A Rapid and Visual Method for Nucleic Acid Detection of Escherichia coli O157:H7 Based on CRISPR/Cas12a-PMNT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chromogenic Reaction of PMNT after Binding to ssDNA of Various Sequence Length
2.3. Cas12a-Mediated Cleavage Assay
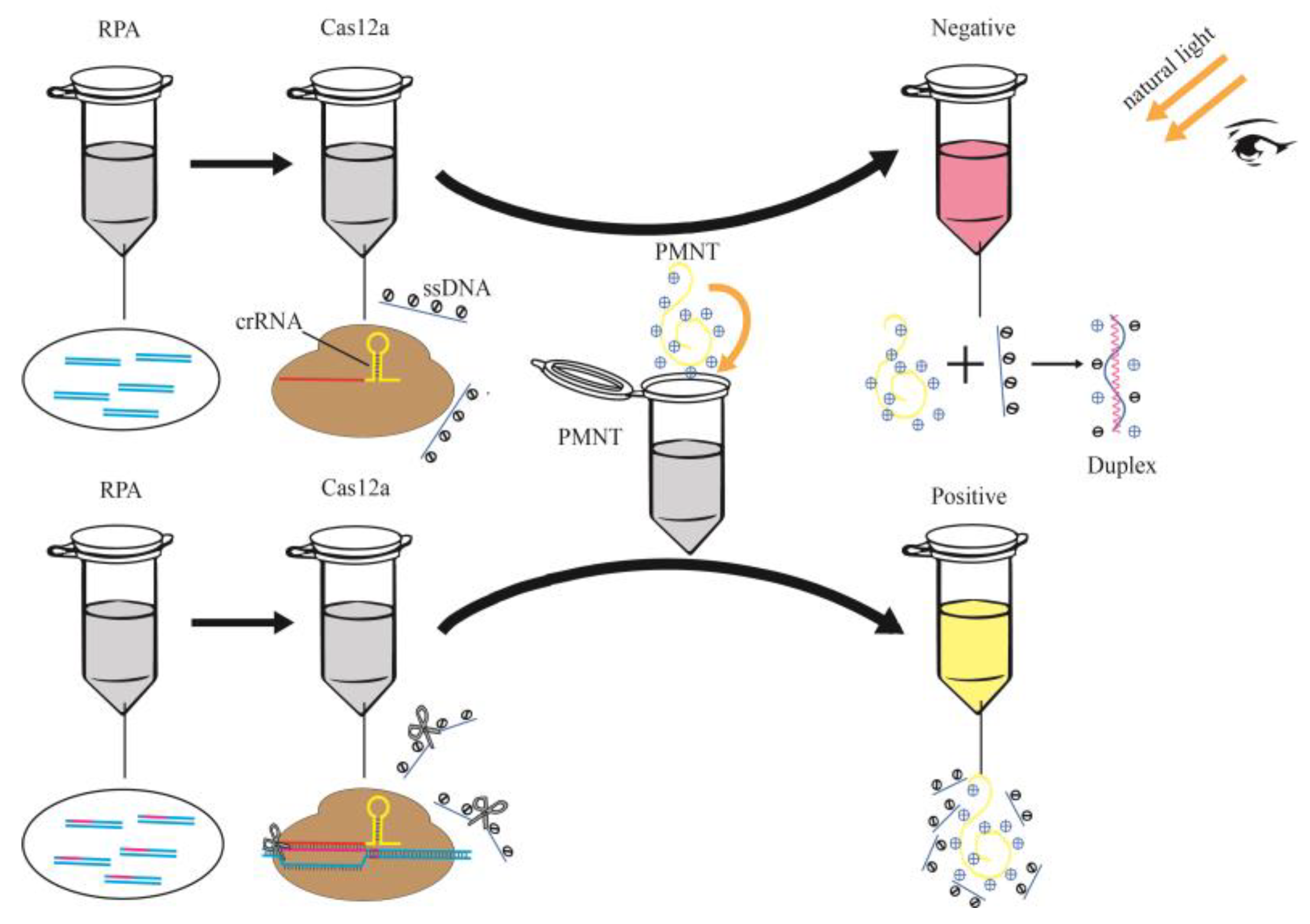
2.4. Feasibility Test
2.5. RPA Reaction Assay
2.6. Detection of Bacterial DNA by Cas12aVIP
3. Results
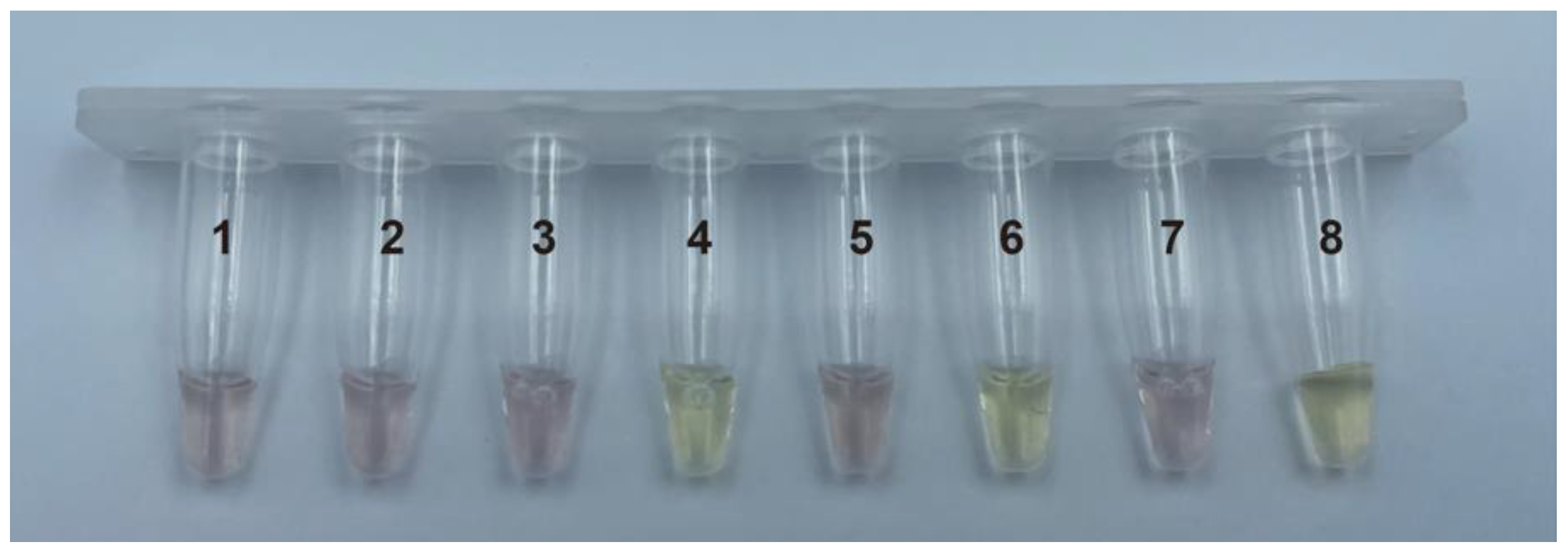
3.1. Color of PMNT after Binding to ssDNA of Various Sequence Length
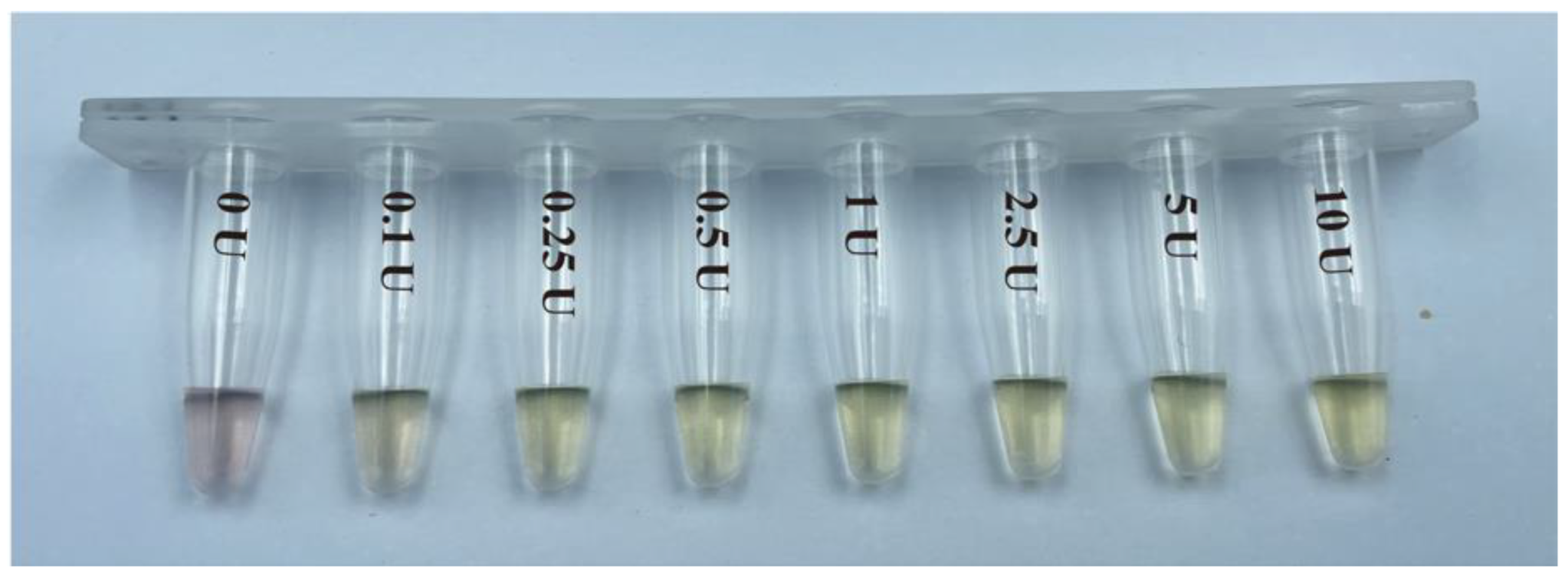
3.2. Color Change of PMNT after Binding to ssDNA-15nt Cleaved by S1 Endonuclease
3.3. Feasibility of Cas12a-PMNT-Based Visual Method for Nucleic Acid Detection
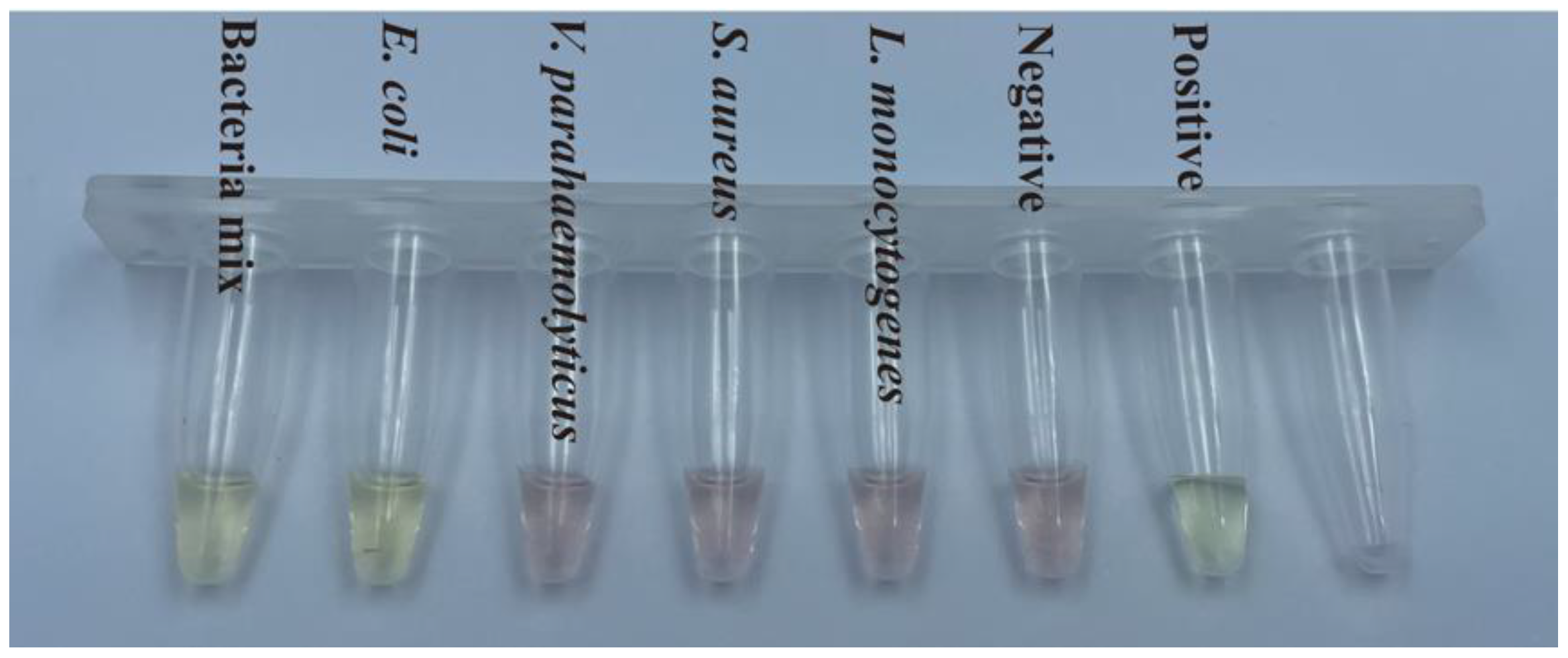
3.4. Detection of Bacterial Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.; Xu, W.; Zhang, P.; Jiang, M.; Chen, Y.; Kwok, R.T.K.; Lee, M.M.S.; Shan, G.; Qi, R.; Zhou, X.; et al. Engineering Sensor Arrays Using Aggregation-Induced Emission Luminogens for Pathogen Identification. Adv. Funct. Mater. 2019, 29, 1805986. [Google Scholar] [CrossRef]
- Fournier, P.E.; Drancourt, M.; Colson, P.; Rolain, J.M.; Scola, B.L.; Raoult, D. Modern Clinical Microbiology: New Challenges and Solutions. Nat. Rev. Microbiol. 2013, 11, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Guevara, C.; Swaminathan, V.V.; Reddy, B.; Huang, J.C.; Liu, Y.S.; Bashir, R. On-Chip Electrical Detection of Parallel Loop-Mediated Isothermal Amplification with DG-BioFETs for the Detection of Foodborne Bacterial Pathogens. RSC Adv. 2016, 6, 103872–103887. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, S.; Wang, J.; Nan, H.; Liu, L.; Sun, X.; Li, D.; Liu, M.; Wang, J.; Tan, K. Rapid Detection of Staphylococcus Aureus in Food Using a Recombinase Polymerase Amplification-Based Assay. Food Anal. Methods 2018, 11, 2847–2856. [Google Scholar] [CrossRef]
- Aquino-Jarquin, G. Recent Progress on Rapid SARS-CoV-2/COVID-19 Detection by CRISPR-Cas13-Based Platforms. Drug Discov. Today 2021, 26, 2025–2035. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Liao, Y.; Yang, Y.; Sun, S.; Zhao, Y.; Yang, P.; Tang, Y.; Chen, B.; Liu, Y.; et al. Highly Sensitive CRISPR/Cas12a-Based Fluorescence Detection of Porcine Reproductive and Respiratory Syndrome Virus. ACS Synth. Biol. 2021, 10, 2499–2507. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, T.; Huang, R. A Universal CRISPR/Cas9-Based Electrochemiluminescence Probe for Sensitive and Single-Base-Specific DNA Detection. Sens. Actuators B Chem. 2022, 357, 131411. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic Acid Detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Cheng, Q.X.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J.; Wang, J.M. CRISPR-Cas12a-Assisted Nucleic Acid Detection. Cell Discov. 2018, 4, 18–21. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Feng, Z.; Song, M.; Li, Q.; Jiang, B.; Qin, F.; Liu, H.; Lan, L.; Yang, M. Rapid Nucleic Acid Detection of Escherichia Coli O157:H7 Based on CRISPR/Cas12a System. Food Control 2021, 130, 108194. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, C.; Zhou, X.; Qi, R.; Liu, L.; Lv, F.; Li, Z.; Wang, S. Cationic Conjugated Polymers for Enhancing Beneficial Bacteria Adhesion and Biofilm Formation in Gut Microbiota. Colloids Surf. B Biointerfaces 2020, 188, 110815. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Baycan, F. A New Donor-Acceptor Conjugated Polymer-Gold Nanoparticles Biocomposite Materials for Enzymatic Determination of Glucose. Polymer 2020, 210, 123066. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, J.; Jang, E.S. Recent Progress in Multifunctional Conjugated Polymer Nanomaterial-Based Synergistic Combination Phototherapy for Microbial Infection Theranostics. Coord. Chem. Rev. 2022, 470, 214701. [Google Scholar] [CrossRef]
- Li, Z.; Lu, W.; Jia, S.; Yuan, H. Backbone-Regulated Cationic Conjugated Polymers for Combating and Monitoring Pathogenic Bacteria. ACS Appl. Polym. Mater. 2022, 4, 29–35. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, C.; An, H.; Niu, R.; Li, R.; Yan, W.; Zhan, Y. Ca2+-Controlled Assembly for Visualized Detection of Conformation Changes of Calmodulin. ACS Appl. Mater. Interfaces 2014, 6, 14790–14794. [Google Scholar] [CrossRef]
- Gao, A.; Liu, X.; Li, T.; Zhou, P.; Wang, Y.; Yang, Q.; Wang, L.; Fan, C. Digital Microfluidic Chip for Rapid Portable Detection of Mercury(II). IEEE Sens. J. 2011, 11, 2820–2824. [Google Scholar] [CrossRef]
- Liu, M.; Li, B.; Cui, X. Conjugated Polyelectrolytes-Initiated Chemiluminescence: A Biosensing Platform for Label-Free and Homogeneous DNA Detection. Biosens. Bioelectron. 2013, 47, 26–31. [Google Scholar] [CrossRef]
- Ho, H.A.; Boissinot, M.; Bergeron, M.G.; Corbeil, G.; Doré, K.; Boudreau, D.; Leclerc, M. Colorimetric and Fluorometric Detection of Nucleic Acids Using Cationic Polythiophene Derivatives. Angew. Chem. Int. Ed. 2002, 41, 1548–1551. [Google Scholar] [CrossRef]
- Franz, E.; Klerks, M.M.; De Vos, O.J.; Termorshuizen, A.J.; Van Bruggen, A.H.C. Prevalence of Shiga Toxin-Producing Escherichia Coli Stx1, Stx2, EaeA, and RfbE Genes and Survival of E. Coli O157:H7 in Manure from Organic and Low-Input Conventional Dairy Farms. Appl. Environ. Microbiol. 2007, 73, 2180–2190. [Google Scholar] [CrossRef]
- Lin, Z.; Kumagai, K.; Baba, K.; Mekalanos, J.J.; Nishibuchi, M. Vibrio Parahaemolyticus Has a Homolog of the Vibrio Cholerae ToxRS Operon That Mediates Environmentally Induced Regulation of the Thermostable Direct Hemolysin Gene. J. Bacteriol. 1993, 175, 3844–3855. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Khan, M.; Morin, D.E.; Hurley, W.L.; Tripathy, D.N.; Kehrli, M.; Oluoch, A.O.; Kakoma, I. Optimization of the PCR for Detection of Staphylococcus Aureus Nuc Gene in Bovine Milk. J. Dairy Sci. 2001, 84, 74–83. [Google Scholar] [CrossRef]
- Behari, J.; Youngman, P. Regulation of Hly Expression in Listeria Monocytogenes by Carbon Sources and PH Occurs through Separate Mechanisms Mediated by PrfA. Infect. Immun. 1998, 66, 3635–3642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A Frontline Nucleic Acid Rapid Detection System for Food Safety Based on CRISPR-Cas12a Combined with Recombinase Polymerase Amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, R.; Wang, D.; Wu, J.; Li, J.; Wang, J.; Liu, H.; Wang, Y. Cas12aVDet: A CRISPR/Cas12a-Based Platform for Rapid and Visual Nucleic Acid Detection. Anal. Chem. 2019, 91, 12156–12161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Wang, J.; Zhang, Y.; Zeng, W.; Liu, Y.; Zhang, X. Photoactivatable CRISPR/Cas12a Strategy for One-Pot DETECTR Molecular Diagnosis. Anal. Chem. 2022, 94, 9724–9731. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; He, C.; Bai, L.; Chen, Y.; Jia, J.; Pan, A.; Lv, B.; Tang, X.; Wu, X. A Rapid and Visual Method for Nucleic Acid Detection of Escherichia coli O157:H7 Based on CRISPR/Cas12a-PMNT. Foods 2023, 12, 236. https://doi.org/10.3390/foods12020236
Jiang W, He C, Bai L, Chen Y, Jia J, Pan A, Lv B, Tang X, Wu X. A Rapid and Visual Method for Nucleic Acid Detection of Escherichia coli O157:H7 Based on CRISPR/Cas12a-PMNT. Foods. 2023; 12(2):236. https://doi.org/10.3390/foods12020236
Chicago/Turabian StyleJiang, Wei, Chuan He, Lan Bai, Yifan Chen, Junwei Jia, Aihu Pan, Beibei Lv, Xueming Tang, and Xiao Wu. 2023. "A Rapid and Visual Method for Nucleic Acid Detection of Escherichia coli O157:H7 Based on CRISPR/Cas12a-PMNT" Foods 12, no. 2: 236. https://doi.org/10.3390/foods12020236




