Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extraction
2.2. Analysis of Chemical Composition
2.3. Measurement of Amino Acid
2.4. Availability of Antioxidant Activity
2.5. Cell Culture
2.6. Measurement of Cell Viability
2.7. Evaluation of Intracellular ROS Level
2.8. Apoptosis Analysis
2.9. Western Blotting
2.10. Statistical Analysis
3. Results and Discussion
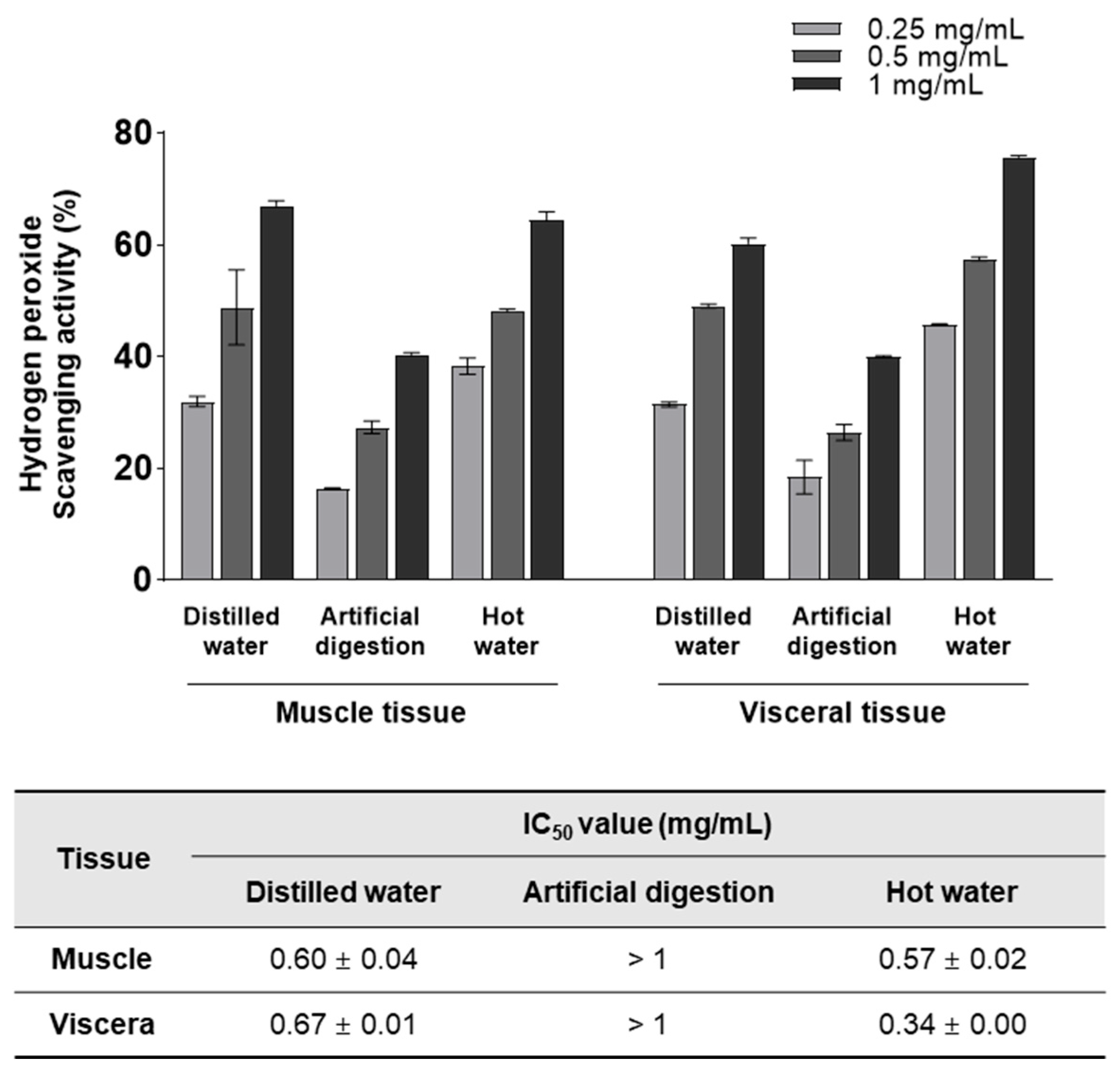
3.1. H2O2-scavenging Activity of Various T. cornutus Extracts
3.2. Extraction Yield and Proximate Composition of TVE
3.3. Amino Acid Composition in TVE
3.4. Antioxidant Activities of TVE
3.5. Effect of TVE on Cell Viability and ROS Generation in H2O2-Stimulated Vero Cells
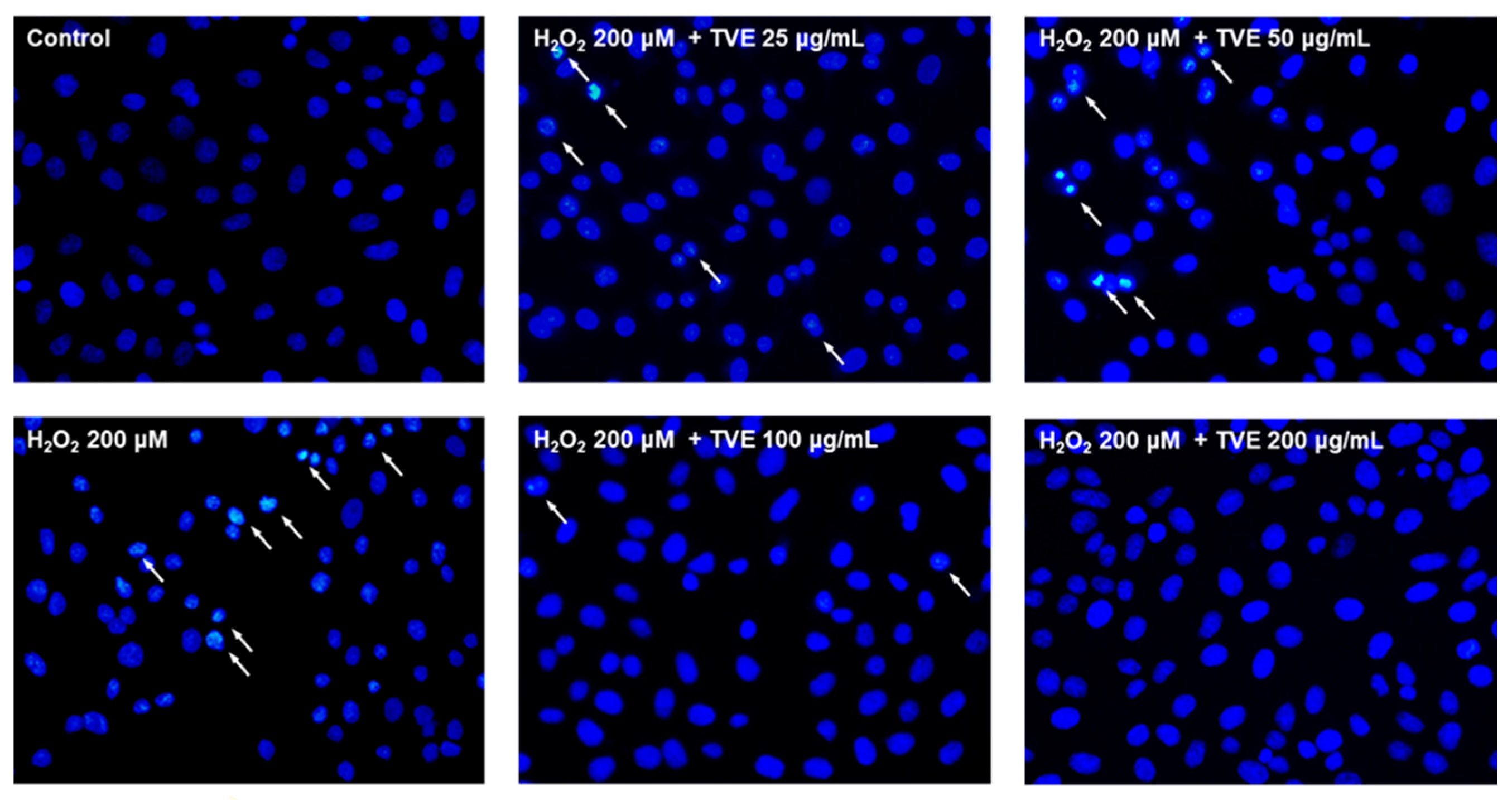
3.6. Effect of TVE on H2O2-Induced Apoptosis in Vero Cells
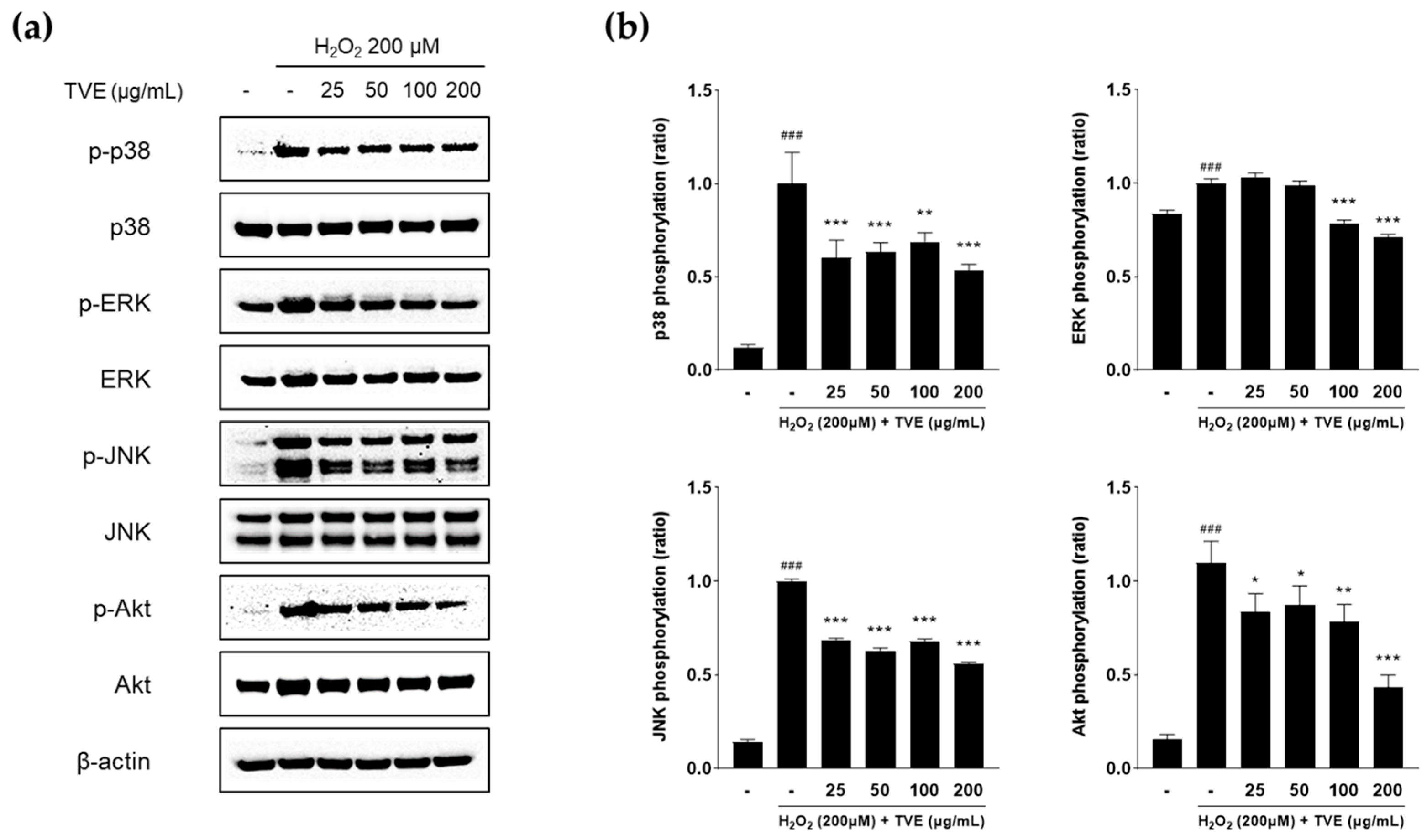
3.7. Effect of TVE on Regulation of MAPK and Akt Signaling Pathways
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fisheries Production and Distribution Industry Survey; Ministry of Oceans and Fisheries: Sejong-si, Republic of Korea, 2021.
- Kang, N.; Kim, E.A.; Kim, J.; Lee, S.H.; Heo, S.J. Identifying potential antioxidant properties from the viscera of sea snails (Turbo cornutus). Mar. Drugs 2021, 19, 567. [Google Scholar] [CrossRef]
- Kim, E.A.; Kang, N.; Kim, J.; Yang, H.W.; Ahn, G.; Heo, S.J. Anti-inflammatory effect of turbo cornutus viscera ethanolic extract against lipopolysaccharide-stimulated inflammatory response via the regulation of the JNK/NF-kB signaling pathway in murine macrophage RAW 264.7 cells and a zebrafish model: A preliminary study. Foods 2022, 11, 364. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, S.Y.; Hwang, J.-Y.; Ahn, C.-B. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods 2015, 16, 94–103. [Google Scholar] [CrossRef]
- Kang, N.; Kim, S.Y.; Rho, S.; Ko, J.Y.; Jeon, Y.J. Antifatigue activity of a mixture of sea horse (hippocampus abdominalis) hydrolysate and red ginseng. Fish Aquat. Sci. 2017, 20, 1–8. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflam. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, J.S.; Lee, J.S.; Jeon, Y.J. Antioxidant activity of olive flounder (Paralichthya olivaceus) surimi digest in in vitro and in vivo. J. Food Sci. Technol. 2022, 59, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-J.; Han, E.J.; Shin, E.-J.; Jung, K.; Heo, S.J.; Kim, E.A.; Kim, K.-N.; Kwak, I.S.; Kim, S.C.; Seo, M.J. Antioxidant effects of an alcalase hydrolysate from Batillus cornutus meat. In Taurine 11; Springer: Singapore, 2019; Volume 11, pp. 643–659. [Google Scholar]
- Dolowy, M.; Pyka, A. Application of TLC, HPLC and GC methods to the study of amino acid and peptide enantiomers: A review. Biomed. Chromatogr. 2014, 28, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Chatatikun, M.; Chiabchalard, A. Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J. Chem. Pharm. Res. 2013, 5, 97–102. [Google Scholar]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, antiviral, and anti-inflammatory activities of lutein-enriched extract of Tetraselmis species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Um, J.H.; Kim, E.A.; Lee, W.; Kang, N.; Han, E.J.; Oh, J.Y.; Park, S.Y.; Jeon, Y.J.; Lee, S.H.; Ahn, G. Protective effects of an enzymatic hydrolysate from Octopus ocellatus meat against hydrogen peroxide-induced oxidative stress in Chang liver cells and zebrafish embryo. In Taurine 10; Springer: Dordrecht, The Netherlands, 2017; Volume 10, pp. 603–620. [Google Scholar] [CrossRef]
- El-Sayed, W.M.; Al-Kahtani, M.A.; Abdel-Moneim, A.M. Prophylactic and therapeutic effects of taurine against aluminum-induced acute hepatotoxicity in mice. J. Hazard. Mater. 2011, 192, 880–886. [Google Scholar] [CrossRef]
- Learn, D.B.; Fried, V.A.; Thomas, E.L. Taurine and hypotaurine content of human leukocytes. J. Leukoc. Biol. 1990, 48, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Zhivotovsky, B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef]
- Miura, M.; Yuan, J. Transient transfection assay of cell death genes. Methods Enzymol. 2000, 322, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Brzezianska, E.; Pastuszak-Lewandoska, D. A minireview: The role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front. Biosci. 2011, 16, 422–439. [Google Scholar] [CrossRef] [PubMed]


| Sample | Yield (%) | Proximate Composition (%) | |
|---|---|---|---|
| Protein | Carbohydrate | ||
| TVE 1 | 37.31 ± 0.41 | 41.08 ± 1.01 | 19.69 ± 1.70 |
| Amino Acid | TVE 1 |
|---|---|
| % Amino Acid | |
| Taurine | 40.75 ± 1.65 |
| Aspartic acid | 7.01 ± 0.00 |
| Glutamic acid | 10.98 ± 0.75 |
| Serine | 3.12 ± 0.05 |
| Histidine | 1.43 ± 0.07 |
| Glycine | 6.65 ± 1.00 |
| Threonine | 3.03 ± 0.36 |
| Arginine | 4.57 ± 0.26 |
| Alanine | 3.46 ± 0.21 |
| Tyrosine | 1.53 ± 0.04 |
| Valine | 2.50 ± 0.14 |
| Methionine | 1.10 ± 0.09 |
| Phenylalanine | 2.27 ± 0.10 |
| Isoleucine | 1.86 ± 0.02 |
| Leucine | 3.12 ± 0.05 |
| Lysine | 1.82 ± 0.31 |
| Proline | 4.80 ± 0.27 |
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Kim, E.-A.; Kang, N.; Park, A.; Heo, S.-J. Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells. Foods 2023, 12, 3660. https://doi.org/10.3390/foods12193660
Lee Y-J, Kim E-A, Kang N, Park A, Heo S-J. Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells. Foods. 2023; 12(19):3660. https://doi.org/10.3390/foods12193660
Chicago/Turabian StyleLee, Yeon-Ji, Eun-A Kim, Nalae Kang, Areumi Park, and Soo-Jin Heo. 2023. "Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells" Foods 12, no. 19: 3660. https://doi.org/10.3390/foods12193660
APA StyleLee, Y.-J., Kim, E.-A., Kang, N., Park, A., & Heo, S.-J. (2023). Antioxidant Effects of Turbo cornutus By-Products Visceral Extract against Hydrogen Peroxide-Induced Oxidative Stress by Regulating MAPK and Akt Signaling Pathways in Vero Cells. Foods, 12(19), 3660. https://doi.org/10.3390/foods12193660







