Dynamic Changes in Cell Wall Polysaccharides during Fruit Development and Ripening of Two Contrasting Loquat Cultivars and Associated Molecular Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
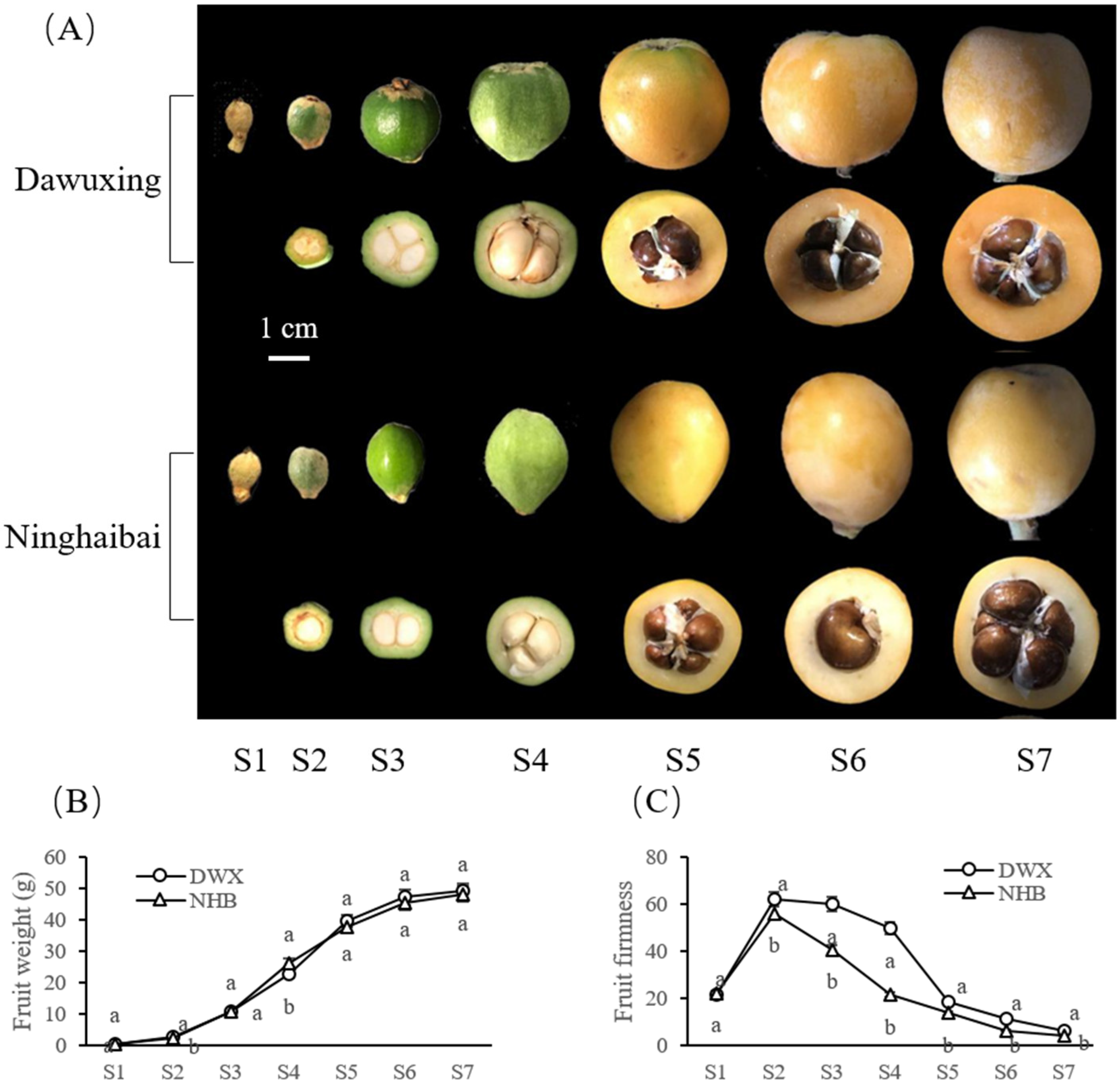
2.2. Determination of Fruit Firmness and Weight
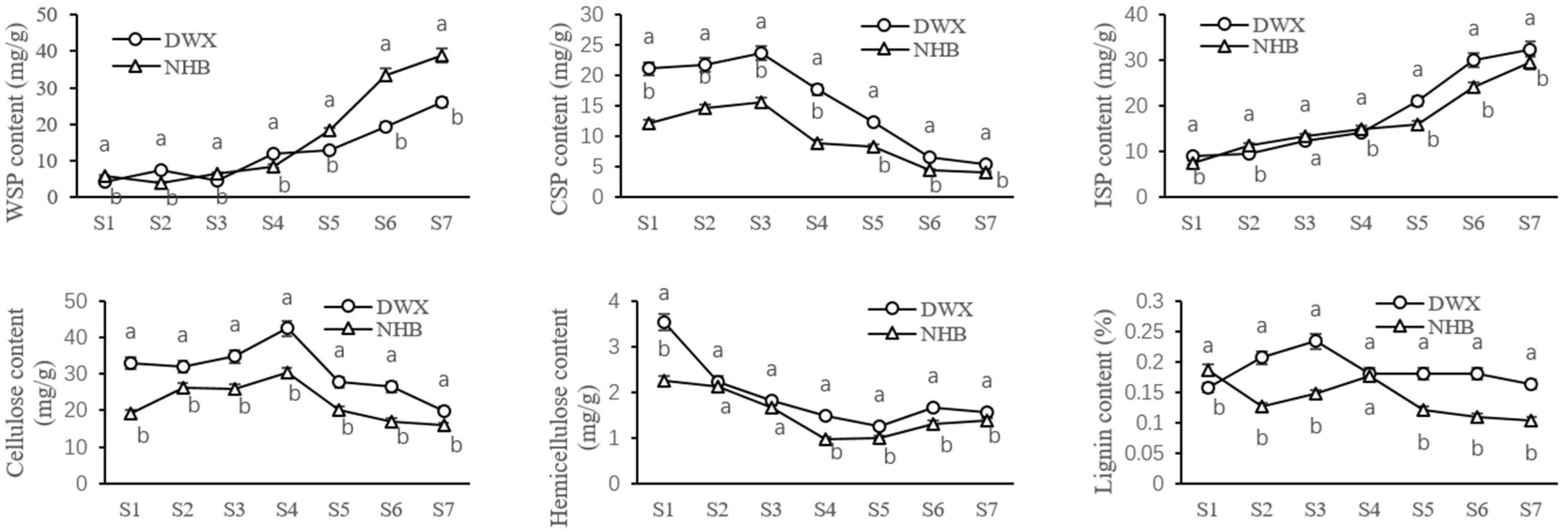
2.3. Extraction and Determination of Cell Wall Components
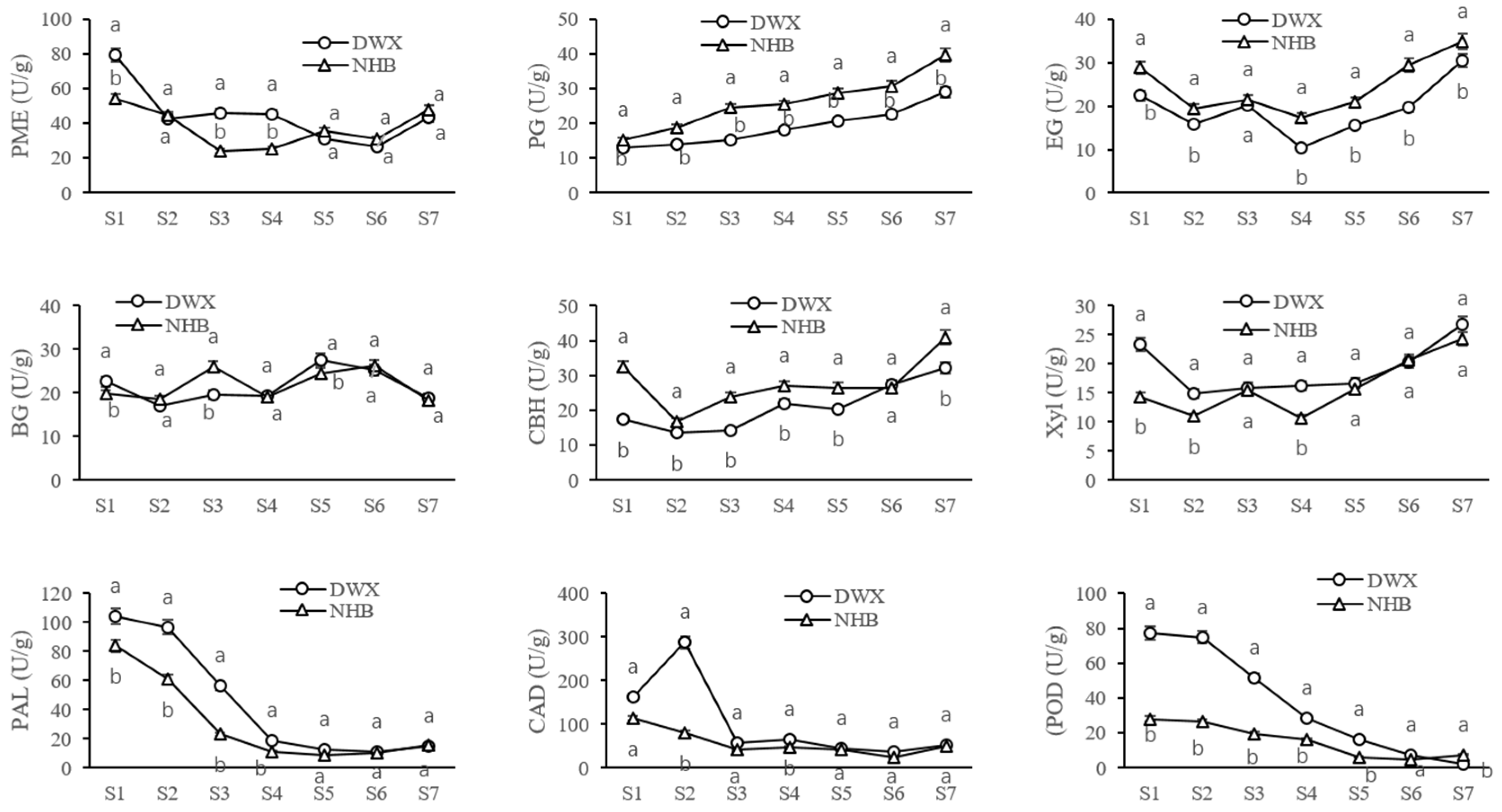
2.4. Extraction of Cell-Wall-Degrading Enzymes and Determination of Activity
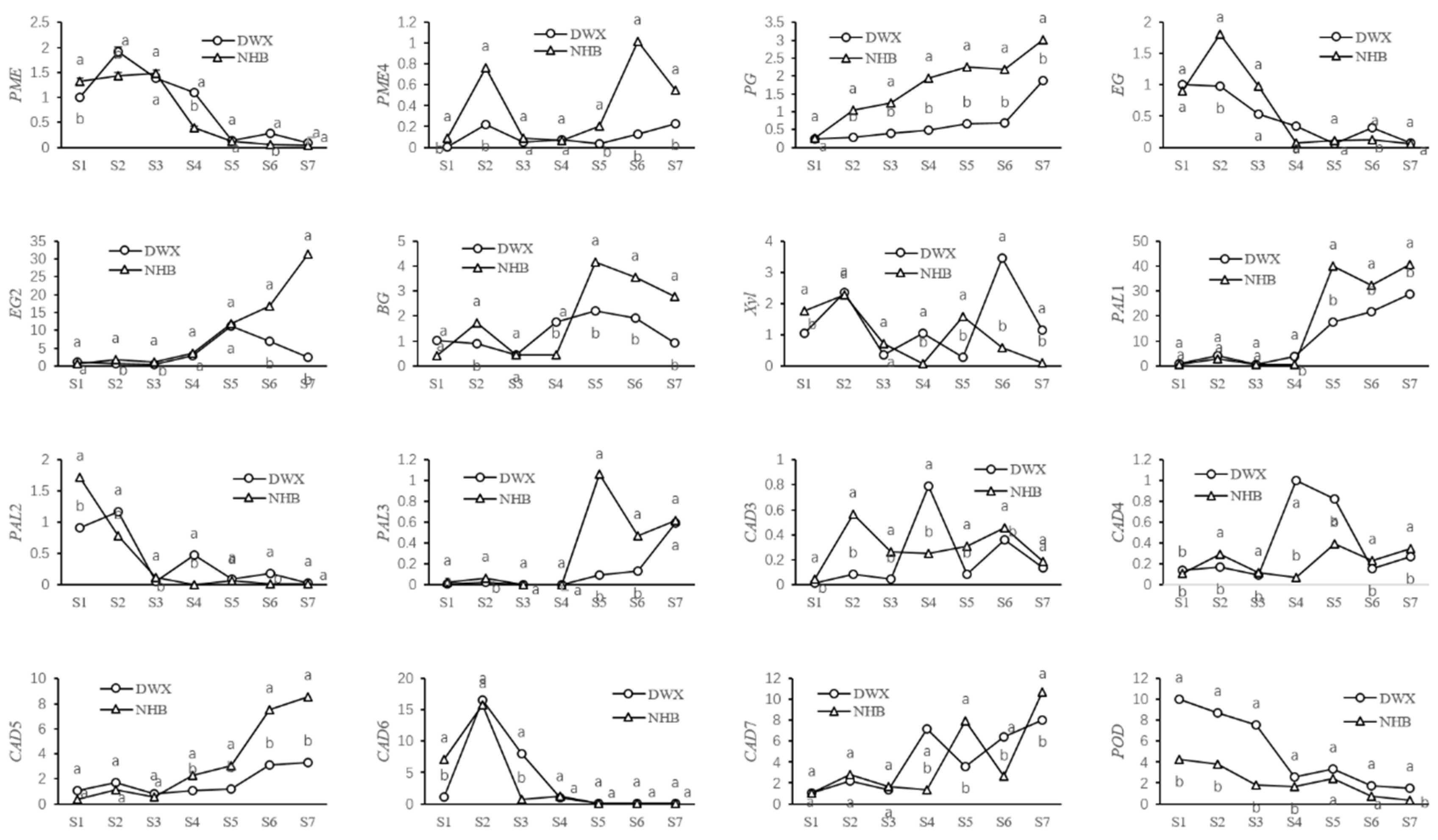
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analyses
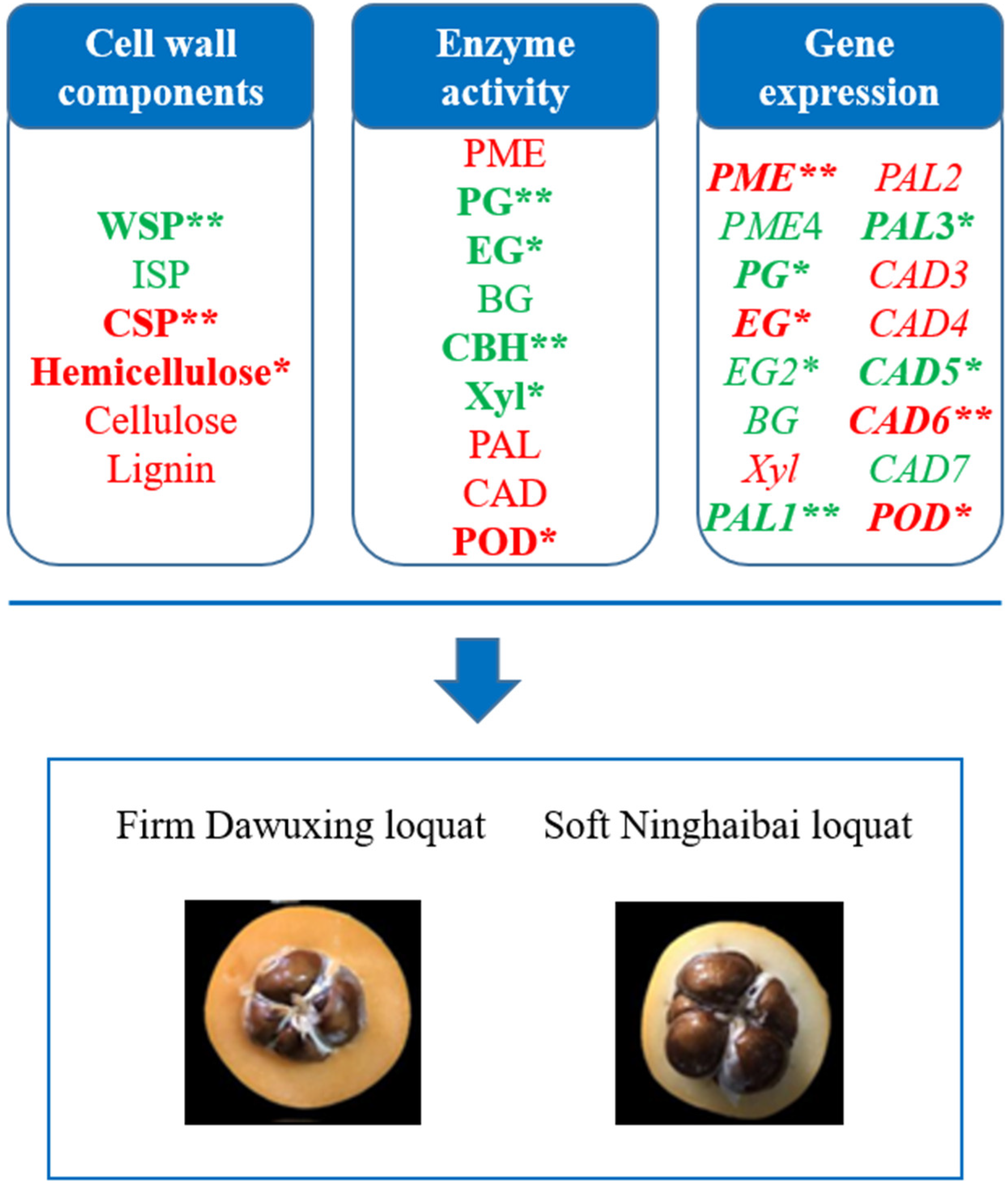
3. Results
3.1. Comparative Analyses of Fruit Phenotype, Weight, and Firmness Changes of Two Contrasting Loquat Cultivars
3.2. Comparative Analyses of Cell Wall Compositions of Two Contrasting Loquat Cultivars
3.3. Comparative Analyses of Enzyme Activities for Cell Wall Metabolism of Two Contrasting Loquat Cultivars
3.4. Correlation Analysis of Cell Wall Components and the Cell-Wall-Degrading Enzyme Activities of Two Contrasting Loquat Cultivars
3.5. Comparative Analyses of Gene Expressions for Cell Wall Metabolism of Two Contrasting Loquat Cultivars
3.6. Correlation Analysis of Cell Wall Components, Cell-Wall-Degrading Enzyme Activities, and Gene Expressions of Two Contrasting Loquat Cultivars
4. Discussion
4.1. Flesh Firmness Changes during Loquat Fruit Development and Ripening
4.2. Pectin Metabolism in Flesh Firmness Changes during Loquat Fruit Development and Ripening
4.3. Cellulose and Hemicellulose Metabolism in Flesh Firmness Changes during Loquat Fruit Development and Ripening
4.4. Lignin Metabolism in Flesh Firmness Changes during Loquat Fruit Development and Ripening
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, S.; Sharpe, R.H.; Janick, J. Loquat: Botany and horticulture. Hortic. Rev. 2010, 23, 233–276. [Google Scholar]
- Badenes, M.L.; Janick, J.; Zhang, Z.; Liang, G.; Wu, W. Breeding loquat. Plant Breed. Rev. 2013, 37, 259–296. [Google Scholar]
- Pareek, S.; Benkeblia, N.; Janick, J.; Cao, S.; Yahia, E.M. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J. Sci. Food Agric. 2014, 94, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Chen, J.W. Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) fruits. J. Sci. Food Agric. 2011, 91, 1057–1063. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 2019, 10, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S. World loquat production and research with special reference to China. Acta Hortic. 2007, 750, 37–43. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Korban, S.S.; Chen, K. Regulatory mechanisms of textural changes in ripening fruits. CRC. Crit. Rev. Plant Sci. 2010, 29, 222–243. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Longhi, S.; Guerra, W.; Magnago, P.; Porro, D.; Soukoulis, C.; Salvi, S.; Velasco, R.; Biasioli, F.; et al. Assessment of apple (Malus × domestica Borkh.) fruit texture by a combined acoustic-mechanical profiling strategy. Postharvest Biol. Technol. 2011, 61, 21–28. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Wang, D.; Ding, C.; Feng, Z.; Ji, S.; Cui, D. Recent advances in portable devices for fruit firmness assessment. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Höfte, H.; Voxeur, A. Plant cell walls. Curr. Biol. 2017, 27, R865–R870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of multienzyme co-immobilized hybrid nanoflowers for an efficient conversion of cellulose into glucose in a cascade reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Li, S.Y.; Xi, Y.F. Changes of cell wall substances in relation to flesh woodiness in cold-stored loquat fruits. Acta Phytopathol. Sin. 2000, 26, 306–310. [Google Scholar]
- Shan, L.L.; Li, X.; Wang, P.; Cai, C.; Zhang, B.; Sun, C.D.; Zhang, W.S.; Xu, C.J.; Ferguson, I.; Chen, K.S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta 2008, 227, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kong, W.; Peng, G.; Zhou, J.; Azam, M.; Xu, C.; Grierson, D.; Chen, K. Plastid structure and carotenogenic gene expression in red-and white-fleshed loquat (Eriobotrya japonica) fruits. J. Exp. Bot. 2012, 63, 341–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Hou, Y.; Zheng, Y.; Jin, P. 2,4-Epibrassinolide enhance chilling tolerance of loquat fruit by regulating cell wall and membrane fatty acid metabolism. Sci. Hortic. 2022, 295, 110813. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Yan, H.; Wang, H.; Wu, D.; Grierson, D.; Chen, K. The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, M.X.; Shi, Y.N.; Liu, X.F.; Li, X.; Grierson, D.; Chen, K.S. EjHAT1 participates in heat alleviation of loquat fruit lignification by suppressing the promoter activity of key lignin monomer synthesis gene EjCAD5. J. Agric. Food Chem. 2019, 67, 5204–5211. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria × ananassa fruits. Sci. Hortic. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Guo, W.; Dai, L.; Chen, W. Modification of cell wall polysaccharide during ripening of Chinese bayberry fruit. Sci. Hortic. 2013, 160, 155–162. [Google Scholar] [CrossRef]
- Anees, M.; Gao, L.; Umer, M.J.; Yuan, P.; Zhu, H.; Lu, X.; He, N.; Gong, C.; Kaseb, M.O.; Zhao, S.; et al. Identification of key gene networks associated with cell wall components leading to flesh firmness in watermelon. Front. Plant Sci. 2021, 12, 630243. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Guan, S.C.; Chen, J.Q.; Wen, C.J.; Cai, J.F.; Chen, X. Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol. 2020, 20, 13. [Google Scholar] [CrossRef] [Green Version]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus x domestica) fruit. BMC Plant Biol. 2012, 12, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Li, X.; Wang, L.; Wang, B.; Feng, Y.; Yang, H.; Zhao, Z. Variation in cell wall metabolism and flesh firmness of four apple cultivars during fruit development. Foods 2022, 11, 3518. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Zepeda, B.; Rojas, B.; Silva-Sanzana, C.; Delgado-Rioseco, J.; Fernández, K.; Balic, I.; Arriagada, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell wall calcium and hemicellulose have a role in the fruit firmness during storage of blueberry (Vaccinium spp.). Plants 2021, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Cappellin, L.; Fontanari, M.; Longhi, S.; Guerra, W.; Magnago, P.; Gasperi, F.; Biasioli, F. Texture dynamics during postharvest cold storage ripening in apple (Malus × domestica Borkh.). Postharvest Biol. Technol. 2012, 69, 54–63. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Hung, Y.C.; Zhang, S.; Lin, Y.; Lin, T. Paper-based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. J. Sci. Food Agric. 2017, 97, 2547–2552. [Google Scholar] [CrossRef]
- Bianchi, T.; Guerrero, L.; Gratacós-Cubarsí, M.; Claret, A.; Argyris, J.; Garcia-Mas, J.; Hortós, M. Textural properties of different melon (Cucumis melo L.) fruit types: Sensory and physical-chemical evaluation. Sci. Hortic. 2016, 201, 46–56. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yin, X.R.; Li, H.; Xu, M.; Zhang, M.X.; Li, S.J.; Liu, X.F.; Shi, Y.N.; Grierson, D.; Chen, K.S. ETHYLENE RESPONSE FACTOR39-MYB8 complex regulates low-temperature-induced lignification of loquat fruit. J. Exp. Bot. 2020, 71, 3172–3184. [Google Scholar] [CrossRef]
| Gene | F (Sequence 5′–3′) | R (Sequence 5′–3′) |
|---|---|---|
| Actin | AATGGAACTGGAATGGTCAAGGC | TGCCAGATCTTCTCCATGTCATCCCA |
| PME1 | ATACAGGCGGATATTATC | ATCAAGTTGGTCTTCTTC |
| PME4 | CACTATAATCTCTGGAACTC | TTAGGTCTCTTGCTATGA |
| PG | GTAACAGGTGATAATGGAA | CAGTATGTCGGATGAATG |
| EG1 | TTACATCTCGCTCCACAA | AGACATAATCCGCCTGAG |
| EG2 | TGATGCCTATGACAACTT | TGCCAATATACCGAGAAG |
| BG | ATTACTACTCAGGAACTTATG | TAGGAACGCCATTATACT |
| Xyl | AACCTCTTCCTTGACAAC | ACATATCCTTCGCTTACAG |
| PAL1 | AACCAAGATGTCAACTCA | CAACTAGGAATGTGGATGA |
| PAL2 | TTTGCCTACATTGATGAC | CATTCTTCTCACTCTCAC |
| PAL3 | CAGTGCTACATATCCTCTAA | CATCTCCTTCTCACCATT |
| CAD3 | AGATTGCGACTATTGTAGAG | TTGTAATCGTGCCATCAG |
| CAD4 | GAGTAGGAGATGTGGTAG | GTATTGCTCATTGTCTGT |
| CAD5 | CTGATGAGTTCTTGGTTAG | CTGATGAGTTCTTGGTTAG |
| CAD6 | GCAGCAAAACATAACATAACGGC | CTAGCAGCCAGTGTGTTTCCAA |
| CAD7 | ACTCGAGAGGCTAGTGAAGAAAG | CTAGGCTAGGCGGGAACTTTTAG |
| POD | TCTTGTGCTGATATTCTT | TTAGTCCATCCAATCTTC |
| Index | Firmness | PME | PG | EG | CBH | BG | Xyl | PAL | CAD | POD |
|---|---|---|---|---|---|---|---|---|---|---|
| firmness | 1.000 | 0.097 | −0.672 ** | −0.618 * | −0.764 ** | −0.412 | −0.603 * | 0.500 | 0.473 | 0.625 * |
| WSP | −0.710 ** | −0.220 | 0.876 ** | - | - | - | - | - | - | - |
| CSP | 0.841 ** | 0.438 | −0.840 ** | - | - | - | - | - | - | - |
| ISP | −0.380 | −0.182 | 0.354 | - | - | - | - | - | - | - |
| hemicellulose | 0.628 * | - | - | −0.712 ** | −0.531 | −0.323 | −0.218 | - | - | - |
| cellulose | 0.285 | - | - | 0.022 | −0.398 | −0.207 | 0.161 | - | - | - |
| lignin | 0.525 | - | - | - | - | - | - | 0.382 | 0.377 | 0.525 |
| Index | PME | PME4 | PG | EG | EG2 | BG | XYL | PAL1 | PAL2 | PAL3 | CAD3 | CAD4 | CAD5 | CAD6 | CAD7 | POD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| firmness | 0.884 ** | −0.16 | −0.600 * | 0.647 * | −0.597* | −0.46 | 0.16 | −0.720 ** | 0.35 | −0.592 * | 0.16 | 0.03 | −0.619 * | 0.756 ** | −0.45 | 0.552 * |
| WSP | −0.766 ** | 0.549* | 0.778 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CSP | 0.836 ** | −0.45 | −0.778 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| ISP | −0.573 * | −0.08 | 0.16 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| hemicellulose | - | - | - | 0.14 | −0.33 | −0.37 | −0.10 | - | - | - | - | - | - | - | - | - |
| cellulose | - | - | - | 0.677 ** | −0.45 | −0.43 | 0.30 | - | - | - | - | - | - | - | - | - |
| lignin | - | - | - | - | - | - | - | −0.634 * | 0.28 | −0.633 * | −0.35 | −0.06 | −0.650 * | 0.32 | −0.44 | 0.551 * |
| PME | 0.29 | −0.13 | −0.35 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PG | −0.751 ** | 0.48 | 0.959 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| EG | - | - | - | −0.18 | 0.52 | 0.18 | −0.19 | - | - | - | - | - | - | - | - | - |
| CBH | - | - | - | −0.51 | 0.625 * | 0.25 | −0.18 | - | - | - | - | - | - | - | - | - |
| BG | - | - | - | −0.27 | 0.18 | 0.42 | −0.06 | - | - | - | - | - | - | - | - | - |
| XYL | - | - | - | −0.42 | 0.42 | 0.23 | −0.10 | - | - | - | - | - | - | - | - | - |
| PAL | - | - | - | - | - | - | - | −0.600 * | 0.834 ** | −0.48 | −0.43 | −0.36 | −0.46 | 0.676 ** | −0.570 * | 0.881 ** |
| CAD | - | - | - | - | - | - | - | −0.41 | 0.715 ** | −0.33 | −0.35 | −0.20 | −0.30 | 0.663 ** | −0.34 | 0.767 ** |
| POD | - | - | - | - | - | - | - | −0.641 * | 0.579 * | −0.550* | −0.37 | −0.20 | −0.49 | 0.556 * | −0.549 * | 0.961 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Wang, X.; Wang, Y.; Xiang, Y.; Chen, M.; Zhang, H.; Luo, X.; Xia, H.; Liang, D.; Lv, X.; et al. Dynamic Changes in Cell Wall Polysaccharides during Fruit Development and Ripening of Two Contrasting Loquat Cultivars and Associated Molecular Mechanisms. Foods 2023, 12, 309. https://doi.org/10.3390/foods12020309
Deng H, Wang X, Wang Y, Xiang Y, Chen M, Zhang H, Luo X, Xia H, Liang D, Lv X, et al. Dynamic Changes in Cell Wall Polysaccharides during Fruit Development and Ripening of Two Contrasting Loquat Cultivars and Associated Molecular Mechanisms. Foods. 2023; 12(2):309. https://doi.org/10.3390/foods12020309
Chicago/Turabian StyleDeng, Honghong, Xi Wang, Yang Wang, Yinchun Xiang, Mingmin Chen, Huifen Zhang, Xian Luo, Hui Xia, Dong Liang, Xiulan Lv, and et al. 2023. "Dynamic Changes in Cell Wall Polysaccharides during Fruit Development and Ripening of Two Contrasting Loquat Cultivars and Associated Molecular Mechanisms" Foods 12, no. 2: 309. https://doi.org/10.3390/foods12020309






