Effect of Soy Wax/Rice Bran Oil Oleogel Replacement on the Properties of Whole Wheat Cookie Dough and Cookies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Oleogel
2.2.2. Cookie Dough and Cookies Preparation
Cookie Dough Preparation
Preparation of Cookies
2.3. Analysis of the Cookie Dough
2.3.1. Microscopic Analysis
2.3.2. Moisture Content
2.3.3. FTIR
2.3.4. Texture Analysis
2.3.5. Impedance
2.4. Analysis of the Cookies
2.4.1. Surface Topology of Cookies
2.4.2. Physical Dimension of Cookies
2.4.3. Moisture Content of Cookies
2.4.4. FTIR of Cookies
2.4.5. Texture Analysis of Cookies
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Dough
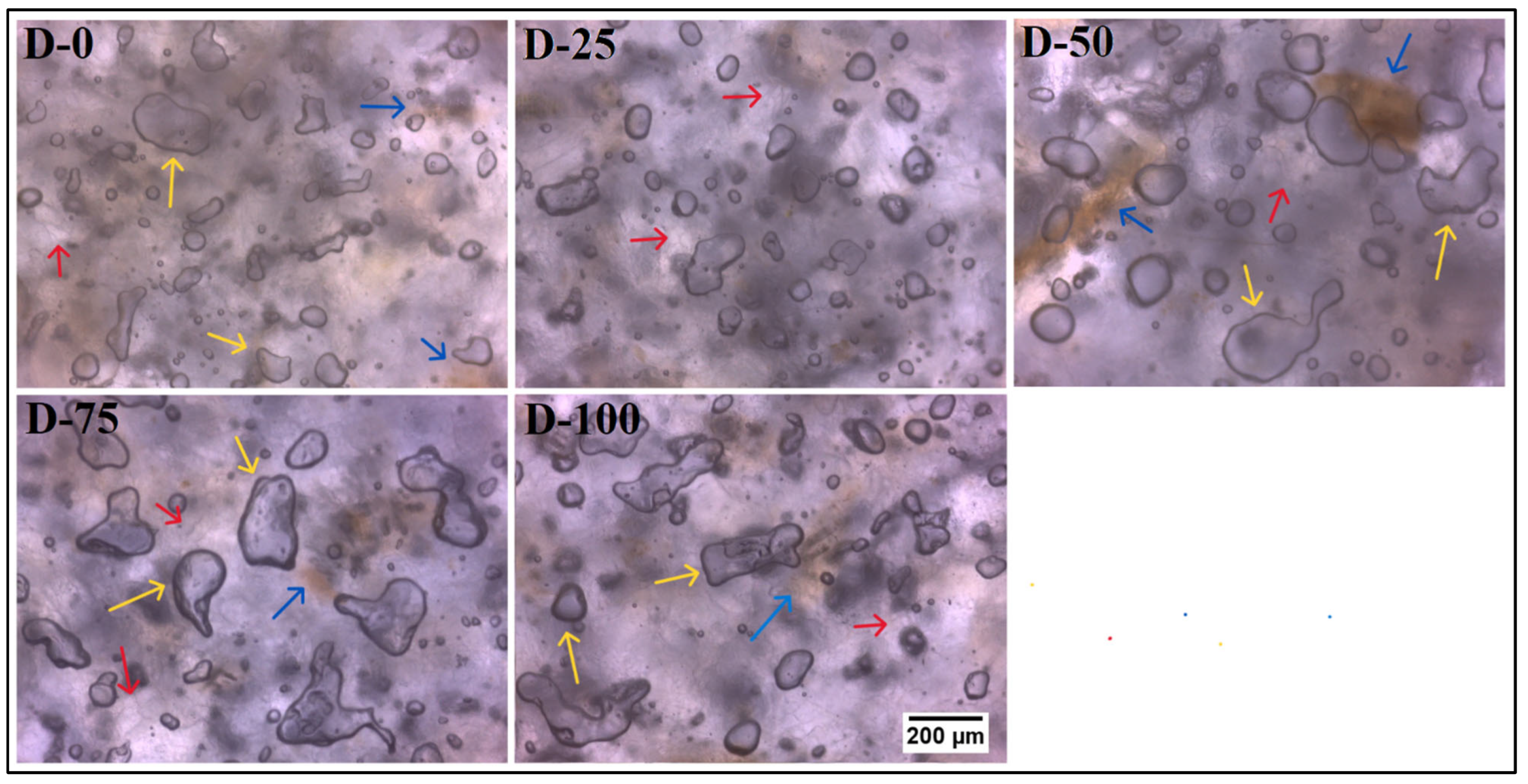
3.1.1. Microscopic Analysis
Brightfield Microscopy
Confocal Laser Scanning Microscopy (CLSM)
3.1.2. Moisture Analysis
3.1.3. FTIR Analysis of Dough
3.1.4. Texture Analysis of Dough
3.1.5. Impedance Analysis
3.2. Analysis of Cookies
3.2.1. Visual Appearance of Cookies
3.2.2. Surface Topology of Cookies
3.2.3. Physical Dimension
3.2.4. Moisture Content of Cookies
3.2.5. FTIR of Cookies
3.2.6. Texture Analysis of Cookies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seal, C.; Courtin, C.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Liou, L.; Kaptoge, S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241993. [Google Scholar] [CrossRef]
- Cybulska, B.; Kłosiewicz-Latoszek, L. Landmark studies in coronary heart disease epidemiology. The Framingham Heart Study after 70 years and the Seven Countries Study after 60 years. Kardiol. Pol. Pol. Heart J. 2019, 77, 173–180. [Google Scholar] [CrossRef]
- Ray, S. Global Nutrition Report 2021—India’s Nutrition Profile and How to Meet Global Nutrition Target. 2021. Available online: https://globalnutritionreport.org/reports/2021-global-nutrition-report/ (accessed on 26 September 2023).
- Manzoor, S.; Masoodi, F.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Demirci, M.; Lee, C.-C.; Çavuş, M.; Çağlar, M.Y. Oleogels for food applications. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 781–811. [Google Scholar]
- Lindoso, J.V.D.S.; Alencar, S.R.; Santos, A.A.D.; Mello Neto, R.S.; Mendes, A.V.D.S.; Furtado, M.M.; Silva, M.G.D.; Brito, A.K.D.S.; Batista, E.K.F.; Baêta, S.A.F.; et al. Effects of “Bacuri” Seed Butter (Platonia insignis Mart.), a Brazilian Amazon fruit, on oxidative stress and diabetes mellitus-related parameters in STZ-Diabetic Rats. Biology 2022, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Derewiaka, D.; Florowska, A.; Szymańska, I.J.A.S. Comparison of the spreadability of butter and butter substitutes. Appl. Sci. 2023, 13, 2600. [Google Scholar] [CrossRef]
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil: A review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Sowiński, M.; Szymańska, I.; Żbikowska, K.; Marciniak-Łukasiak, K.; Werpachowski, W. Analysis of stability, rheological and structural properties of oleogels obtained from peanut oil structured with yellow beeswax. Gels 2022, 8, 448. [Google Scholar] [CrossRef]
- Pang, M.; Kang, S.; Liu, L.; Ma, T.; Zheng, Z.; Cao, L.J.G. Physicochemical properties and cookie-making performance as fat replacer of wax-based rice bran oil oleogels. Gels 2022, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Alongi, M.; Lucci, P.; Anese, M. Effect of different oleogelators on lipolysis and curcuminoid bioaccessibility upon in vitro digestion of sunflower oil oleogels. Food Chem. 2020, 314, 126146. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in microstructural and physicochemical properties of candelilla wax/rice bran oil–derived oleogels using sunflower lecithin and soya lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Dhal, S.; Alhamidi, A.; Al-Zahrani, S.M.; Anis, A.; Pal, K. The Influence of Emulsifiers on the Physiochemical Behavior of Soy Wax/Rice Bran Oil-Based Oleogels and Their Application in Nutraceutical Delivery. Gels 2023, 9, 47. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Zbikowska, K.; Kowalska, M.; Kowalska, H.; Rutkowska, J. Study on the introduction of solid fat with a high content of unsaturated fatty acids to gluten-free muffins as a basis for designing food with higher health value. Int. J. Mol. Sci. 2021, 22, 9220. [Google Scholar] [CrossRef]
- Jekle, M.; Becker, T. Dough microstructure: Novel analysis by quantification using confocal laser scanning microscopy. Food Res. Int. 2011, 44, 984–991. [Google Scholar] [CrossRef]
- Ahmad, S.; Naz, A.; Usman, M.; Amjad, A.; Pasha, I.; Farooq, U. Impediment effect of chemical agents (additives) on gluten development in cookie dough. J. Food Sci. Technol. 2022, 59, 1396–1406. [Google Scholar] [CrossRef]
- Bhat, N.A.; Wani, I.A.; Hamdani, A.M.; Gani, A.; Masoodi, F. Physicochemical properties of whole wheat flour as affected by gamma irradiation. LWT-Food Sci. Technol. 2016, 71, 175–183. [Google Scholar] [CrossRef]
- Garcia-Valle, D.E.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effects of mixing, sheeting, and cooking on the starch, protein, and water structures of durum wheat semolina and chickpea flour pasta. Food Chem. 2021, 360, 129993. [Google Scholar] [CrossRef]
- Tebben, L.; Li, Y. Effect of xanthan gum on dough properties and bread qualities made from whole wheat flour. Cereal Chem. 2019, 96, 263–272. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, E.; Zhang, T.; Li, M.; Bian, K. Influence of water addition methods on water mobility characterization and rheological properties of wheat flour dough. J. Cereal Sci. 2019, 89, 102791. [Google Scholar] [CrossRef]
- Alam, S.S.; Bharti, D.; Pradhan, B.K.; Sahu, D.; Dhal, S.; Kim, N.M.; Jarzębski, M.; Pal, K. Analysis of the Physical and Structure Characteristics of Reformulated Pizza Bread. Foods 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.F.R.; Sahagún, M.; Ribotta, P.D.; Ferrero, C.; Gómez, M. Particle size and hydration properties of dried apple pomace: Effect on dough viscoelasticity and quality of sugar-snap cookies. Food Bioprocess Technol. 2019, 12, 1083–1092. [Google Scholar] [CrossRef]
- Rojhani, A.; Naranjo, J.; Ouyang, P. Physiochemical properties and sensory characteristics of resistant starch enriched cookies. Nutr. Food Sci. 2022, 52, 791–800. [Google Scholar] [CrossRef]
- Korese, J.K.; Chikpah, S.K.; Hensel, O.; Pawelzik, E.; Sturm, B. Effect of orange-fleshed sweet potato flour particle size and degree of wheat flour substitution on physical, nutritional, textural and sensory properties of cookies. Eur. Food Res. Technol. 2021, 247, 889–905. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Chanona-Perez, J.J.; Necoechea-Mondragon, H. Comparison of crumb microstructure from pound cakes baked in a microwave or conventional oven. LWT Food Sci. Technol. 2008, 41, 620–627. [Google Scholar]
- Sim, S.Y.J.; Wong, K.X.; Henry, C.J. Healthier pineapple tart pastry using oleogel-based solid fat replacement. Mal. J. Nutr. 2021, 27, 2. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef]
- Oterhals, Å.; Ahmad, R.; Samuelsen, T.A. A novel approach to determine optimal protein texturization conditions—A critical moisture level with increased effect of temperature on viscosity reduction in the rubbery state. J. Food Eng. 2019, 261, 66–75. [Google Scholar] [CrossRef]
- Kannan, P.; Paramasivan, M.; Marimuthu, S.; Swaminathan, C.; Bose, J. Applying both biochar and phosphobacteria enhances Vigna mungo L. growth and yield in acid soils by increasing soil pH, moisture content, microbial growth and P availability. Agric. Ecosyst. Environ. 2021, 308, 107258. [Google Scholar]
- Sert, D.; Mercan, E. Characterisation of physicochemical, microbiological, thermal, oxidation properties and fatty acid composition of butter produced from thermosonicated cream. Int. Dairy J. 2020, 109, 104777. [Google Scholar] [CrossRef]
- Fanari, F.; Carboni, G.; Desogus, F.; Grosso, M.; Wilhelm, M. A chemometric approach to assess the rheological properties of durum wheat dough by indirect FTIR measurements. Food Bioprocess Technol. 2022, 15, 1040–1054. [Google Scholar] [CrossRef]
- Pezzana, L.; Melilli, G.; Guigo, N.; Sbirrazzuoli, N.; Sangermano, M. Cross-Linking of Biobased Monofunctional Furan Epoxy Monomer by Two Steps Process, UV Irradiation and Thermal Treatment. Macromol. Chem. Phys. 2023, 224, 2200012. [Google Scholar] [CrossRef]
- Naseer, K.; Ali, S.; Mubarik, S.; Hussain, I.; Mirza, B.; Qazi, J. FTIR spectroscopy of freeze-dried human sera as a novel approach for dengue diagnosis. Infrared Phys. Technol. 2019, 102, 102998. [Google Scholar] [CrossRef]
- Zheng, M.; Lin, Y.; Wu, H.; Zeng, S.; Zheng, B.; Zhang, Y.; Zeng, H. Water migration depicts the effect of hydrocolloids on the structural and textural properties of lotus seed starch. Food Chem. 2020, 315, 126240. [Google Scholar] [CrossRef]
- Walrafen, G. Water: A Comprehensive Treatise; Franks, F., Ed.; Prenum Press: New York, NY, USA, 1972; Volume 1, p. 151. [Google Scholar]
- Laurson, P.; Raudsepp, P.; Kaldmäe, H.; Kikas, A.; Mäeorg, U. The deconvolution of FTIR-ATR spectra to five Gaussians for detection of small changes in plant-water clusters. AIP Adv. 2020, 10, 085214. [Google Scholar] [CrossRef]
- Alvarez-Ramirez, J.; Carrera-Tarela, Y.; Carrillo-Navas, H.; Vernon-Carter, E.; Garcia-Diaz, S. Effect of leavening time on LAOS properties of yeasted wheat dough. Food Hydrocoll. 2019, 90, 421–432. [Google Scholar] [CrossRef]
- Chen, G.; Hu, R.; Li, Y. Potassium bicarbonate improves dough and cookie characteristics through influencing physicochemical and conformation properties of wheat gluten. Food Chem. X 2020, 5, 100075. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Wang, S.-L.; Luo, R.-M. Evaluation of key aroma compounds and protein secondary structure in the roasted Tan mutton during the traditional charcoal process. Front. Nutr. 2022, 9, 1003126. [Google Scholar] [CrossRef]
- Pulatsu, E.; Su, J.-W.; Kenderes, S.M.; Lin, J.; Vardhanabhuti, B.; Lin, M. Effects of ingredients and pre-heating on the printing quality and dimensional stability in 3D printing of cookie dough. J. Food Eng. 2021, 294, 110412. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Papastergiadis, E. Acorn and water interactions on the microstructure of gluten-free yeasted dough. J. Food Process. Preserv. 2022, 46, e15876. [Google Scholar]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Meraz, M.; Bello-Perez, L.A.; Garcia-Diaz, S. Canola oil/candelilla wax oleogel improves texture, retards staling and reduces in vitro starch digestibility of maize tortillas. J. Sci. Food Agric. 2020, 100, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, J.; Bevanda, C.; Wu, D. Effect of different edible fats on the spread ratio of sugar cookies. The Expedition 2022, 13. [Google Scholar]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods 2021, 10, 1929. [Google Scholar] [CrossRef]
- Kouhsari, F.; Saberi, F.; Kowalczewski, P.Ł.; Lorenzo, J.M.; Kieliszek, M.J.L. Effect of the various fats on the structural characteristics of the hard dough biscuit. LWT 2022, 159, 113227. [Google Scholar] [CrossRef]
- Pareyt, B.; Wilderjans, E.; Goesaert, H.; Brijs, K.; Delcour, J.A. The role of gluten in a sugar-snap cookie system: A model approach based on gluten–starch blends. J. Cereal Sci. 2008, 48, 863–869. [Google Scholar] [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Development of saturated fat replacers: Conventional and nano-emulsions stabilised by lecithin and hydroxylpropyl methylcellulose. Foods 2022, 11, 2536. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef]
- Schädle, C.N.; Bader-Mittermaier, S.; Sanahuja, S. The effect of corn dextrin on the rheological, tribological, and aroma release properties of a reduced-fat model of processed cheese spread. Molecules 2022, 27, 1864. [Google Scholar] [CrossRef]
- Markgren, J.; Rasheed, F.; Hedenqvist, M.S.; Skepö, M.; Johansson, E. Clustering and cross-linking of the wheat storage protein α-gliadin: A combined experimental and theoretical approach. Int. J. Biol. Macromol. 2022, 211, 592–615. [Google Scholar] [CrossRef]
- Rani, M.; Siddiqi, R.A.; Sharma, R.; Gill, B.S.; Sogi, D.S. Functional and structural properties of gliadin as influenced by pH, extraction protocols, and wheat cultivars. Int. J. Biol. Macromol. 2023, 234, 123484. [Google Scholar] [CrossRef]
- Muskat, L.C.; Jiang, L.; Brikmann, J.; Rostás, M.; Patel, A.V. Development of a Self-Adhesive Oleogel Formulation Designed for the Slow Release of Semiochemicals. Macromol. Mater. Eng. 2022, 307, 2200276. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guan, E.Q.; Zhang, L.L.; Pang, J.Y.; Li, M.M.; Bian, K. Effects of vacuum degree, mixing speed, and water amount on the moisture distribution and rheological properties of wheat flour dough. J. Food Sci. 2021, 86, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, S. Development and evaluation of a novel oleogel system based on starch–water–wax–oil. Food Funct. 2020, 11, 7727–7735. [Google Scholar] [CrossRef] [PubMed]
- Jumadi, M.; Jamaludin, D. Non-destructive measurement of rock melon fruit properties using Electrical Impedance Spectroscopy (EIS) technique. ASM Sci. J. 2020, 13, 20–26. [Google Scholar]
- Singh, V.K.; Anis, A.; Al-Zahrani, S.; Pradhan, D.K.; Pal, K. Molecular and electrochemical impedance spectroscopic characterization of the carbopol based bigel and its application in iontophoretic delivery of antimicrobials. Int. J. Electronchem. Sci. 2014, 9, 5049–5060. [Google Scholar] [CrossRef]
- Hamam, K.J.T. Organic Solar Cells Based on High Dielectric Constant Materials: An Approach to Increase Efficiency. Ph.D. Thesis, Western Michigan University, Kalamazoo, MI, USA, June 2013. [Google Scholar]
- Kertész, Á.; Hlavacova, Z.; Vozáry, E.; Staronova, L. Relationship between moisture content and electrical impedance of carrot slices during drying. Int. Agrophysics 2015, 29, 1. [Google Scholar] [CrossRef]
- Huang, Z.; Li, K.; Ma, L.; Chen, F.; Hu, X.; Miao, S.; Ji, J. The effect of Maillard reaction on the lactose crystallization and flavor release in lactose/WPI/inulin encapsulation. Food Chem. X 2023, 18, 100650. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Gökmen, V. Investigations on the formation of Maillard reaction products in sweet cookies made of different cereals. Food Res. Int. 2021, 144, 110352. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sudha, M. Effect of fat and sugar replacement on rheological, textural and nutritional characteristics of multigrain cookies. J. Food Sci. Technol. 2021, 58, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Celia, J.A.; Resende, O.; LIMA, M.S.D.; Correia, J.S.; Oliveira, K.B.D.; Takeuchi, K.P. Technological properties of gluten-free biscuits from sorghum flour granifero (Sorghum bicolor (L.) Moench). Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Dhal, S.; Anis, A.; Shaikh, H.M.; Alhamidi, A.; Pal, K. Effect of Mixing Time on Properties of Whole Wheat Flour-Based Cookie Doughs and Cookies. Foods 2023, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, S.; Zhang, W.; Zhang, H.; Guo, L.; Zheng, S.; Du, C. Effect of black soybean flour particle size on the nutritional, texture and physicochemical characteristics of cookies. LWT 2022, 164, 113649. [Google Scholar] [CrossRef]
- Paciulli, M.; Littardi, P.; Carini, E.; Paradiso, V.M.; Castellino, M.; Chiavaro, E. Inulin-based emulsion filled gel as fat replacer in shortbread cookies: Effects during storage. LWT 2020, 133, 109888. [Google Scholar] [CrossRef]
- Tyagi, P.; Chauhan, A.K.; Aparna. Optimization and characterization of functional cookies with addition of Tinospora cordifolia as a source of bioactive phenolic antioxidants. LWT 2020, 130, 109639. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.U.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT 2021, 142, 111035. [Google Scholar] [CrossRef]
- Sogabe, T.; Kobayashi, R.; Thanatuksorn, P.; Suzuki, T.; Kawai, K. Physical and structural characteristics of starch-based and conventional cookies: Water sorption, mechanical glass transition, and texture properties of their crust and crumb. J. Texture Stud. 2021, 52, 347–357. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Muhammad, K. Functional Properties, Antioxidant Activities and Storage Stability of Cookies from Germinated Brown Rice and Rice-Potato Starch Composite Flour. Pertanika J. Trop. Agric. Sci. 2019, 42, 2. [Google Scholar]
- Alvarez, M.D.; Cofrades, S.; Espert, M.; Salvador, A.; Sanz, T. Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel. Foods 2021, 10, 793. [Google Scholar] [CrossRef]
















| Sample | Composition (g) | Weight of Dough Cutouts (Avg ± SD) (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| WWF | Butter | Oleogel | Sugar | Water | Salt | Baking Soda | ||
| D-0 | 100.00 | 40.00 | 0 | 40.00 | 25.00 | 1.00 | 3.00 | 15.40 ± 0.84 |
| D-25 | 100.00 | 30.00 | 10.00 | 40.00 | 25.00 | 1.00 | 3.00 | 15.60 ± 0.55 |
| D-50 | 100.00 | 20.00 | 20.00 | 40.00 | 25.00 | 1.00 | 3.00 | 15.20 ± 0.45 |
| D-75 | 100.00 | 10.00 | 30.00 | 40.00 | 25.00 | 1.00 | 3.00 | 15.20 ± 0.84 |
| D-100 | 100.00 | 0 | 40.00 | 40.00 | 25.00 | 1.00 | 3.00 | 15.40 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, A.; Anis, A.; Alam, M.A.; Al-Zahrani, S.M.; Jarzebski, M.; Pal, K. Effect of Soy Wax/Rice Bran Oil Oleogel Replacement on the Properties of Whole Wheat Cookie Dough and Cookies. Foods 2023, 12, 3650. https://doi.org/10.3390/foods12193650
Pradhan A, Anis A, Alam MA, Al-Zahrani SM, Jarzebski M, Pal K. Effect of Soy Wax/Rice Bran Oil Oleogel Replacement on the Properties of Whole Wheat Cookie Dough and Cookies. Foods. 2023; 12(19):3650. https://doi.org/10.3390/foods12193650
Chicago/Turabian StylePradhan, Aditi, Arfat Anis, Mohammad Asif Alam, Saeed M. Al-Zahrani, Maciej Jarzebski, and Kunal Pal. 2023. "Effect of Soy Wax/Rice Bran Oil Oleogel Replacement on the Properties of Whole Wheat Cookie Dough and Cookies" Foods 12, no. 19: 3650. https://doi.org/10.3390/foods12193650






