso-Rodríguez, E.; López-Mahía, P.; Bermejo-Barrera, P. Trace metals in marine foodstuff: Bioavailability estimation and effect of major food constituents. Food Chem. 2012, 134, 339–345. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents 1. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Belattmania, Z.; Engelen, A.H.; Pereira, H.; Serrão, E.A.; Barakate, M.; Elatouani, S.; Zrid, R.; Bentiss, F.; Chahboun, N.; Reani, A.; et al. Potential uses of the brown seaweed Cystoseira humilis biomass: 2-Fatty acid composition, antioxidant and antibacterial activities. J. Mater. Environ. Sci. 2016, 7, 2074–2081. [Google Scholar]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? N Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. N Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef]
- Treml, J.; Smejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Tanna, B.; Yadav, S.; Mishra, A. Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol. Biol. Rep. 2020, 47, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.G. Tannins and Polyphenols. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5729–5733. [Google Scholar]
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.; Pereira, L. Antitumour potential of Gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing: Philadelphia, PN, USA, 2013; pp. 181–204. [Google Scholar]
- Mannino, A.M.; Micheli, C. Ecological function of phenolic compounds from Mediterranean fucoid algae and seagrasses: An overview on the genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of Bromophenols in Species of Marine Algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A. Antioxidant Potential of Two Red Seaweeds from the Brazilian Coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Aschwin, E.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Giada, M.D.L.R. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 87–112. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Valentão, P.; Andrade, P.B. Sterols in algae and health. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; Hernández-Ledesma, B., Herrero, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 173–191. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A.; Chauhan, O.P.; Patel, M.K.; Shokralla, S.; El-Abedin, T.K.Z.; Elansary, H.O.; Mahmoud, E.A. Antioxidant, scavenging, reducing, and anti-proliferative activities of selected tropical brown seaweeds confirm the nutraceutical potential of Spatoglossum asperum. Foods 2021, 10, 2482. [Google Scholar] [CrossRef]
- Li, Y.X.; Kim, S.K. Utilization of seaweed derived ingredients as potential antioxidants and functional ingredients in the food industry: An overview. Food Sci. Biotechnol. 2011, 20, 1461–1466. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Santoso, J.; Yoshie, Y.; Suzuki, T. Polyphenolic compounds from seaweeds: Distribution and their antioxidative effect. Devel Food Sci. 2004, 42, 169–177. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskal) C. Agardh. Arabian J. Chem. 2013, 10 (Suppl. S2), S2608–S2614. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese, Laminariaceae. In Nineteenth International Seaweed Symposium; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 255–261. [Google Scholar] [CrossRef]
- El Shoubaky, G.A.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Isolation and identification of a flavone apigenin from marine red alga Acanthophora spicifera with antinociceptive and anti-inflammatory activities. J. Exp. Neurosci. 2016, 10, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. 2018, 148, 230–237. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; Perveen, S., Ed.; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Gaysinski, M.; Ortalo-Magné, A.; Thomas, O.P.; Culioli, G. Extraction, purification, and NMR analysis of terpenes from brown algae. In Natural Products from Marine Algae; Humana Press: New York, NY, USA, 2015; pp. 207–223. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical composition of seaweeds. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA, 2015; pp. 79–124. [Google Scholar] [CrossRef]
- Obando, J.M.C.; dos Santos, T.C.; Martins, R.C.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Current and promising applications of seaweed culture in laboratory conditions. Aquaculture 2022, 560, 738596. [Google Scholar] [CrossRef]
- Polzin, J.P.; Rorrer, G.L. Halogenated monoterpene production by microplantlets of the marine red alga Ochtodes secundiramea within an airlift photobioreactor under nutrient medium perfusion. Biotechnol. Bioeng. 2003, 82, 415–428. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Sudatti, D.B.; Fujii, M.T.; Rodrigues, S.V.; Pereira, R.C. Inter-and intrapopulation variation in the defensive chemistry of the red seaweed Laurencia dendroidea (Ceramiales, Rhodophyta). Phycologia 2013, 52, 130–136. [Google Scholar] [CrossRef]
- Dumay, O.; Pergent, G.; Pergent-Martini, C.; Amade, P. Variations in caulerpenyne contents in Caulerpa taxifolia and Caulerpa racemosa. J. Chem. Ecol. 2002, 28, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Burney, M.; Mathew, L.; Gaikwad, A.; Nugent, E.K.; Gonzalez, A.O.; Smith, J.A. Evaluation fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus in combination with anticancer drugs in human cancer orthotopic mouse models. Integr. Cancer Ther. 2018, 17, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Ponce, N.M.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Hou, Y.; Zhang, H. In-vitro anticoagulant activity of fucoidan derivatives from brown seaweed Laminaria japonica. Chin. J. Oceanol. Limnol. 2011, 29, 679–685. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 199–218. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune activation of RAW264. 7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.; Fernandes, C.; Silva, P.; Pereira, L.; Gonçalves, T. Antifungal activity of carrageenan extracts from the red alga Chondracanthus teedei var. lusitanicus. J. Appl. Phycol. 2016, 28, 2991–2998. [Google Scholar] [CrossRef]
- Ghannam, A.; Murad, H.; Jazzara, M.; Odeh, A.; Allaf, A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018, 108, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, S.; Soegima, R.; Yamaguchi, K.; Oda, T. Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 2009, 73, 961–964. [Google Scholar] [CrossRef]
- Teo, B.S.X.; Gan, R.Y.; Abdul Aziz, S.; Sirirak, T.; Mohd Asmani, M.F.; Yusuf, E. In vitro evaluation of antioxidant and antibacterial activities of Eucheuma cottonii extract and Its in vivo evaluation of the wound-healing activity in mice. J. Cosmet. Dermatol. 2021, 20, 993–1001. [Google Scholar] [CrossRef]
- Pushpamali, W.A.; Nikapitiya, C.; De Zoysa, M.; Whang, I.; Kim, S.J.; Lee, J. Isolation and purification of an anticoagulant from fermented red seaweed Lomentaria catenata. Carbohydr. Polym. 2008, 73, 274–279. [Google Scholar] [CrossRef]
- Ana, P.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomas, M.S.; Yolanda, F.P. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Abirami, R.G.; Kowsalya, S. Antidiabetic activity of Ulva fasciata and its impact on carbohydrate metabol-ism enzymes in alloxan induced diabetic rats. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 136–141. [Google Scholar]
- Arunkumar, K.; Raja, R.; Kumar, V.S.; Joseph, A.; Shilpa, T.; Carvalho, I.S. Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. J. Food Meas. Charact. 2021, 15, 567–576. [Google Scholar] [CrossRef]
- Palani, K.; Balasubramanian, B.; Malaisamy, A.; Maluventhen, V.; Arumugam, V.A.; Al-Dhabi, N.A.; Arasu, M.V.; Pushparaj, K.; Liu, W.-C.; Arumugam, M. Sulfated Polysaccharides Derived from Hypnea valentiae and Their Potential of Antioxidant, Antimicrobial, and Anticoagulant Activities with In Silico Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 3715806. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Al-Badaani, A.A.; Hifney, A.F.; Adam, M.S. Utilization of cellulose and ulvan from the green seaweed Ulva lactuca in the development of composite edible films with natural antioxidant properties. J. Appl. Phycol. 2022, 34, 2615–2626. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, R.R. Anti-proliferative and apoptotic efficacies of ulvan polysaccharides against different types of carcinoma cells in vitro and in vivo. J. Cancer Sci. Ther. 2014, 6, 202–208. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Rizk, M.Z.; Aly, H.F.; Matloub, A.A.; Fouad, G.I. The anti-hypercholesterolemic effect of ulvan polysaccharide extracted from the green alga Ulva fasciata on aged hypercholesterolemic rats. Asian J. Pharm. Clin. Res. 2016, 9, 165–176. [Google Scholar]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K.; et al. Structural features and anti-coagulant activity of the sulphated polysaccharide SPS-CF from a green alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci. Rep. 2018, 8, 3062. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Berri, M.; Slugocki, C.; Olivier, M.; Helloin, E.; Jacques, I.; Salmon, H.; Demais, H.; Le Goff, M.; Collen, P.N. Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. J. Appl. Phycol. 2016, 28, 2999–3008. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G.; Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; et al. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yu, S.; Yin, L.; Zhao, M.; Han, Z. Synthesized oversulfated and acetylated derivatives of polysaccharide extracted from Enteromorpha linza and their potential antioxidant activity. Int. J. Biol. Macromol. 2011, 49, 1012–1015. [Google Scholar] [CrossRef]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’en, L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Luo, H.; Yang, Y. Selenium-containing polysaccharide-protein complex in Se-enriched Ulva fasciata induces mitochondria-mediated apoptosis in A549 human lung cancer cells. Mar. Drugs 2017, 15, 215. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Jayanthi, S. Partial characterization and anticancer activities of purified glycoprotein extracted from green seaweed Codium decorticatum. J. Funct. Foods 2016, 25, 323–332. [Google Scholar] [CrossRef]
- da Conceição Rivanor, R.L.; Chaves, H.V.; do Val, D.R.; de Freitas, A.R.; Lemos, J.C.; Rodrigues, J.A.G.; Pereira, K.M.A.; de Araújo, I.W.F.; Bezerra, M.M.; Benevides, N.M.B. A lectin from the green seaweed Caulerpa cupressoides reduces mechanical hyper-nociception and inflammation in the rat temporomandibular joint during zymosan-induced arthritis. Int. Immuno pharmacol. 2014, 21, 34–43. [Google Scholar] [CrossRef]
- Oh, J.H.; Nam, T.J. Hydrophilic glycoproteins of an edible green alga Capsosiphon fulvescens prevent aging-induced spatial memory impairment by suppressing GSK-3β-mediated ER stress in dorsal hippocampus. Mar. Drugs 2019, 17, 168. [Google Scholar] [CrossRef]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the Nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of algae and plant lectins on planktonic growth and biofilm formation in clinically relevant bacteria and yeasts. Biomed Res. Int. 2014, 2014, 365272. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Garzón, A.G.; Ancona, D.B.; Guerrero, L.C.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Moon, I.S.; Kim, J.-M.; Kong, I.S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Alagarsamy, S.; Arnous, A.; Jacobsen, C. Enzymatic extraction of antioxidant ingredients from Danish seaweeds and characterization of active principles. Algal Res. 2021, 56, 102292. [Google Scholar] [CrossRef]
- Park, S.J.; Ryu, J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Induction of apoptosis by a peptide from Porphyra yezoensis: Regulation of the insulin-like growth factor I receptor signaling pathway in MCF-7 cells. Int. J. Oncol. 2014, 45, 1011–1016. [Google Scholar] [CrossRef]
- Kim, E.Y.; Rafiquzzaman, S.M.; Lee, J.M.; Noh, G.; Jo, G.A.; Lee, J.H.; Kong, I.S. Structural features of glycoprotein purified from Saccharina japonica and its effects on the selected probiotic properties of Lactobacillus plantarum in Caco-2 cell. J. Appl. Phycol. 2015, 27, 965–973. [Google Scholar] [CrossRef]
- Go, H.; Hwang, H.J.; Nam, T.J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. In Vitro Toxicol. 2010, 24, 1546–1553. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Mendes, M.; Pereira, R.; Pinto, I.S.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Pirian, K.; Jeliani, Z.Z.; Sohrabipour, J.; Arman, M.; Faghihi, M.M.; Yousefzadi, M. Nutritional and bioactivity evaluation of common seaweed species from the Persian Gulf. Iran J. Sci. Technol. Trans. A Sci. 2018, 42, 1795–1804. [Google Scholar] [CrossRef]
- Airanthi, M.W.A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2–ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Ara, J.; Sultana, V.; Qasim, R.; Ehteshamul-Haque, S.; Ahmad, V.U. Biological activity of Spatoglossum asperum: A brown alga. Phytother. Res. 2005, 19, 618–623. [Google Scholar] [CrossRef]
- Park, N.H.; Choi, J.S.; Hwang, S.Y.; Kim, Y.C.; Hong, Y.K.; Cho, K.K.; Choi, I.S. Antimicrobial activities of stearidonic and gamma-linolenic acids from the green seaweed Enteromorpha linza against several oral pathogenic bacteria. Bot. Stud. 2013, 54, 39. [Google Scholar] [CrossRef]
- Cortés, Y.; Hormazábal, E.; Leal, H.; Urzúa, A.; Mutis, A.; Parra, L.; Quiroz, A. Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta: Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica, agents that cause diseases in salmonids. Electron. J. Biotechnol. 2014, 17, 126–131. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and antibacterial activity of red seaweed Kappaphycus alvarezii against pathogenic bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar] [CrossRef]
- Bonde, C.S.; Bornancin, L.; Lu, Y.; Simonsen, H.T.; Martínez-Valladares, M.; Peña-Espinoza, M.; Mejer, H.; Williams, A.R.; Thamsborg, S.M. Bio-guided fractionation and molecular networking reveal fatty acids to be principal anti-parasitic compounds in nordic seaweeds. Front. Pharmacol. 2021, 12, 674520. [Google Scholar] [CrossRef]
- Barot, M.; Kumar, N.J.I.; Kumar, R.N. Bioactive compounds and antifungal activity of three different seaweed species Ulva lactuca, Sargassum tenerrimum and Laurencia obtusa collected from Okha coast, Western India. J. Coast. Life Med. 2016, 4, 284–289. [Google Scholar] [CrossRef]
- Horincar, V.B.; Parfene, G.; Tyagi, A.K.; Gottardi, D.; Dinică, R.; Guerzoni, M.E.; Bahrim, G. Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J. Appl. Phycol. 2014, 26, 551–559. [Google Scholar] [CrossRef]
- Jiang, R.W.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Le Roch, K.; Aalbersberg, W.; Kubanek, J. Antineoplastic unsaturated fatty acids from Fijian macroalgae. Phytochemistry 2008, 69, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, M.; Kim, S.H.; Siriwardhana, N.; Ha, J.H.; Lee, K.W.; Jeon, Y.J. Antioxidant potential of ecklonia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Int. J. Food Sci. 2006, 12, 27–38. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar] [CrossRef]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Pirian, K.; Moein, S.; Sohrabipour, J.; Rabiei, R.; Blomster, J. Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. J. Appl. Phycol. 2017, 29, 3151–3159. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shenody, R.A.E.K.; Bases, E.A.; EL Shafay, S.M. Comparative assessment of antioxidant activity and biochemical composition of four seaweeds, Rocky Bay of Abu Qir in Alexandria, Egypt. Food Sci. Technol. 2020, 41, 29–40. [Google Scholar] [CrossRef]
- VijaySankar, N.P.; Jagtap, A.S.; Baghel, R.S.; Imchen, T.; Manohar, C.S. Elucidation of the antioxidant potential of marine macroalgal biomolecules for healthcare applications: Current status and future prospects. In Marine Antioxidants: Preparations, Syntheses, and Applications; Se-Kwon Kim, S.-K., Shin, K.H., Venkatesan, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Salinity and desiccation induced oxidative stress acclimation in seaweeds. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 91–123. [Google Scholar] [CrossRef]
- Jagtap, A.S.; Manohar, C.S.; Ayyapankutty, A.M.T.; Meena, S.N. Antioxidant and Antiglycemic Properties of Macroalgae, an Underutilized Blue Economy Bioresource in India. Russ. J. Mar. Biol. 2021, 47, 489–497. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocoll. 2018, 75, 229–236. [Google Scholar] [CrossRef]
- Saluri, K.; Tuvikene, R. Anticoagulant and antioxidant activity of lambda-and theta-carrageenans of different molecular weights. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100243. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.M.; Xu, S.Y.; Liu, Y.; Cheong, K.L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different levels of skin whitening activity among 3, 6-anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. The brown seaweed Cystoseira schiffneri as a source of sodium alginate: Chemical and structural characterization, and antioxidant activities. Food Biosci. 2021, 40, 100873. [Google Scholar] [CrossRef]
- Zeng, J.; An, D.; Jiao, C.; Xiao, Q.; Weng, H.; Yang, Q.; Xiao, A. Cloning, expression, and characterization of a new pH-and heat-stable alginate lyase from Pseudoalteromonas carrageenovora ASY5. J. Food Biochem. 2019, 43, e12886. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, H.J.; Kim, J.H.; Lee, J.W. Enhanced biological activities of laminarin degraded by gamma-ray irradiation. J. Food Biochem. 2012, 36, 465–469. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Zhu, P.; Lin, J. Antioxidant activity and hepatoprotective potential of agaro-oligosaccharides in vitro and in vivo. Nutr. J. 2006, 5, 31. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Chandran, M.N.; Suganya, A.M.; Immanuel, G. Investigation on bio-properties and in-vivo antioxidant potential of carrageenans against alloxan induced oxidative stress in Wistar albino rats. Int. J. Biol. Macromol. 2020, 151, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Q.; Dai, X. Carrageenan oligosaccharides extend life span and health span in male Drosophila melanogaster by modulating antioxidant activity, immunity, and gut microbiota. J. Med. Food 2021, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.K.; Lee, J.C.; Yang, G.E.; Her, Y.; et al. Laminarin attenuates ultraviolet-induced skin damage by reducing superoxide anion levels and increasing endogenous antioxidants in the dorsal skin of mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Lee, A.S.; Lin, C.H.; Tseng, K.W. Cytoprotective potential of fucoxanthin in oxidative stress-induced age-related macular degeneration and retinal pigment epithelial cell senescence in vivo and in vitro. Mar. Drugs 2021, 19, 114. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfatedulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.; Liao, X.; Hu, X.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447–455. [Google Scholar] [CrossRef]
- Ciancia, M.; Quintana, I.; Cerezo, A.S. Overview of anticoagulant activity of sulfated polysaccharides from seaweeds in relation to their structures, focusing on those of green seaweeds. Curr. Med. Chem. 2010, 17, 2503–2529. [Google Scholar] [CrossRef]
- de Carvalho, M.M.; de Freitas, R.A.; Ducatti, D.R.; Ferreira, L.G.; Gonçalves, A.G.; Colodi, F.G.; Mazepa, E.; Aranha, E.M.; Noseda, M.D.; Duarte, M.E.R. Modification of ulvans via periodate-chlorite oxidation: Chemical characterization and anticoagulant activity. Carbohydr. Polym. 2018, 197, 631–640. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Nakamura, M.; Yogi, T.; Teruya, T.; Konishi, T.; Uechi, S.; Tako, M. Anticoagulant activity of rhamnansulfate isolated from commercially cultured Monostroma nitidum. Int. J. Biomed. Mater. Res. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulphated rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef]
- dos Santos-Fidencio, G.C.; Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydr. Polym. 2019, 214, 286–293. [Google Scholar] [CrossRef]
- Castro, L.S.E.P.W.; de Sousa Pinheiro, T.; Castro, A.J.G.; da Silva Nascimento Santos, M.; Soriano, E.M.; Leite, E.L. Potential anti-angiogenic, antiproliferative, antioxidant, and anticoagulant activity of anionic polysaccharides, fucans, extracted from brown algae Lobophora variegata. J. Appl. Phycol. 2015, 27, 1315–1325. [Google Scholar] [CrossRef]
- Abd-Ellatef, G.E.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.-H.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer Targets Ther. 2017, 9, 67. [Google Scholar] [CrossRef]
- Zainal Ariffin, S.H.; Yeen, W.W.; ZainolAbidin, I.Z.; Megat Abdul Wahab, R.; Zainal Ariffin, Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement. Altern. Med. 2014, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, Z.J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and potential antitumor activity of polysaccharide from Gracilariopsis lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.A.R.; Dore, C.M.P.; Castro, A.J.; de Azevedo, T.C.; de Oliveira, M.T.B.; Maria de Fátima, V.M.; Benevides, N.M.; Leite, E.L. Galactans from the red seaweed Amansia multifida and their effects on inflammation, angiogenesis, coagulation and cell viability. Biomed. Prev. Nutr. 2012, 2, 154–162. [Google Scholar] [CrossRef]
- Ropellato, J.; Carvalho, M.M.; Ferreira, L.G.; Noseda, M.D.; Zuconelli, C.R.; Gonçalves, A.G.; Ducatti, D.R.; Kenski, J.C.; Nasato, P.L.; Winnischofer, S.M.; et al. Sulfated heterorhamnans from the green seaweed Gayralia oxysperma: Partial depolymerization, chemical structure and antitumor activity. Carbohydr. Polym. 2015, 117, 476–485. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure–function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Li, G.; Tian, C.; Zhang, T. Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin. Herb. Med. 2022, 14, 528–534. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The seaweed diet in prevention and treatment of the neurodegenerative diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother. Res. 2013, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Banskota, A.H.; Critchley, A.T.; Hafting, J.; Prithiviraj, B. Neuroprotective effects of the cultivated Chondrus crispus in a C. elegans model of Parkinson’s disease. Mar. Drugs 2015, 13, 2250–2266. [Google Scholar] [CrossRef]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl-and butyryl-cholinesterase inhibitory activities of the edible brown alga Eiseniabicyclis. Arch. Pharm. Res. 2015, 38, 1477–1487. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl-and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivative of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Q.; Peng, J.; Jin, L.; Zhu, X.; Zheng, D.; Zhang, Y.; Wang, R.; Song, Y.; Hu, W.; et al. Fucoxanthin regulates Nrf2 signaling to decrease oxidative stress and improves renal fibrosis depending on Sirt1 in HG-induced GMCs and STZ-induced diabetic rats. Eur. J. Pharmacol. 2021, 913, 174629. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Kolender, A.A.; Matulewicz, M.C.; Cerezo, A.S. Structural analysis of antiviral sulfated α-D-(1→3)-linked mannans. Carbohydr. Res. 1995, 273, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Neyts, J.; Pujol, C.A.; Snoeck, R.; Andrei, G.; Ikeda, S.; Witvrouw, M.; Reymen, D.; Haines, H.; Matulewicz, M.C.; et al. Antiviral activity of a sulphated polysaccharide from the red seaweed Nothogenia fastigiata. Biochem. Pharmacol. 1994, 47, 2187–2192. [Google Scholar] [CrossRef]
- Witvrouw, M.; Este, J.A.; Mateu, M.Q.; Reymen, D.; Andrei, G.; Snoeck, R.; Ikeda, S.; Pauwels, R.; Bianchini, N.V.; Desmyter, J.; et al. Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antivir. Chem. Chemother. 1994, 5, 297–303. [Google Scholar] [CrossRef]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef]
- Gomaa, H.H.; Elshoubaky, G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 34–42. [Google Scholar]
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Trejo-Avila, L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cirne-Santos, C.C.; Barros, C.D.S.; Nogueira, C.C.R.; Azevedo, R.C.; Yamamoto, K.A.; Meira, G.L.S.; Vasconcelos, Z.F.M.D.; Ratcliffe, N.A.; Teixeira, V.L.; Schmidt-Chanasit, J.; et al. Inhibition by marine algae of chikungunya virus isolated from patients in a recent disease outbreak in Rio de Janeiro. Front. Microbiol. 2019, 10, 2426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The inhibitory effects and mechanisms of 3, 6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018, 198, 329–338. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. 3 Biotech. 2022, 12, 154. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Arunkumar, M.; Aravind, M.K.; Gayathri, S.; Rajkeerthana, S.; Mohankumar, V.; Varalakshmi, P. Probing marine brown macroalgal phlorotannins as antiviral candidate against SARS-CoV-2: Molecular docking and dynamics simulation approach. Mol. Divers 2022, 26, 3205–3224. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Jena, M. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551. [Google Scholar] [CrossRef]
- Ambika, S.; Sujatha, K. Antifungal activity of aqueous and ethanol extracts of seaweeds against sugarcane red rot pathogen (Colletotrichum falcatum). Sci. Res. Essays 2015, 10, 232–235. [Google Scholar] [CrossRef]
- Lotfi, A.; Kottb, M.; Elsayed, A.; Shafik, H. Antifungal activity of some Mediterranean seaweed against Macrophomina phaseolina and Fusarium oxysporum in Vitro. Alfarama J. Basic Appl. Sci. 2021, 2, 81–96. [Google Scholar] [CrossRef]
- El-Bilawy, E.H.; Al-Mansori, A.N.A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Teiba, I.I.; Sabry, A.E.N.; Elsharkawy, M.M.; Heflish, A.A.; Behiry, S.I.; et al. Antiviral and Antifungal of Ulva fasciata Extract: HPLC Analysis of Polyphenolic Compounds. Sustainability 2022, 14, 12799. [Google Scholar] [CrossRef]
- El Barky, A.R.; Ezz, A.A.H.; Alm-Eldeen, A.A.-E.; Hussein, S.A.; Hafez, Y.A.; Mohamed, T.M. Can stem cells ameliorate the pancreatic damageinduced by streptozotocin in rats? Can. J. Diabetes 2018, 42, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, O.H.; Abo-Shady, A.M.; Ismail, G.A.; Gheda, S.F. Potential effect of Turbinariadecurrens acetone extract on the biochemical and histological parameters of alloxan-induced diabetic rats. Int. J. Environ. Health Res. 2022, 32, 1447–1468. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Singh, V.; Chauhan, K. Antidiabetic potential of seaweed and their bioactive compounds: A review of developments in last decade. Crit. Rev. Food Sci. Nutr. 2022, 63, 5739–5770. [Google Scholar] [CrossRef]
- García, V.; Uribe, E.; Vega Gálvez, A.; Delporte Vergara, C.; Valenzuela Barra, G.; López, J.; Pastén, A. Health-promoting activities of edible seaweed extracts from Chilean coasts: Assessment of antioxidant, anti-diabetic, anti-inflammatory and antimicrobial potential. Rev. Chil. Nutr. 2020, 47, 792–800. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive polyphenols from southern Chile seaweed as inhibitors of enzymes for starch digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef]
- Shafay, S.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, antidiabetic, anti-inflammatory and anticancer potential of some seaweed extracts. Food Sci. Technol. 2021, 42, e20521. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padinatetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Jia, R.B.; Wu, J.; Li, Z.R.; Ou, Z.R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Comparison of physicochemical properties and antidiabetic effects of polysaccharides extracted from three seaweed species. Int. J. Biol. Macromol. 2020, 149, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, Y.; Famurewa, A.C.; Olatunji, O.J. Antidiabetic and nephroprotective effects of polysaccharide extract from the seaweed Caulerpa racemosa in high fructose-streptozotocin induced diabetic nephropathy. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, ume 14, 2121–2131. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassumangustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef]
- Awang, A.N.; Ng, J.L.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B. Anti-obesity property of the brown seaweed, Sargassum polycystum using an in vivo animal model. J. Appl. Phycol. 2014, 26, 1043–1048. [Google Scholar] [CrossRef]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef]
- Lee, H.G.; Lu, Y.A.; Li, X.; Hyun, J.M.; Kim, H.S.; Lee, J.J.; Kim, T.H.; Kim, H.M.; Kang, M.C.; Jeon, Y.J. Anti-Obesity effects of Grateloupiaelliptica, a red seaweed, in mice with high-fat diet-induced obesity via suppression of adipogenic factors in white adipose tissue and increased thermogenic factors in brown adipose tissue. Nutrients 2020, 12, 308. [Google Scholar] [CrossRef]
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.M.; Kim, H.S.; Kim, T.H.; Kim, H.M.; Lee, J.J.; Kang, M.C.; Jeon, Y.J. Anti-obesity effects of red seaweed, Plocamiumtelfairiae, in C57BL/6 mice fed a high-fat diet. Food Funct. 2020, 11, 2299–2308. [Google Scholar] [CrossRef]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Jaworowska, A.; Murtaza, A. Seaweed derived lipids are a potential anti-inflammatory agent: A review. Int. J. Environ. Res. Public Health 2022, 20, 730. [Google Scholar] [CrossRef]
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive andanti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Lima, V.; Holanda, M.L.; Pinheiro, P.G.; Rodrigues, J.A.; Lima, M.E.; Benevides, N.M. Antinociceptive and anti-inflammatory activities oflectin from marine red alga Pterocladiella capillacea. Biol. Pharm. Bull. 2010, 33, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.G.; Bitencourt, F.S.; Cunha, T.M.; Luz, P.B.; Nascimento, K.S.; Mota, M.R.; Sampaio, A.H.; Cavada, B.S.; Cunha, F.Q.; Alencar, N.M. Agglutinin isolated from the red marine alga Hypnea cervicornis J. Agardh reduces inflammatory hypernociception: Involvement of nitric oxide. Pharmacol. Biochem. Behav. 2010, 96, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Hwang, H.J.; Kim, I.H.; Nam, T.J. A glycoprotein from Porphyra yezoensis produces anti-inflammatory effects in liposaccharide-stimulated macrophages via the TLR4 signaling pathway. Int. J. Mol. Med. 2011, 28, 809–815. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activityof edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Kwon, M.-S.; Choi, J.-W.; Shin, T.; No, H.K.; Choi, J.-S.; Byun, D.-S.; Kim, J.-I.; Kim, H.-R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Lee, S.-H.; Park, P.-J.; Kang, D.-H.; Oh, C.; Kim, D.-W.; Han, J.-S.; Jeon, Y.-J.; et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2010, 48, 1448–1454. [Google Scholar] [CrossRef]
- Manzoor, Z.; Mathema, V.B.; Chae, D.; Kang, H.K.; Yoo, E.S.; Jeon, Y.J.; Koh, Y.S. Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-kappaB pathways. Biosci. Biotechnol. Biochem. 2013, 77, 1970–1972. [Google Scholar] [CrossRef]
- Heras, B.; Hortelano, S. Molecular Basis of the Anti-Inflammatory Effects of Terpenoids. Inflamm. Allergy Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Mishra, A. Algal Functional Foods and Nutraceuticals: Benefits, Opportunities, and Challenges; Bentham Science Publishers: Singapore, 2022; 540p. [Google Scholar] [CrossRef]
- Bouga, M.; Combet, E. Emergence of seaweed and seaweed-containing foods in the UK: Focus on labeling, iodine content, toxicity and nutrition. Foods 2015, 4, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhuang, D.; Chew, K.W.; Ling, T.C.; Khoo, K.S.; Van Quyen, D.; Feng, S.; Show, P.L. Current status and future trends in removal, control, and mitigation of algae food safety risks for human consumption. Molecules 2022, 27, 6633. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Akama, A. Radioactive contamination of aquatic insects in a stream impacted by the fukushima nuclear power plant accident. Hydrobiologia 2014, 722, 19–30. [Google Scholar] [CrossRef]
- Kawai, H.; Kitamura, A.; Mimura, M.; Mimura, T.; Tahara, T.; Aida, D.; Sato, K.; Sasaki, H. Radioactive cesium accumulation in seaweeds by the fukushima 1 nuclear power plant accident-two years’ monitoring at iwaki and its vicinity. J. Plant Res. 2014, 127, 23–42. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the commercial seaweed nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, L.; Wu, M.; Yu, H.; Shang, T.; Zhang, T.; Zhao, M. Total and inorganic arsenic contents in seaweeds: Absorption, accumulation, transformation and toxicity. Aquaculture 2018, 497, 49–55. [Google Scholar] [CrossRef]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef]
- de Oliveira, M.N.; Freitas, A.L.P.; Carvalho, A.F.U.; Sampaio, T.M.T.; Farias, D.F.; Teixeira, D.I.A.; Gouveia, S.T.; Pereira, J.G. Nutritive and non-nutritive attributes of washed-up seaweeds from the coast of Ceará, Brazil. Food Chem. 2009, 115, 254–259. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.R.; Oh, S.; Paik, M.J.; Je, J.G.; Heo, J.H.; Lee, T.K.; Fu, X.; Xu, J.; Gao, X.; et al. Arsenic removal from the popular edible seaweed Sargassum fusiforme by sequential processing involving hot water, citric acid, and fermentation. Chemosphere 2022, 292, 133409. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Concepcion, A.; Duinker, A.; Tiwari, B.; Elvevoll, E.O.; Yukawa, G.; Levine, I.A.; Banach, J.L.; Sato, J.; Hayes, M.; et al. Report of the expert meeting on food safety for seaweed–Current status and future perspectives. Food and Agriculture Organization of the United Nations (FAO). In Food Safety and Quality Series; FAO: Roma, Italy; WHO: Geneva, Switzerland, 2022. [Google Scholar]
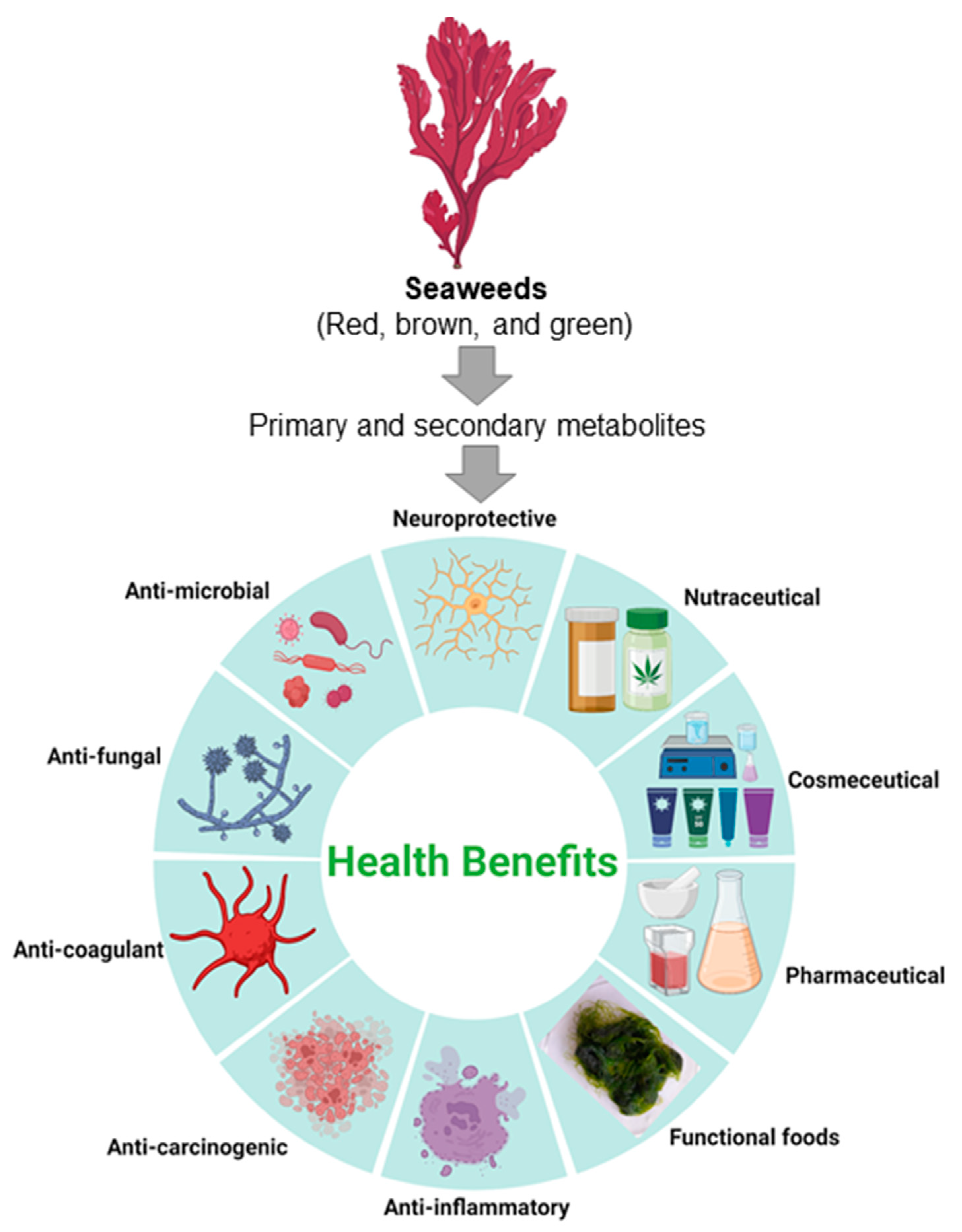

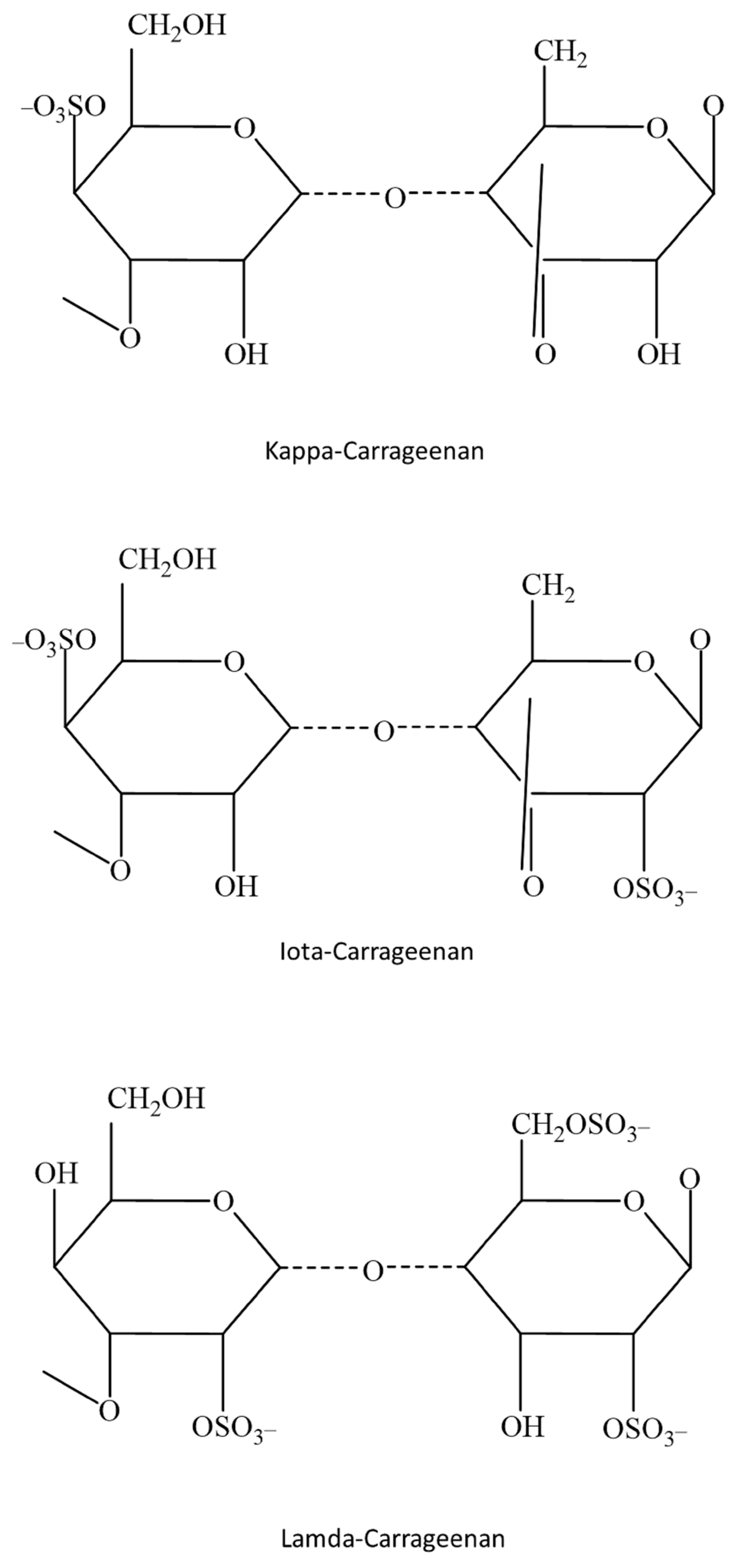
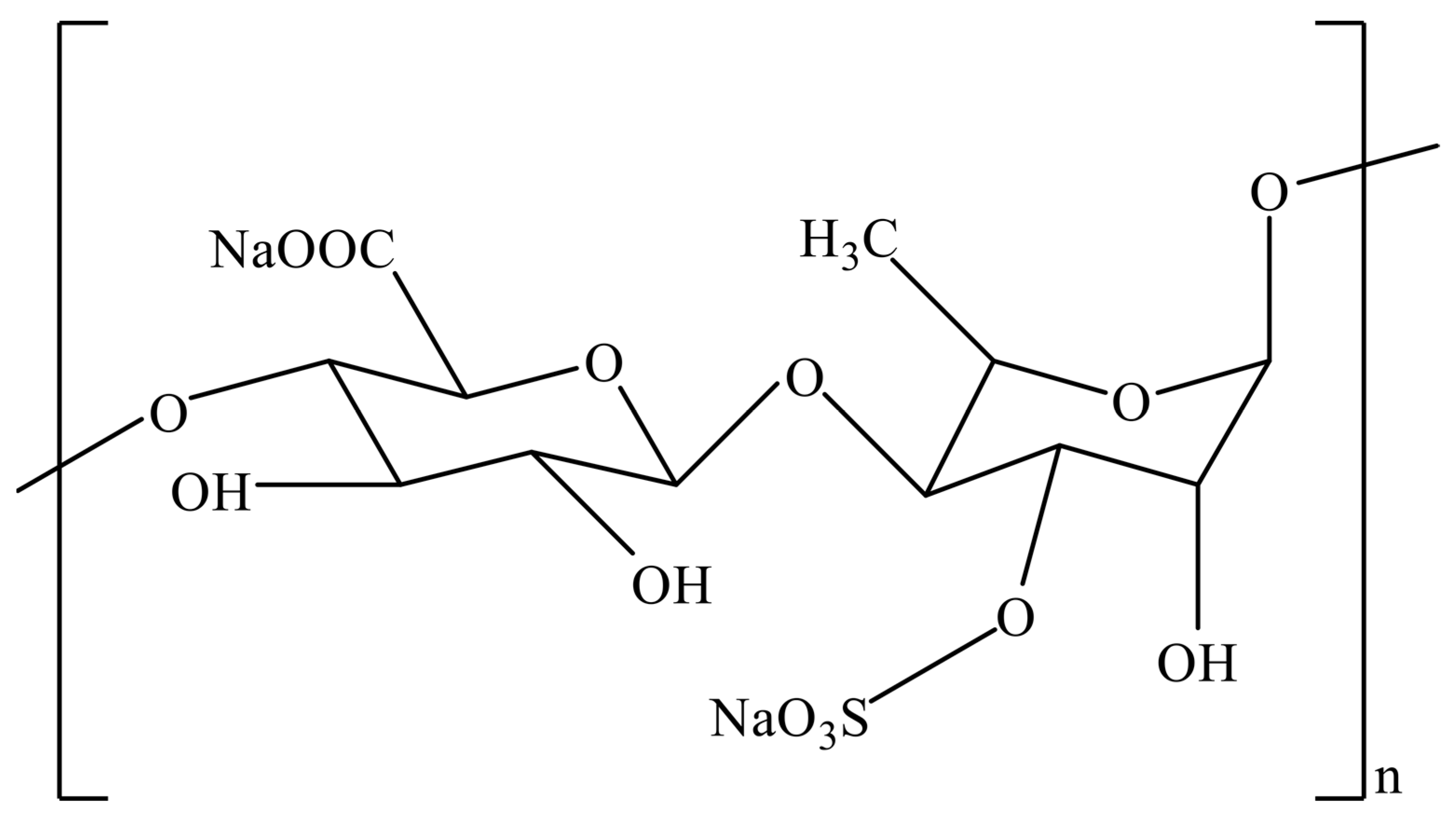
| No. | Species | Key Minerals in Selected Seaweeds | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca (mg/ 100 g DW) | Mg (mg/ 100 g DW) | K (mg/ 100 g DW) | Na (mg/ 100 g DW) | Cu (mg/100 g DW) | Zn (mg/100 g DW) | Fe (mg/ 100 gDW) | I (mg/100 g DW) | |||
| 1 | Padina australis | 28.3 | 4 | 0.5 | 1 | 0.005 | 0.013 | 0.446 | - | [26] |
| 2 | Fucus vesiculosus | 938 | 994 | 4322 | 5469 | <0.5 | 3.71 | 4.2 | - | [26] |
| 3 | Laminaria digitata | 1005 | 659 | 11,579 | 3818 | <0.5 | 1.77 | 3.29 | - | [27] |
| 4 | Chondrus crispus | 420 | 732 | 3184 | 4270 | <0.5 | 7.14 | 3.97 | - | [27] |
| 5 | Porphyra tenera | 390 | 565 | 3500 | 3627 | <0.5 | 2.21 | 10.3 | - | [27] |
| 6 | Caulerpa racemosa | - | - | - | - | 5.35 | 2.96 | 404.94 | - | [28] |
| 7 | Ulva lactuca CRM 279 | - | - | - | - | 13.83 | 76.88 | 883.28 | - | [28] |
| 8 | Colpomenia sinuosa | 5226.9 | - | 3510 | 423.83 | 0.94 | - | 1581 | - | [29] |
| 9 | Dictyota dichotoma | 3256.5 | - | 3417.01 | 633.85 | 1.29 | - | 1196 | - | [29] |
| 10 | Caulerpa lentillifera | 1874 | 1029 | 1143 | 8917 | 0.11 | 3.5 | 21.4 | - | [30] |
| 11 | Porphyra columbina | 443.7 | 491.53 | 1444.17 | 414.22 | 0.51 | 1.46 | 22.0 | - | [31] |
| 12 * | Halimeda opuntia | - | 20.14 | 91.74 | 34.76 | 8.93 | 0.72 | 19.88 | - | [32] |
| 13 * | Kappaphycus alvarezii | - | 90.5 | 672 | 25.66 | 5.25 | 7.75 | 8.15 | - | [32] |
| 14 ** | Ascophyllum nodosum | 575 | 225.0 | 765.0 | 1173.8 | 0.8 | - | 14.9 | 18.2 | [33] |
| 15 ** | Undaria pinnatifida | 112.3 | 78.7 | 62.4 | 448.7 | 0.2 | 0.3 | 3.9 | 3.9 | [33] |
| 16 ** | Chondrus crispus | 373.8 | 573.8 | 827.5 | 1572.5 | 0.1 | - | 6.6 | 6.1 | [33] |
| 17 *** | Eucheuma cottonii | 329.69 | 271.33 | 13,155.2 | 1771.84 | 0.03 | 4.30 | 2.61 | 9.42 | [34] |
| 18 *** | Caulerpa lentillifera | 1874.74 | 1028.6 | 1142.68 | 8917.46 | 0.11 | 3.51 | 21.37 | 4.78 | [34] |
| 19 *** | Sargassum polycystum | 3792.06 | 487.81 | 8371.23 | 1362.13 | 0.03 | 2.15 | 68.21 | 7.66 | [34] |
| 20 | Ulva intestinalis | 794.5 | 4115.2 | 2456.8 | 1711.9 | 0.6 | 1.5 | - | - | [35] |
| Seaweed Species | Polysaccharide | Activities | References |
|---|---|---|---|
| Undaria pinnatifida, Fucus vesiculosus | Fucoidan | Anticancer activity against MCF-7 and ZR-75D breast cancer | [169] |
| Sargassum polycystum | Fucoidan | Antioxidant and Anticancer activities | [170] |
| Scytosiphon lomentaria | Fucoidan | Antiviral activity against HSV-1 and HSV-2 | [171] |
| Laminaria japonica | Fucoidan | Anticoagulant and Antithrombotic activities | [172,173] |
| Hizikia fusiforme, Laminaria japonica | Fucoidan | Antioxidant activity | [85,174] |
| Eclonia cava, Sargassum hornery, Costaria costata, Cladosiphon okamuranus | Fucoidan | Antitumor activity | [175,176,177] |
| Sargassum wightii, Undaria pinnatifida | Fucoidan | Immunomodulatory activity | [178,179] |
| Ecklonia cava, Lessonia vadosa | Fucoidan | Anticoagulant activity | [180,181] |
| Chondracanthus teedei var. lusitanicus | Carrageenan | Antifungal activity | [182] |
| Laurencia papillosa, Ascophyllum nodosum, Undaria pinnatifida | Carrageenan | Antiproliferative activity | [183,184] |
| Eucheuma Cottonii | Carrageenan | Wound healing | [185] |
| Lomentaria catenata | Carrageenan | Anticoagulant activity | [186] |
| Solieria filiformis | Carrageenan | Herpes simplex virus type 1 (HSV-1) and Antioxidant activities | [187] |
| Chondrus ocellatus | Carrageenan | Antitumor and Immunomodulation activities | [188] |
| Kappaphycus alvarezii, Portieria hornemannii, Spyridia hypnoides, Asparagopsis taxiformis, Centroceras clavulatum, Padina pavonica | Carrageenan | Antioxidant, Anticancer, and Antidiabetic activities | [189,190,191] |
| Hypnea valentiae | Carrageenan | Antimicrobial, Antioxidant, and Anticoagulant activities | [192] |
| Ulva lactuca | Ulvan | Antioxidant properties, Cytotoxic activity Anti-proliferative and Apoptotic effects | [193,194,195] |
| Ulva armoricana | Ulvan | Antiviral and Antioxidant activities | [196] |
| Ulva fasciata | Ulvan | Anti-hypercholesterolemic and Antidiabetic activities | [197] |
| Capsosiphon fulvescens, Ulva fasciata, Enteromorpha prolifera | Ulvan | Anti-coagulant activity | [198,199,200] |
| Ulva reticulate, Ulva armoricana | Ulvan | Antimicrobial activity | [201,202] |
| Ulvaintestinalis, Ulva pertusa, Ulva intestinalis | Ulvan | Immunomodulatory activity | [203,204,205] |
| Ulva pertusa, Enteromorpha linza | Ulvan | Antioxidant activity | [206,207] |
| Ulva pertusa, Enteromorpha prolifera | Ulvan | Antioxidant and Antihyperlipidemic activities | [208,209,210,211] |
| Enteromorpha intestinalis, Ulva fasciata | Ulvan | Anticancer activities | [212,213] |
| Seaweeds | Proteins and Peptides | Biological Activity | References |
|---|---|---|---|
| Codium decorticatum | Glycoprotein | Anti-cancer activities | [214] |
| Caulerpa cupressoides | Lectin | Anti-nociceptive and anti-inflammatory | [215] |
| Capsosiphon fulvescens | Glycoprotein | Anti-aging agent | [216] |
| Porphyra yezoensis | Peptide | Anticoagulant activity | [217] |
| Halimeda renschii | Glycoprotein | Antiviral activity | [113] |
| Saccharina longicruris | Peptides | Antibacterial | [218] |
| Solieria filiformis | Lectin | Anticancer activity | [112] |
| Cratylia floribunda, Vatairea macrocarpa, Bauhinia bauhinioides, Bryothamnion seaforthii & Hypnea musciformis | Lectin | Antimicrobial activity | [219] |
| Pyropia columbina | Peptides | Antioxidant and ACE I inhibitory | [220] |
| Undaria pinnatifida | Glycoprotein | Anti-Alzheimer’s and anti-inflammatory activities | [221] |
| Fucus serratus & Fucus vesiculosus | Glycoprotein | Antioxidant activity | [222] |
| Porphyra yezoensis | Peptides | Anticancer activity | [223] |
| Saccharina japonica | Glycoprotein | Probiotic properties | [224] |
| Laminaria japonica | Glycoprotein | Anticancer activity | [225] |
| Palmaria palmata | Protein fraction | Cardioprotective, anti-diabetic, and antioxidant activity | [226] |
| Seaweed | Lipid/Fatty Acid | Biological Activity | References |
|---|---|---|---|
| Gracilaria vermiculophylla, Porphyra dioica and Chondrus crispus | Palmitic acid (16:0)-(PUFA); monounsaturated fatty acids (MUFAs) | Antimicrobial activity | [227] |
| Ulva linza, Sargassum vulgar, and Gracilaria corticata | C18:1n-9- MUFA and C18:4-PUFA | Antibacterial activity | [228] |
| Undaria pinnatifida | Omega-3 PUFA | Antioxidant activity | [229] |
| Ulva lactuca | Unsaturated fatty acid | Chemo-preventive | [230] |
| Ulva armoricana and Solieria chordalis | 6:4n-3, 18:4n-3, 18:2n-3, 18:2n-6, 22:6n-3, 20:4n-6, and 20:5n-3 | Antiproliferative activity | [231] |
| C. racemosa, S. tenerrimum, C. indica and C. veravalnensis | Palmitic acid (C16:0)-PUFAs | Antioxidant activity | [6] |
| Spatoglossum asperum | Mixture of fatty acids | Antifungal and nematicidal activities | [232] |
| Enteromorpha linza | Stearidonic acid (SA, C18:4 n-3) and gamma-linolenic acid (GLA, C18:3 n-6) | Antimicrobial activity | [233] |
| Ceramium rubrum | Mixture of fatty acids | Antibacterial and antifungal activities | [234] |
| Kappaphycus alvarezii | Levoglucosenone, 4-Pyridinemethanol, and Hexamethyl- cyclotrisiloxane | Antioxidant and antibacterial activity | [235] |
| Palmaria palmata, Laminaria digitata, Saccharina latissima, and Ascophyllum nodosum | Stearidonic acid, Eicosapentaenoic acid, Alpha-Linolenic acid, Docosahexaenoic acid, and Arachidonic acid | Anti-parasitic | [236] |
| Ulva lactuca, Sargassum tenerrimum, and Laurencia obtusa | Fatty acids and esters of fatty acids | Antifungal activity | [237] |
| C. vagabunda, C. virgatum, and U. intestinalis | Palmitic acid (C16:0), arachidonic acid (C20:4n-6), and oleic acid (C18:1ω-9cis) | Antimicrobial activity | [238] |
| Tydemania expeditionis | 3(ζ)-hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid (1) and 3(ζ)-hydroxy-hexadeca-4(E),6(Z)-dienoic acid | Antitumor activity | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods 2023, 12, 3642. https://doi.org/10.3390/foods12193642
Baghel RS, Choudhary B, Pandey S, Pathak PK, Patel MK, Mishra A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods. 2023; 12(19):3642. https://doi.org/10.3390/foods12193642
Chicago/Turabian StyleBaghel, Ravi S., Babita Choudhary, Sonika Pandey, Pradeep Kumar Pathak, Manish Kumar Patel, and Avinash Mishra. 2023. "Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals" Foods 12, no. 19: 3642. https://doi.org/10.3390/foods12193642
APA StyleBaghel, R. S., Choudhary, B., Pandey, S., Pathak, P. K., Patel, M. K., & Mishra, A. (2023). Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods, 12(19), 3642. https://doi.org/10.3390/foods12193642