Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains
2.3. Activation of Microorganisms
2.4. Encapsulation of Probiotics
2.5. Characterization of the Obtained Formulations
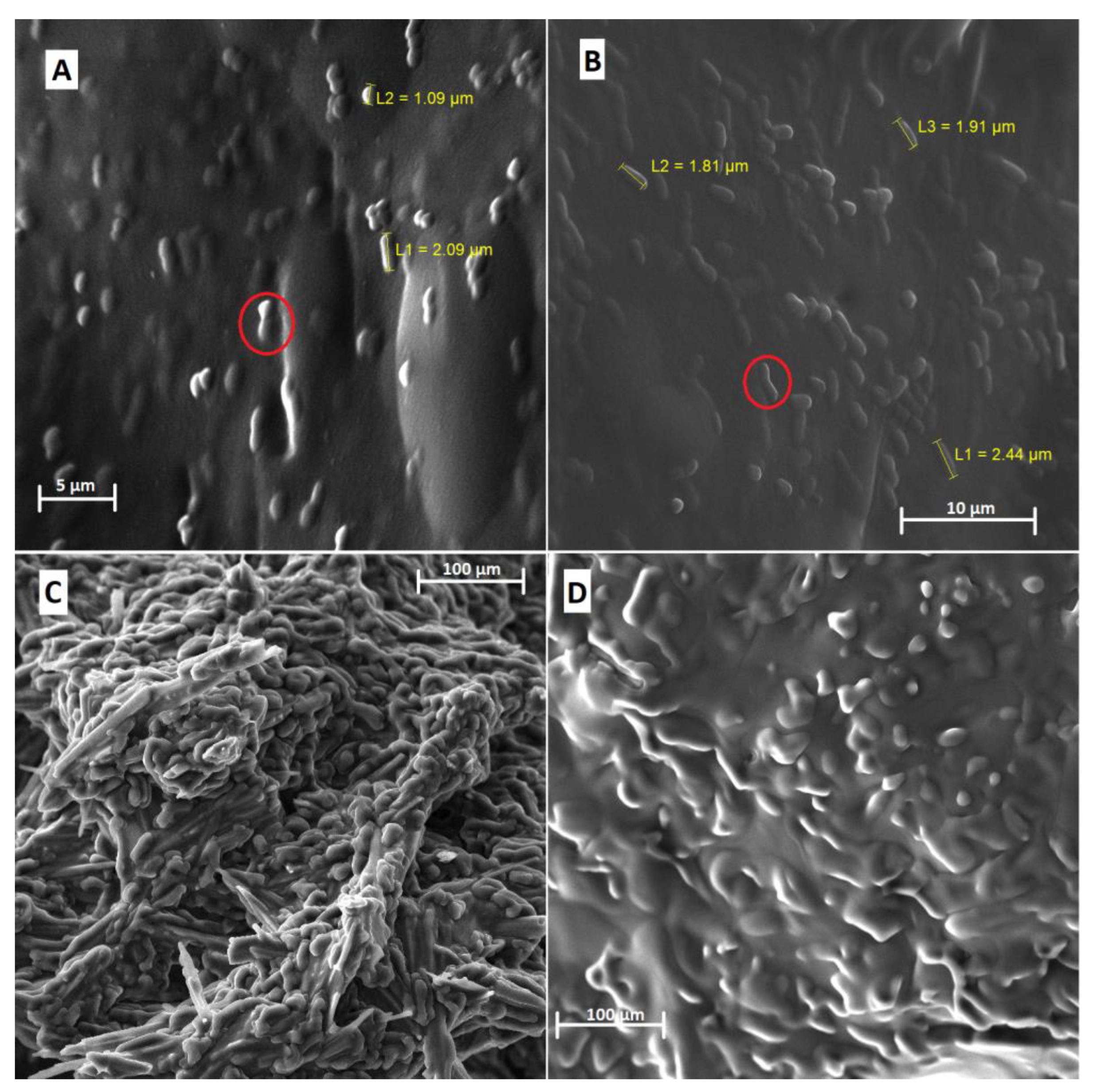
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Laser Diffraction
2.5.3. Zeta Potential
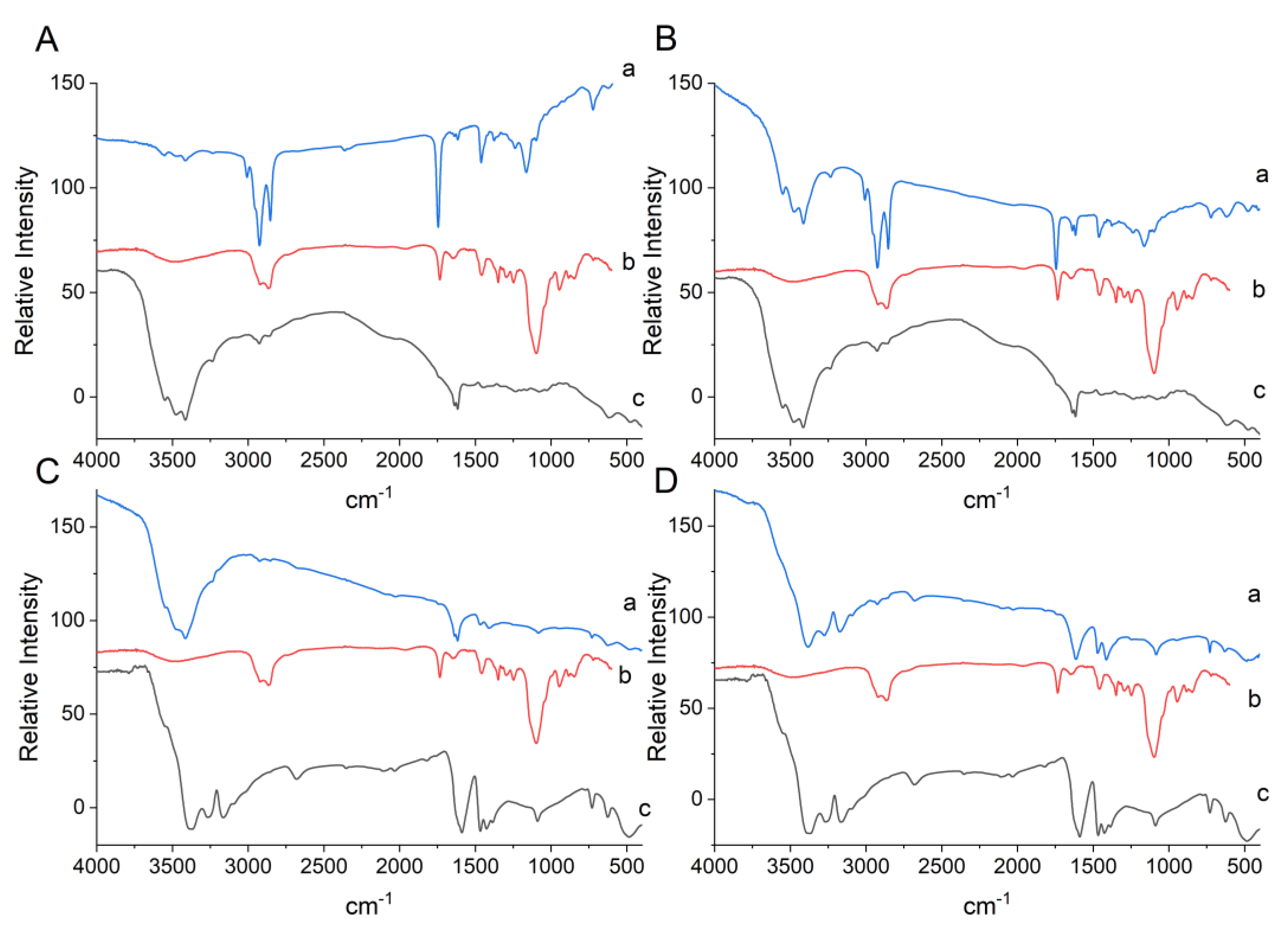
2.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.5. X-ray Diffraction
2.6. Enumeration of Viable Cells and Determination of Encapsulation Efficiency
2.7. Dispersibility
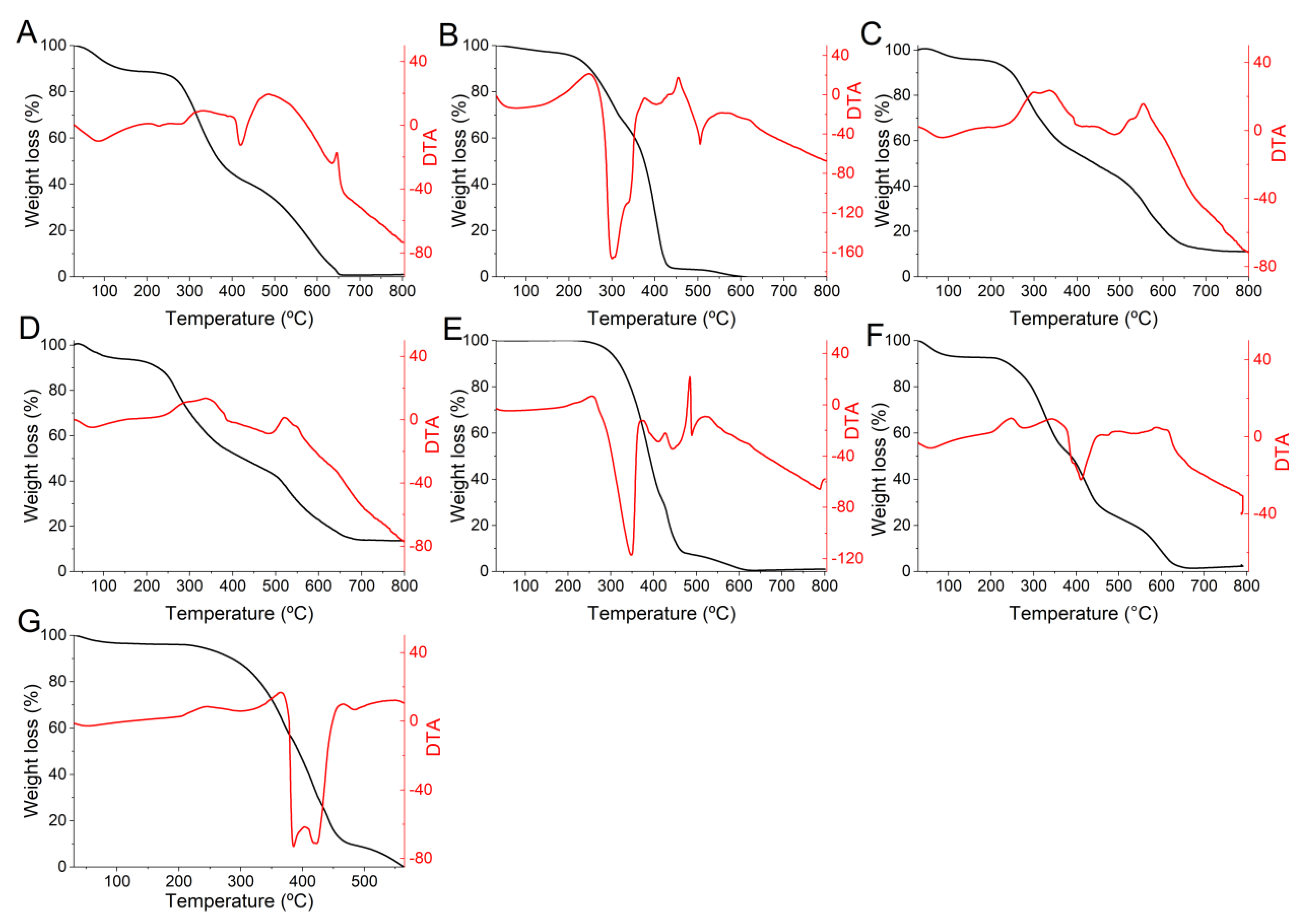
2.8. Thermogravimetry (TG) and Differential Thermal Analysis (DTA)
2.9. Evaluation of the Stability of Encapsulated Probiotics during Storage
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microparticle Characterization
3.1.1. Scanning Electron Microscope
3.1.2. Laser Diffraction
3.1.3. Zeta Potential
3.1.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.5. X-ray Diffraction
3.2. Enumeration of Viable Cells and Encapsulation Efficiency
3.3. Dispersibility
3.4. Thermal Analysis
3.5. Evaluation of the Stability of Encapsulated Probiotics during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food: Report of a Joint (FAO/WHO) Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 10 October 2022).
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated Probiotic Cells: Relevant Techniques, Natural Sources as Encapsulating Materials and Food Applications—A Narrative Review. Food Res. Inter. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Paula, D.; Martins, E.M.F.; Costa, N.D.A.; De Oliveira, P.M.; De Oliveira, E.B.; Ramos, A.M. Use of Gelatin and Gum Arabic for Microencapsulation of Probiotic Cells from Lactobacillus plantarum by a Dual Process Combining Double Emulsification Followed by Complex Coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef]
- Ribeiro, E.S.S.; Damasceno, K.S.F.S.C.; Da Costa Dantas, L.M.; De Azevedo, W.M.; Leite, P.I.P.; De Assis, C.F.; De Sousa Junior, F.C. Fermented Yellow Mombin Juice Using Lactobacillus acidophilus NRRL B-4495: Chemical Composition, Bioactive Properties and Survival in Simulated Gastrointestinal Conditions. PLoS ONE 2020, 15, e0239392. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of Alginate Molecular Weight and M/G Ratio in Beads Properties Foreseeing the Protection of Probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef]
- How, Y.; Pui, L. Survivability of Microencapsulated Probiotics in Nondairy Beverages: A Review. J. Food Process Preserv. 2021, 45, e15641. [Google Scholar] [CrossRef]
- Shu, G.; He, Y.; Chen, L.; Song, Y.; Cao, J.; Chen, H. Effect of Xanthan-Chitosan Microencapsulation on the Survival of Lactobacillus acidophilus in Simulated Gastrointestinal Fluid and Dairy Beverage. Polymers 2018, 10, 588. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of Various Polysaccharides (Alginate, Carrageenan, Gums, Chitosan) and Their Combination with Prebiotic Saccharides (Resistant Starch, Lactosucrose, Lactulose) on the Encapsulation of Probiotic Bacteria Lactobacillus casei 01 Strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent Developments on Encapsulation of Lactic Acid Bacteria as Potential Starter Culture in Fermented Foods—A Review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating Polymers for Probiotics Delivery Systems: Preparation, Characterization, and Applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Machado Vasconcelos, L.I.; Da Silva-Buzanello, R.A.; Kalschne, D.L.; Scremin, F.R.; Stival Bittencourt, P.R.; Gaudêncio Dias, J.T.; Canan, C.; Corso, M.P. Functional Fermented Sausages Incorporated with Microencapsulated Lactobacillus plantarum BG 112 in Acrycoat S100. LWT 2021, 148, 111596. [Google Scholar] [CrossRef]
- De Matos-Jr, F.E.; Da Silva, M.P.; Kasemodel, M.G.C.; Santos, T.T.; Burns, P.; Reinheimer, J.; Vinderola, G.; Favaro-Trindade, C.S. Evaluation of the Viability and the Preservation of the Functionality of Microencapsulated Lactobacillus paracasei BGP1 and Lactobacillus rhamnosus 64 in Lipid Particles Coated by Polymer Electrostatic Interaction. J. Funct. Foods 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Miguel, M.G.; Esquena, J. On the Encapsulation and Viability of Probiotic Bacteria in Edible Carboxymethyl Cellulose-Gelatin Water-in-Water Emulsions. Food Hydrocoll. 2018, 75, 41–50. [Google Scholar] [CrossRef]
- Qin, X.S.; Gao, Q.Y.; Luo, Z.G. Enhancing the Storage and Gastrointestinal Passage Viability of Probiotic Powder (Lactobacillus plantarum) through Encapsulation with Pickering High Internal Phase Emulsions Stabilized with WPI-EGCG Covalent Conjugate Nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Valorization of Okara Oil for the Encapsulation of Lactobacillus plantarum. Food Res. Inter. 2018, 106, 81–89. [Google Scholar] [CrossRef]
- Camelo-Silva, C.; Verruck, S.; Ambrosi, A.; Di Luccio, M. Innovation and Trends in Probiotic Microencapsulation by Emulsification Techniques. Food Eng. Rev. 2022, 14, 462–490. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Sun, W.; McClements, D.J.; Cheng, C.; Zeng, H.; Zou, L.; Liu, W. Impact of Encapsulation of Probiotics in Oil-in-Water High Internal Phase Emulsions on Their Thermostability and Gastrointestinal Survival. Food Hydrocoll. 2022, 126, 107478. [Google Scholar] [CrossRef]
- Castro, G.M.M.A.; Passos, T.S.; Nascimento, S.S.C.; Medeiros, I.; Araújo, N.K.; Maciel, B.L.L.; Padilha, C.E.A.; Ramalho, A.M.Z.; Sousa Júnior, F.C.; Assis, C.F. Gelatin nanoparticles enable water dispersibility and potentialize the antimicrobial activity of Buriti (Mauritia flexuosa) oil. BMC Biotechnol. 2020, 20, 55. [Google Scholar] [CrossRef]
- Morais, N.S.; Passos, T.S.; Ramos, G.R.; Ferreira, V.A.F.; Moreira, S.M.G.; Filho, G.P.C.; Barreto, A.P.G.; Leite, P.I.P.; De Almeida, R.S.; Paulo, C.L.R.; et al. Nanoencapsulation of Buriti Oil (Mauritia Flexuosa L.f.) in Porcine Gelatin Enhances the Antioxidant Potential and Improves the Effect on the Antibiotic Activity Modulation. PLoS ONE 2022, 17, e0265649. [Google Scholar] [CrossRef]
- De Oliveira, G.L.R.; Medeiros, I.; Nascimento, S.S.C.; Viana, R.L.S.; Porto, D.L.; Rocha, H.A.O.; Aragão, C.F.S.; Maciel, B.L.L.; De Assis, C.F.; De Araujo Morais, A.H.; et al. Antioxidant Stability Enhancement of Carotenoid Rich-Extract from Cantaloupe Melon (Cucumis Melo L.) Nanoencapsulated in Gelatin under Different Storage Conditions. Food Chem. 2021, 348, 129055. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.Y.; Marshall, R.T.; Heymann, H. Improving Survival of Culture Bacteria in Frozen Desserts by Microentrapment. J. Dairy Sci. 1993, 76, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of Microbial Cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, 1st ed.; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; Volume 1, pp. 43–58. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of Probiotic Saccharomyces cerevisiae var. boulardii with Different Wall Materials by Spray Drying. LWT 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of Unmodified Starch on Viability of Alginate-Encapsulated Lactobacillus fermentum CECT5716. LWT 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Rakshit, R.; Pal, M.; Mandal, M.; Mandal, K. Charge Transfer Mediated Magnetic Response of Cobalt Ferrite Nanoparticles. Mater Lett. 2015, 151, 64–67. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2005; pp. 1–550. [Google Scholar]
- Santos, M.I.; Gerbino, E.; Tymczyszyn, E.; Gomez-Zavaglia, A. Applications of Infrared and Raman Spectroscopies to Probiotic Investigation. Foods 2015, 4, 283–305. [Google Scholar] [CrossRef]
- Saad, M.A.; Abdurahman, N.H.; Yunus, R.M. Synthesis, Characterization, and Demulsification of Water in Crude Oil Emulsion via a Corn Oil-Based Demulsifier. Mater Today Proc. 2021, 42, 251–258. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Anis, A.; Pal, K. Review on Encapsulation of Vegetable Oils: Strategies, Preparation Methods, and Applications. Polym. Plast. Technol. Eng. 2016, 55, 291–311. [Google Scholar] [CrossRef]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in Alginate-Coated Gelatin Microspheres Improves Survival of the Probiotic Bifidobacterium adolescentis 15703T during Exposure to Simulated Gastro-Intestinal Conditions. Food Res. Inter. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Khalil, K.A.; Mustafa, S.; Mohammad, R.; Bin Ariff, A.; Ahmad, S.A.; Dahalan, F.A.; Abdul Manap, M.Y. Encapsulation of Bifidobacterium pseudocatenulatum Strain G4 within Bovine Gelatin-Genipin-Sodium Alginate Combinations: Optimisation Approach Using Face Central Composition Design-Response Surface Methodology (FCCD-RSM). Int. J. Microbiol. 2019, 2019, 4208986. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic Survival and in Vitro Digestion of L. salivarius spp. salivarius Encapsulated by High Homogenization Pressures and Incorporated into a Fruit Matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on Microencapsulation of Lycopene by Spray-Drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of Gelatin-Polyphenol and Gelatin–Genipin Cross-Linking on the Structure of Gelatin Hydrogels. Int. J. Food Prop. 2018, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Lacroix, M. Gelatin and Low Methoxyl Pectin Films Containing Probiotics: Film Characterization and Cell Viability. Food Biosci. 2020, 36, 100660. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Improvement of the Water Resistance and Ductility of Gelatin Film by Zein. Food Hydrocoll. 2020, 105, 105804. [Google Scholar] [CrossRef]
- Botrel, D.A.; De Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of Wall Matrix Systems on the Properties of Spray-Dried Microparticles Containing Fish Oil. Food Res. Inter. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Kuo, C.C.; Clark, S.; Qin, H.; Shi, X. Development of a Shelf-Stable, Gel-Based Delivery System for Probiotics by Encapsulation, 3D Printing, and Freeze-Drying. LWT 2022, 157, 113075. [Google Scholar] [CrossRef]


| Formulation | Mean Diameter (µm) * | Polydispersion Index * | Viable Cell Count (Log CFU/g) | EE% * | |
|---|---|---|---|---|---|
| Before | After | ||||
| LAEG | 26.08 ± 1.74 a | 0.5 ± 0 a | 12.1 ± 1.0 | 10.9 ± 0.9 | 89.6 ± 4.2 a |
| LPEG | 21.56 ± 4.17 a | 0.6 ± 0.1 a | 12.3 ± 1.4 | 9.9 ± 0.8 | 81.1 ± 9.7 a |
| LAEA | 5.24 ± 1.32 b | 0.1 ± 0.1 b | 12.8 ± 0.0 | <1 | <1 b |
| LPEA | 5.52 ± 0.45 b | 0.1 ± 0 b | 12.7 ± 0.2 | <1 | <1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, S.Â.D.; Batista, L.d.S.P.; Diniz, D.S.; Nascimento, S.S.d.C.; Morais, N.S.; de Assis, C.F.; Passos, T.S.; de Sousa Júnior, F.C. Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions. Foods 2023, 12, 252. https://doi.org/10.3390/foods12020252
da Silva SÂD, Batista LdSP, Diniz DS, Nascimento SSdC, Morais NS, de Assis CF, Passos TS, de Sousa Júnior FC. Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions. Foods. 2023; 12(2):252. https://doi.org/10.3390/foods12020252
Chicago/Turabian Styleda Silva, Sebastião Ânderson Dantas, Leonam da Silva Pereira Batista, Dara Souza Diniz, Sara Sayonara da Cruz Nascimento, Neyna Santos Morais, Cristiane Fernandes de Assis, Thaís Souza Passos, and Francisco Canindé de Sousa Júnior. 2023. "Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions" Foods 12, no. 2: 252. https://doi.org/10.3390/foods12020252
APA Styleda Silva, S. Â. D., Batista, L. d. S. P., Diniz, D. S., Nascimento, S. S. d. C., Morais, N. S., de Assis, C. F., Passos, T. S., & de Sousa Júnior, F. C. (2023). Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions. Foods, 12(2), 252. https://doi.org/10.3390/foods12020252








