Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Physiological Indices in Leaves and Roots
2.3. Determination of Amino Acids Contents and Total Nitrogen Contents
2.4. Metabonomic Analysis (UPLC-MS/MS)
2.4.1. Sample Preparation and Extraction
2.4.2. UPLC Conditions
2.4.3. ESI-Q TRAP-MS/MS
2.5. Phosphoproteomic Analysis
2.5.1. Protein Extraction and Trypsin Digestion
2.5.2. Affinity Enrichment
2.5.3. LC-MS/MS Analysis
2.5.4. Database Search
3. Results
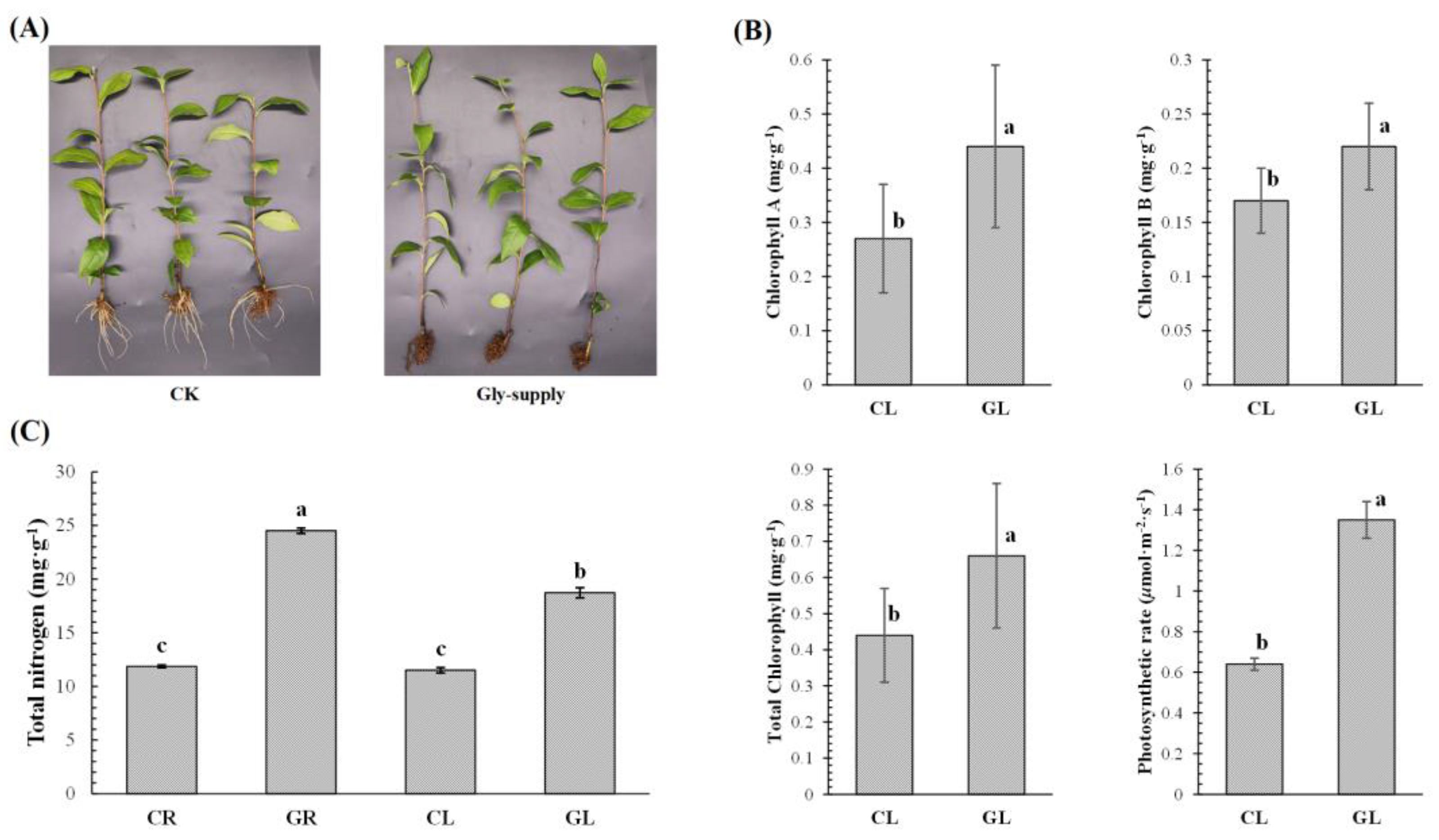
3.1. Analysis of Phenotype and Physiological Indices of Tea Plants in Response to Glycine
3.2. Glycine-Supply Increased Amino Acids Contents of Tea Plants
3.3. Glycine-Supply Changed Enzyme Activities in Tea Plants
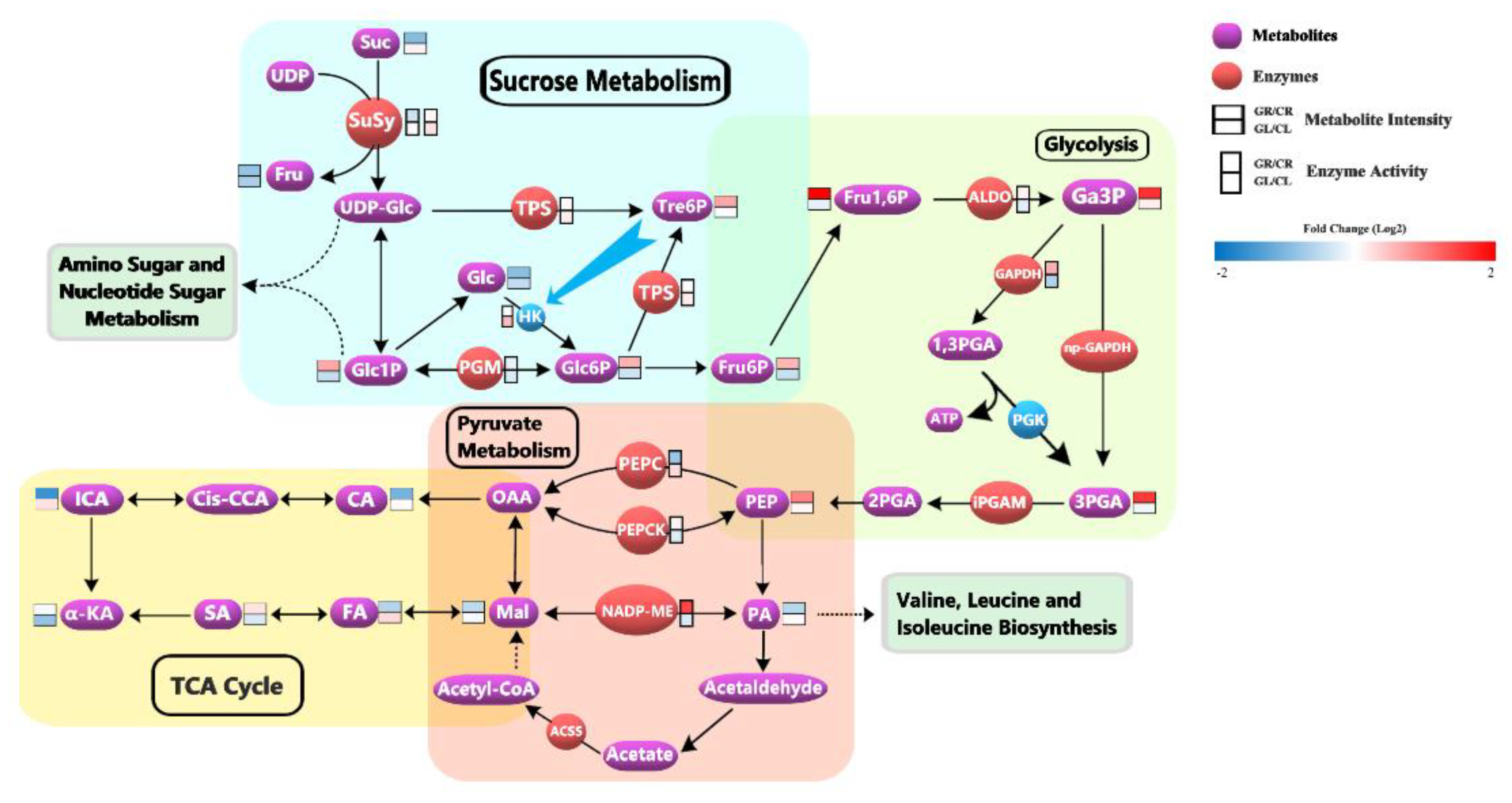
3.4. Glycine-Supply Affected the Primary Metabolism of Tea Plants
3.5. Glycine-Supply Affected the Phosphoproteome of Tea Plants
4. Discussion
4.1. Glycine-Supply Regulated the Formation of New Roots and Promoted the Photosynthesis of Tea Leaves
4.2. Glycine-Supply Affected the Energy Metabolism by Phosphorylation of Proteins in Tea Roots
4.2.1. Gly-Supply Promoted the Accumulation of Glc6P in Sucrose Metabolism
4.2.2. Gly-Supply Increased the Activity of Glycolysis
4.2.3. Gly-Supply Regulated Pyruvate Metabolism and Decreased the Activity of TCA Cycle
4.3. Glycine-Supply Promoted Photosynthesis in Tea Leaves and Regulated Amino Acid Metabolism to Improve Tea Quality
4.3.1. Gly-Supply Promoted the Photosynthesis
4.3.2. Gly-Supply Promoted the Cleavage and Reuse of Glycine
4.3.3. Gly-Supply Improved Tea Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Dong, C.; Yang, T.; Bao, S.; Fang, W.; Lucas, W.J.; Zhang, Z. The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Hortic. Res. 2021, 8, 178. [Google Scholar] [CrossRef]
- Wang, X.; Tang, D.; Huang, D. Proteomic analysis of pakchoi leaves and roots under glycine-nitrogen conditions. Plant Physiol. Bioch. 2014, 75, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, R.; Tang, D.; Huang, D. Comparison of glycine uptake by pak choi in organic and conventional soil under different glycine concentrations: A pot study. J. Plant Nutr. Soil Sci. 2015, 178, 768–775. [Google Scholar] [CrossRef]
- Jin, V.L.; Evans, R.D. Elevated CO2 increases plant uptake of organic and inorganic N in the desert shrub Larrea tridentata. Oecologia 2010, 163, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. Uptake of inorganic and amino acid nitrogen from soil by Eucalyptus regnans and Eucalyptus pauciflora seedlings. Tree Physiol. 2009, 29, 401–409. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Xu, Z.; Näsholm, T. Direct uptake and rapid decrease of organic nitrogen by Wollemia nobilis. Biol. Fert. Soils 2013, 49, 1247–1252. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, L.; Yang, W.; Mao, Y.; Chen, C.; Xing, S. Differential uptake of soluble organic and inorganic nitrogen by two fruit species: Dimocarpus longan Lour. and Eriobotrya japonica Lindl. J. Soils Sediments 2017, 17, 1579–1587. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Jämtgård, S.; Näsholm, T.; Huss-Danell, K. Nitrogen compounds in soil solutions of agricultural land. Soil Biol. Biochem. 2010, 42, 2325–2330. [Google Scholar] [CrossRef]
- Domínguez-May, Á.V.; Carrillo-Pech, M.; Barredo-Pool, F.A.; Martínez-Estévez, M.; Us-Camas, R.Y.; Moreno-Valenzuela, O.A.; Echevarría-Machado, I. A Novel Effect for Glycine on Root System Growth of Habanero Pepper. J. Am. Soc. Hortic. Sci. 2013, 138, 433–442. [Google Scholar] [CrossRef]
- Han, R.; Khalid, M.; Juan, J.; Huang, D. Exogenous glycine inhibits root elongation and reduces nitrate-N uptake in pak choi (Brassica campestris ssp. Chinensis L.). PLoS ONE 2018, 13, e0204488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasuska, U.; Andrzejczak, O.; Staszek, P.; Bogatek, R.; Gniazdowska, A. Canavanine Alters ROS/RNS Level and Leads to Post-translational Modification of Proteins in Roots of Tomato Seedlings. Front. Plant Sci. 2016, 7, 840. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, M.A.; Becana, M. Molecular responses of legumes to abiotic stress: Protein post-translational modifications and redox signaling. J. Exp. Bot. 2021, 72, 5876–5892. [Google Scholar] [CrossRef]
- Polit, J.T.; Ciereszko, I. Sucrose synthase activity and carbohydrates content in relation to phosphorylation status of Vicia faba root meristems during reactivation from sugar depletion. J. Plant Physiol. 2012, 169, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Brechenmacher, L.; Aldrich, J.T.; Clauss, T.R.; Gritsenko, M.A.; Hixson, K.K.; Libault, M.; Tanaka, K.; Yang, F.; Yao, Q.; et al. Quantitative Phosphoproteomic Analysis of Soybean Root Hairs Inoculated with Bradyrhizobium japonicum. Mol. Cell Proteom. 2012, 11, 1140–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijk, K.J.v.; Friso, G.; Walther, D.; Schulze, W.X. Meta-Analysis of Arabidopsis thaliana Phospho-Proteomics Data Reveals Compartmentalization of Phosphorylation Motifs. Plant Cell 2014, 26, 2367–2389. [Google Scholar] [CrossRef] [Green Version]
- Reiland, S.; Messerli, G.l.; Baerenfaller, K.; Gerrits, B.; Endler, A.; Grossmann, J.; Gruissem, W.; Baginsky, S. Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol. 2009, 150, 889–903. [Google Scholar] [CrossRef] [Green Version]
- Marondedze, C.; Groen, A.J.; Thomas, L.; Lilley, K.S.; Gehring, C. A Quantitative Phosphoproteome Analysis of cGMP-Dependent Cellular Responses in Arabidopsis thaliana. Mol. Plant 2016, 9, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lv, D.; Ge, P.; Bian, Y.; Chen, G.; Zhu, G.; Li, X.; Yan, Y. Phosphoproteome analysis reveals new drought response and defense mechanisms of seedling leaves in bread wheat (Triticum aestivum L.). J. Proteom. 2014, 109, 290–308. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Y.; Li, M.; Gao, F.; Yang, M.-k.; Wang, X.; Li, S.; Yang, P. Analysis of phosphoproteome in rice pistil. Proteomics 2014, 14, 2319–2334. [Google Scholar] [CrossRef]
- Li, Y.; Jeyaraj, A.; Yu, H.; Wang, Y.; Ma, Q.; Chen, X.; Sun, H.; Zhang, H.; Ding, Z.; Li, X. Metabolic Regulation Profiling of Carbon and Nitrogen in Tea Plants [Camellia sinensis (L.) O. Kuntze] in Response to Shading. J. Agric. Food Chem. 2020, 68, 961–974. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, J.H.; Son, N.; Kim, S.-H.; Park, J.-D.; Jang, D.-J.; Jeong, Y.; Kim, H.-J. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Y.; Ding, Y.; Qiu, C.; Sun, L.; Gai, Z.; Gu, H.; Ding, Z. Global Ubiquitome Profiling Revealed the Roles of Ubiquitinated Proteins in Metabolic Pathways of Tea Leaves in Responding to Drought Stress. Sci. Rep. 2019, 9, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Damaris, R.N.; Yang, P. Mechanism of GA-mediated leaf sheath growth in rice: A proteomic approach. Plant Growth Regul. 2020, 91, 23–36. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, N.; Zhao, Y.; Zhao, R.; Fu, X.; Zhao, D.; Zhao, Y.; Huang, L.; Li, C.; Qiu, Y.; et al. Global Phosphoproteomic Analysis Reveals Significant Metabolic Reprogramming in the Termination of Liver Regeneration in Mice. J. Proteom. Res. 2020, 19, 1788–1799. [Google Scholar] [CrossRef]
- Fedoreyeva, L.I.; Dilovarova, T.A.; Kononenko, N.V.; Baranova, E.N.; Smirnova, E.A.; Vanyushin, B.F. Influence of Glycylglycine, Glycine, and Glycylaspartic Acid on Growth, Development, and Gene Expression in a Tobacco (Nicotiana tabacum) Callus Culture. Biol. Bull. 2018, 45, 351–358. [Google Scholar] [CrossRef]
- Thornton, B.; Robinson, D. Uptake and assimilation of nitrogen from solutions containing multiple N. Plant Cell Environ. 2005, 28, 813–821. [Google Scholar] [CrossRef]
- Hardin, S.C.; Tang, G.Q.; Scholz, A.; Holtgraewe, D.; Winter, H.; Huber, S.C. Phosphorylation of sucrose synthase at serine 170: Occurrence and possible role as a signal for proteolysis. Plant J. 2003, 35, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Anguenot, R.; Nguyen-Quoc, B.; Yelle, S.; Michaud, D. Protein phosphorylation and membrane association of sucrose synthase in developing tomato fruit. Plant Physiol. Biochem. 2006, 44, 294–300. [Google Scholar] [CrossRef]
- Fedosejevs, E.T.; Gerdis, S.A.; Ying, S.; Pyc, M.; Anderson, E.M.; Snedden, W.A.; Mullen, R.T.; She, Y.-M.; Plaxton, W.C. The calcium-dependent protein kinase RcCDPK2 phosphorylates sucrose synthase at Ser11 in developing castor oil seeds. Biochem. J. 2016, 473, 3667–3682. [Google Scholar] [CrossRef]
- Harthill, J.E.; Meek, S.E.M.; Morrice, N.; Peggie, M.W.; Borch, J.; Wong, B.H.C.; MacKintosh, C. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J. 2006, 47, 211–223. [Google Scholar] [CrossRef]
- Almadanim, M.C.; Alexandre, B.M.; Rosa, M.T.G.; Sapeta, H.; Leitão, A.E.; Ramalho, J.C.; Lam, T.T.; Negrão, S.; Abreu, I.A.; Oliveira, M.M. Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant Cell Environ. 2017, 40, 1197–1213. [Google Scholar] [CrossRef] [Green Version]
- Deranieh, R.M.; He, Q.; Caruso, J.A.; Greenberg, M.L. Phosphorylation regulates myo-inositol-3-phosphate synthase: A novel regulatory mechanism of inositol biosynthesis. J. Biol. Chem. 2013, 288, 26822–26833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Stiers, K.M.; Kain, B.N.; Beamer, L.J. Compromised catalysis and potential folding defects in in vitro studies of missense mutants associated with hereditary phosphoglucomutase 1 deficiency. J. Biol. Chem. 2014, 289, 32010–32019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Allen, K.N.; Dunaway-Mariano, D. Mechanism of Substrate Recognition and Catalysis of the Haloalkanoic Acid Dehalogenase Family Member α-Phosphoglucomutase. Biochemistry 2018, 57, 4504–4517. [Google Scholar] [CrossRef]
- Gururaj, A.; Barnes, C.J.; Vadlamudi, R.K.; Kumar, R. Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene 2004, 23, 8118–8127. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Z.; Everaert, N.; Lametsch, R.; Zhang, D. Quantitative phosphoproteomic analysis of ovine muscle with different postmortem glycolytic rates. Food Chem. 2019, 280, 203–209. [Google Scholar] [CrossRef] [PubMed]
- GM, B. Biology of the p21-Activated Kinases. Annu. Rev. Biochem. 2003, 72, 743–781. [Google Scholar]
- Chen, L.; Bai, Y.; Everaert, N.; Li, X.; Tian, G.; Hou, C.; Zhang, D. Effects of protein phosphorylation on glycolysis through the regulation of enzyme activity in ovine muscle. Food Chem. 2019, 293, 537–544. [Google Scholar] [CrossRef]
- Piattoni, C.V.; Ferrero, D.M.L.; Dellaferrera, I.; Vegetti, A.; Iglesias, A.Á. Cytosolic Glyceraldehyde-3-Phosphate Dehydrogenase Is Phosphorylated during Seed Development. Front. Plant Sci. 2017, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Piattoni, C.V.; Bustos, D.M.n.; Guerrero, S.A.n.; Iglesias, A.A.l. Nonphosphorylating Glyceraldehyde-3-Phosphate Dehydrogenase Is Phosphorylated in Wheat Endosperm at Serine-404 by an SNF1-Related Protein Kinase Allosterically Inhibited by Ribose-5-Phosphate. Plant Physiol. 2011, 156, 1337–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerný, M.; Doubnerová, V.; Müller, K.; Ryslavá, H. Characterization of phosphoenolpyruvate carboxylase from mature maize seeds: Properties of phosphorylated and dephosphorylated forms. Biochimie 2010, 92, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.E.; Hartman, M.D.; Figueroa, C.M.; Leaden, L.; Podestá, F.E.; Iglesias, A.A. Biochemical characterization of phosphoenolpyruvate carboxykinases from Arabidopsis thaliana. Biochem. J. 2019, 476, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Hysková, V.D.; Miedzinska, L.; Dobrá, J.; Vankova, R.; Ryslavá, H. Phosphoenolpyruvate carboxylase, NADP-malic enzyme, and pyruvate, phosphate dikinase are involved in the acclimation of Nicotiana tabacum L. to drought stress. J. Plant Physiol. 2014, 171, 19–25. [Google Scholar] [CrossRef]
- Bovdilova, A.; Alexandre, B.M.; Höppner, A.; Luís, I.M.; Alvarez, C.E.; Bickel, D.; Gohlke, H.; Decker, C.; Nagel-Steger, L.; Alseekh, S.; et al. Posttranslational Modification of the NADP-Malic Enzyme Involved in C4 Photosynthesis Modulates the Enzymatic Activity during the Day. Plant Cell 2019, 31, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, F.; Shi, S.; Feng, X.; Wang, W.; Wang, Y.; Guo, W.; Wang, J.; Gao, S.; Gao, Y.; et al. Prognostic Impact of Metabolism Reprogramming Markers Acetyl-CoA Synthetase 2 Phosphorylation and Ketohexokinase-A Expression in Non-Small-Cell Lung Carcinoma. Front. Oncol. 2019, 9, 1123. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Bian, X.; Zhang, Q.; Xia, Z.; Liu, B.; Chen, Q.; Ke, C.; Wu, J.-L.; Zhao, Y. Shengui Sansheng San Ameliorates Cerebral Energy Deficiency via Citrate Cycle After Ischemic Stroke. Front. Pharmacol. 2019, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Sakamoto, W. Phosphorylation of photosystem II core proteins prevents undesirable cleavage of D1 and contributes to the fine-tuned repair of photosystem II. Plant J. 2014, 79, 312–321. [Google Scholar] [CrossRef]
- Tikkanen, M.; Nurmi, M.; Kangasjärvi, S.; Aro, E.-M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta 2008, 1777, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- XUE, R.L.; WANG, S.Q.; XU, H.L.; ZHANG, P.J.; LI, H.; ZHAO, H.J. Progesterone increases photochemical efficiency of photosystem II in wheat under heat stress by facilitating D1 protein phosphorylation. Photosynthetica 2017, 55, 664–670. [Google Scholar] [CrossRef]
- Chen, L.; Jia, H.; Tian, Q.; Du, L.; Gao, Y.; Miao, X.; Liu, Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynth. Res. 2012, 112, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Betterle, N.; Ballottari, M.; Baginsky, S.; Bassi, R. High light-dependent phosphorylation of photosystem II inner antenna CP29 in monocots is STN7 independent and enhances nonphotochemical quenching. Plant Physiol. 2015, 167, 457–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betterle, N.; Poudyal, R.S.; Rosa, A.; Wu, G.; Bassi, R.; Lee, C.-H. The STN8 kinase-PBCP phosphatase system is responsible for high-light-induced reversible phosphorylation of the PSII inner antenna subunit CP29 in rice. Plant J. 2017, 89, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, S.d.; Ballottari, M.; Dall’Osto, L.; Bassi, R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem. Soc. Trans. 2010, 38, 651–660. [Google Scholar]
- Rodriguez, R.E.; Lodeyro, A.; Poli, H.O.; Zurbriggen, M.; Peisker, M.; Palatnik, J.F.; Tognetti, V.B.; Tschiersch, H.; Hajirezaei, M.-R.; Valle, E.M.; et al. Transgenic Tobacco Plants Overexpressing Chloroplastic Ferredoxin-NADP(H) Reductase Display Normal Rates of Photosynthesis and Increased Tolerance to Oxidative Stress. Plant Physiol. 2007, 143, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Schönberg, A.; Rödiger, A.; Mehwald, W.; Galonska, J.; Christ, G.; Helm, S.; Thieme, D.; Majovsky, P.; Hoehenwarter, W.; Baginsky, S. Identification of STN7/STN8 kinase targets reveals connections between electron transport, metabolism and gene expression. Plant J. 2017, 90, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Simmons, R.M.; McKnight, S.M.; Edwards, A.K.; Wu, G.; Satterfield, M.C. Obesity increases hepatic glycine dehydrogenase and aminomethyltransferase expression while dietary glycine supplementation reduces white adipose tissue in Zucker diabetic fatty rats. Amino Acids 2020, 52, 1413–1423. [Google Scholar] [CrossRef]
- Singh, S.; Okamura, T.; Ali-Osman, F. Serine phosphorylation of glutathione S-transferase P1 (GSTP1) by PKCα enhances GSTP1-dependent cisplatin metabolism and resistance in human glioma cells. Biochem. Pharmacol. 2010, 80, 1343–1355. [Google Scholar] [CrossRef]
- Tatli, M.; Hebert, A.S.; Coon, J.J.; Amador-Noguez, D. Genome Wide Phosphoproteome Analysis of Zymomonas mobilis Under Anaerobic, Aerobic, and N2-Fixing Conditions. Front. Microbiol. 2019, 10, 1986. [Google Scholar] [CrossRef]
- Lima, L.; Seabra, A.; Melo, P.; Cullimore, J.; Carvalho, H. Phosphorylation and subsequent interaction with 14-3-3 proteins regulate plastid glutamine synthetase in Medicago truncatula. Planta 2006, 223, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, D.; Denninger, A.R.; Voss, C.M.; Frank, P.; Gao, N.; Brandon, N.; Waagepetersen, H.S.; Ferguson, A.D.; Pangalos, M.; Doig, P.; et al. Phosphorylation of Glutamine Synthetase on Threonine 301 Contributes to Its Inactivation during Epilepsy. Front. Mol. Neurosci. 2019, 12, 120. [Google Scholar] [CrossRef]
- Tan, F.; Tan, C.; Zhao, A.; Li, M. Simultaneous determination of free amino acid content in tea infusions by using high-performance liquid chromatography with fluorescence detection coupled with alternating penalty trilinear decomposition algorithm. J. Agric. Food Chem. 2011, 59, 10839–10847. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of root zone pH and form and concentration of nitrogen on accumulation of quality-related components in green tea. J. Sci. Food Agric. 2007, 87, 1505–1516. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, Y.; Ding, Z.; Shen, J.; Wang, Y.; Zhao, L.; Xu, M. The identification and evaluation of two different color variations of tea. J. Sci. Food Agric. 2016, 96, 4951–4961. [Google Scholar] [CrossRef]
- Alcázar, A.; Ballesteros, O.; Jurado, J.M.; Pablos, F.; Martín, M.J.; Vilches, J.L.; Navalón, A. Differentiation of green, white, black, Oolong, and Pu-erh teas according to their free amino acids content. J. Agric. Food Chem. 2007, 55, 5960–5965. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011, 5, 2110–2124. [Google Scholar]
- Feng, L.; Gao, M.; Hou, R.; Hu, X.; Zhang, L.; Wan, X.; Wei, S. Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 2014, 155, 98–104. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Song, W.; Zhao, B.; Dou, Y. Rapid and selective quantification of L-theanine in ready-to-drink teas from Chinese market using SPE and UPLC-UV. Food Chem. 2012, 135, 402–407. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef]
- Yu, Y.; Kou, X.; Gao, R.; Chen, X.; Zhao, Z.; Mei, H.; Li, J.; Jeyaraj, A.; Thangaraj, K.; Periakaruppan, R.; et al. Glutamine Synthetases Play a Vital Role in High Accumulation of Theanine in Tender Shoots of Albino Tea Germplasm “Huabai 1”. J. Agric. Food Chem. 2021, 69, 13904–13915. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Wei, K.; Wang, L.; Cheng, H.; Wu, L.; Li, H. Characteristics of Free Amino Acids (the Quality Chemical Components of Tea) under Spatial Heterogeneity of Different Nitrogen Forms in Tea (Camellia sinensis) Plants. Molecules 2019, 24, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806S–822S. [Google Scholar] [CrossRef] [PubMed]
| GR | CR | GL | CL | |
| Methionine (μg/g) | 338.38 ± 3.49 a | 87.19 ± 2.78 c | 328.48 ± 8.59 a | 164.54 ± 2.44 b |
| Isoleucine (μg/g) | 115.48 ± 1.08 c | 30.06 ± 0.61 d | 163.87 ± 3.80 a | 123.86 ± 1.73 b |
| Arginine (μg/g) | 200.62 ± 2.12 b | 46.13 ± 1.61 c | 263.84 ± 14.70 a | 216.54 ± 3.37 b |
| Leucine (μg/g) | 3.50 ± 0.02 c | 2.58 ± 0.04 d | 5.47 ± 0.48 a | 4.82 ± 0.05 b |
| Phenylalanine (μg/g) | 395.38 ± 3.93 c | 95.61 ± 2.56 d | 494.10 ± 10.90 a | 449.26 ± 6.65 b |
| Alanine (μg/g) | 357.51 ± 3.65 a | 68.17 ± 1.99 d | 331.77 ± 6.88 b | 319.13 ± 4.79 c |
| Asparagine (μg/g) | 371.46 ± 1.92 c | 226.62 ± 1.31 d | 434.48 ± 5.09 a | 417.73 ± 3.56 b |
| Histidine (μg/g) | 1216.24 ± 12.72 b | 234.87 ± 7.98 c | 1315.88 ± 20.14 a | 1308.85 ± 20.28 a |
| Glutamic acid (μg/g) | 1218.66 ± 12.81 a | 237.73 ± 8.15 c | 1199.58 ± 16.91 ab | 1183.43 ± 18.36 b |
| Threonine (μg/g) | 365.46 ± 3.68 a | 65.22 ± 1.72 c | 348.32 ± 4.80 b | 346.86 ± 5.15 b |
| Proline (μg/g) | 816.44 ± 8.61 a | 141.24 ± 4.92 c | 775.21 ± 6.79 b | 782.46 ± 12.18 b |
| Lysine (μg/g) | 486.39 ± 4.96 b | 118.44 ± 3.58 c | 626.35 ± 7.81 a | 632.34 ± 9.60 a |
| Glutamine (μg/g) | 1080.90 ± 9.72 b | 338.72 ± 6.29 c | 1177.78 ± 18.21 a | 1186.80 ± 16.01 a |
| Glycine (μg/g) | 496.69 ± 5.19 a | 108.87 ± 3.65 d | 352.13 ± 12.28 c | 383.01 ± 5.89 b |
| Aspartic acid (μg/g) | 993.61 ± 10.22 a | 201.08 ± 6.14 d | 631.53 ± 12.92 c | 749.93 ± 11.28 b |
| Tyrosine (μg/g) | 807.84 ± 8.41 c | 170.90 ± 5.59 d | 975.01 ± 28.25 b | 1160.41 ± 17.90 a |
| Valine (μg/g) | 926.92 ± 9.64 a | 179.49 ± 5.80 d | 454.69 ± 8.32 c | 549.42 ± 8.34 b |
| Serine (μg/g) | 38.84 ± 0.31 a | 15.22 ± 0.20 b | 12.2 ± 0.22 c | 15.62 ± 0.10 b |
| Cysteine (μg/g) | 1188.72 ± 11.06 a | 661.33 ± 18.28 c | 582.93 ± 9.83 d | 1087.27 ± 14.75 b |
| Theanine # | 1,805,733 ± 86,848.00 b | 1,922,700 ± 57,051.26 b | 1,125,000 ± 19,214.75 a | 118,200 ± 3167.09 c |
| Tryptophan # | 10,435,000 ± 332,349.21 b | 36,827,333 ± 532,304.63 a | 1,655,700 ± 23,693.46 d | 4,064,633 ± 35,089.44 c |
| Enzymes | GR | CR | GL | CL |
|---|---|---|---|---|
| GS (U·g−1) | - | - | 58.61 ± 8.85 a | 24.69 ± 0.41 b |
| Fd-GOGAT (nmol·min−1·g−1) | - | - | 21.54 ± 1.18 b | 36.86 ± 1.06 a |
| SuSy-I (Decomposition, μg·min−1·g−1) | 120.49 ± 3.59 c | 158.06 ± 2.90 b | 470.96 ± 7.90 a | 462.32 ± 10.69 a |
| SuSy-II (Synthesis, μg·min−1·g−1) | 490.06 ± 23.53 c | 470.73 ± 27.63 c | 1117.81 ± 50.36 a | 959.36 ± 46.82 b |
| TPS (nmol·min−1·g−1) | 23.04 ± 0.64 a | 22.50 ± 0.95 a | 18.02 ± 0.23 b | 16.31 ± 0.96 c |
| PGM (nmol·min−1·g−1) | 22.28 ± 0.36 d | 25.08 ± 0.67 c | 84.40 ± 2.27 b | 102.67 ± 2.03 a |
| HK (nmol·min−1·g−1) | 192.04 ± 3.40 a | 191.64 ± 9.84 a | 168.49 ± 10.56 b | 118.45 ± 3.91 c |
| ALDO (nmol·min−1·g−1) | 45.67 ± 3.12 c | 42.56 ± 0.84 c | 206.36 ± 0.94 b | 239.06 ± 14.30 a |
| GAPDH (nmol·min−1·g−1) | 34.89 ± 1.52 b | 22.59 ± 1.53 c | 33.81 ± 1.96 b | 51.21 ± 3.86 a |
| PEPC (nmol·min−1·g−1) | 21.17 ± 1.53 d | 36.34 ± 2.47 c | 728.21 ± 11.84 a | 595.28 ± 12.77 b |
| PEPCK (nmol·min−1·g−1) | 831.21 ± 37.99 a | 791.33 ± 60.96 a | 448.56 ± 25.60 c | 535.70 ± 17.79 b |
| NADP-ME (nmol·min−1·g−1) | 80.15 ± 4.19 c | 29.12 ± 1.46 d | 105.25 ± 6.14 b | 128.40 ± 7.74 a |
| Compounds | Formula | Fold Change (GR/CR) | Fold Change (GL/CL) |
|---|---|---|---|
| D-Sucrose | C12H22O11 | 0.53 | 1.08 |
| D-Glucose | C6H12O6 | 0.54 | 0.72 |
| D-Fructose | C6H12O6 | 0.57 | 0.63 |
| D-Trehalose | C12H22O11 | 0.64 | 1.32 |
| Trehalose-6-phosphate | C12H23O14P | 1.60 | 0.99 |
| D-Glucose-6-phosphate | C6H13O9P | 1.51 | 0.76 |
| Glucose-1-phosphate | C6H13O9P | 1.61 | 0.73 |
| D-Fructose-6-phosphate | C6H13O9P | 1.48 | 0.75 |
| D-Fructose-1,6-biphosphate | C6H14O12P2 | 4.18 | 0.88 |
| 3-Phospho-D-glyceric acid | C3H7O7P | 2.93 | 0.93 |
| DL-Glyceraldehyde-3-phosphate | C3H7O6P | 3.10 | 1.10 |
| Pyruvic acid | C3H4O3 | 0.69 | 1.04 |
| Phosphoenolpyruvate | C3H5O6P | 1.95 | 1.05 |
| D-Malic acid | C4H6O5 | 0.71 | 0.95 |
| Citric acid | C6H8O7 | 0.48 | 1.02 |
| Isocitric acid | C6H8O7 | 0.35 | 1.18 |
| Fumaric acid | C4H4O4 | 0.67 | 1.23 |
| Succinic acid | C4H6O4 | 1.16 | 0.82 |
| α-Ketoglutaric acid | C5H6O5 | 0.97 | 0.56 |
| Protein Accession | Phosphorylation Protein | Site | FC (GR/CR) | FC (GL/CL) |
|---|---|---|---|---|
| TEA017533.1 | Sucrose synthase 2 (SuSy) | Ser-11 Ser-151 | 0.262 0.535 | - - |
| TEA006786.1 | Alpha, alpha-trehalose-phosphate synthase (TPS) | Ser-5 | 0.451 | - |
| TEA018596.1 | Phosphoglucomutase (PGM) | Ser-193 | 0.478 | - |
| TEA023229.1 | Fructose-bisphosphate aldolase cytoplasmic isozyme (ALDO) | Ser-76 Ser-85 Ser-384 Ser-394 | 0.613 0.443 0.563 0.340 | 0.723 - - - |
| TEA031641.1 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Ser-306 | 0.660 | - |
| TEA013943.1 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (iPGAM) | Ser-3 Ser-4 Ser-69 | 0.509 0.444 0.449 | - - - |
| TEA009852.1 | Phosphoenolpyruvate carboxylase (PEPC) | Ser-11 Ser-966 | 0.250 0.165 | - - |
| TEA008970.1 | Phosphoenolpyruvate carboxykinase (PEPCK) | Ser-57 | 0.454 | - |
| TEA003598.1 | NADP-dependent malic enzyme (NADP-ME) | Ser-357 | 0.538 | - |
| TEA005053.1 | Acetyl-coenzyme A synthetase (ACSS) | Ser-125 | 0.652 | - |
| TEA001596.1 | Photosystem II D1 protein | Thr-2 Ser-232 | - - | 2.478 4.961 |
| TEA032678.1 | Chlorophyll a-b binding protein CP29.1 | Thr-108 | - | 2.948 |
| TEA032600.1 | Ferredoxin-NADP reductase (FNR) | Thr-171 | - | 3.268 |
| TEA022478.1 | Glycine dehydrogenase (decarboxylating) (GLDC) | Thr-98 | - | 0.369 |
| TEA023186.1 | Aminomethyltransferase (AMT) | Ser-173 | - | 1.668 |
| TEA028194.1 | Glutamine synthetase (GS) | Thr-99 | - | 2.797 |
| TEA030315.1 | Ferredoxin-dependent glutamate synthase (Fd-GOGAT) | Ser-1100 | - | 1.808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fan, K.; Shen, J.; Wang, Y.; Jeyaraj, A.; Hu, S.; Chen, X.; Ding, Z.; Li, X. Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant. Foods 2023, 12, 334. https://doi.org/10.3390/foods12020334
Li Y, Fan K, Shen J, Wang Y, Jeyaraj A, Hu S, Chen X, Ding Z, Li X. Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant. Foods. 2023; 12(2):334. https://doi.org/10.3390/foods12020334
Chicago/Turabian StyleLi, Yuchen, Kai Fan, Jiazhi Shen, Yu Wang, Anburaj Jeyaraj, Shunkai Hu, Xuan Chen, Zhaotang Ding, and Xinghui Li. 2023. "Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant" Foods 12, no. 2: 334. https://doi.org/10.3390/foods12020334
APA StyleLi, Y., Fan, K., Shen, J., Wang, Y., Jeyaraj, A., Hu, S., Chen, X., Ding, Z., & Li, X. (2023). Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant. Foods, 12(2), 334. https://doi.org/10.3390/foods12020334







