Shelf Life Extension and Nutritional Quality Preservation of Sour Cherries through High Pressure Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
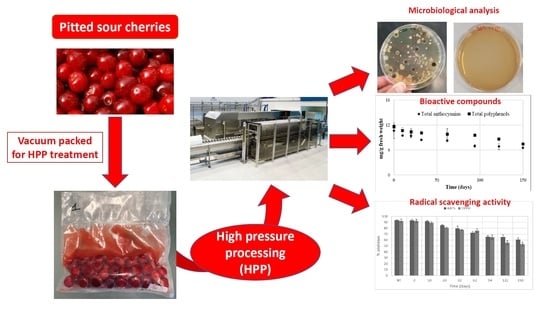
2.2. High Pressure Processing (HPP)
2.3. Microbiological Analysis
2.4. Colorimetric Analysis and pH Determination
2.5. Sour Cherries Extraction Procedure
2.6. Evaluation of Total Phenols, Flavonoids and Anthocyanins
2.7. Evaluation of Radical Scavenging Activity
2.8. Statistical Analysis
3. Results
3.1. Microbiological Analysis
3.2. Monitoring of Color Parameters and pH
3.3. Evaluation of Total Phenols, Flavonoids, and Anthocyanin Contents
3.4. Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oey, I.; Lille, M.; Loey, A.V.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Gutiérrez, J.L.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol. Technol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Oliveira, T.L.C.d.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Natural antimicrobials as additional hurdles to preservation of foods by high pressure processing. Trends Food Sci. Technol. 2015, 45, 60–85. [Google Scholar] [CrossRef]
- Huang, H.W.; Lung, H.M.; Yang, B.B.; Wang, C.Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Sila, D.N.; Duvetter, T.; De Roeck, A.; Verlent, I.; Smout, C.; Moates, K.G.; Hills, P.B.; Waldron, K.K.; Hendrickx, M.; Van Loey, A. Texture changes of processed fruits and vegetables: Potential use of high-pressure processing. Trends Food Sci. Technol. 2008, 19, 309–319. [Google Scholar] [CrossRef]
- Gopal, K.R.; Kalla, A.M.; Srikanth, K. High pressure processing of fruits and vegetable products: A review. Int. J. Pure App. Biosci. 2017, 5, 680–692. [Google Scholar] [CrossRef]
- Schuster, M. Sour cherries for fresh consumption. Acta Hortic. 2019, 1235, 113–118. [Google Scholar] [CrossRef]
- Dalla Rosa, M. Advanced technologies for cherry processing and packaging. Review n. 37. Italus Hortus 2019, 26, 51–58. [Google Scholar] [CrossRef]
- Grafe, C.; Schuster, M. Physicochemical characterization of fruit quality traits in a German sour cherry collection. Sci. Hortic. 2014, 180, 24–31. [Google Scholar] [CrossRef]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Yılmaz, M.E.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Bilek, E.S.; Uygun, Ö.; Bircan, C. Sour cherry by-products: Compositions, functional properties and recovery potentials: A review. Crit. Rev. Food Sci. Nutr. 2018, 52, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT, F.A.O. Food and agriculture organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 13 July 2022).
- Bonacini, L.; Lugli, S. La coltura delle amarene, una tradizione tutta Modenese. In Amarena Brusca di Modena, Tradizione e Trasformazione; Artestampa Ed: Modena, Italy, 2018; pp. 53–58. [Google Scholar]
- Kirakosyan, A.; Seymour, E.M.; Urcuyo Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Ademović, Z.; Mehić, J.; Suljagic, J.; Jašić, M.; Juul, N. Influence of processing technology on bioactive components of sour cherry. Acta Technol. 2017, 10, 5–11. [Google Scholar]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crops Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Separ. Sci. Technol. 2016, 51, 1472–1479. [Google Scholar] [CrossRef]
- Quero-García, J.; Iezzoni, A.; Pulawska, J.; Lang, G.A. (Eds.) Cherries: Botany, Production and Uses; Centre for Agriculture and Biosciences International (CABI): Wallingford, UK, 2017. [Google Scholar] [CrossRef]
- Proietti, S.S.; Moscatello, S.; Villani, F.; Mecucci, F.; Walker, R.P.; Famiani, F.; Battistelli, A. Quality and nutritional compounds of Prunus Cerasus L. Var. Austera Fruit Grown in Central Italy. HortScience 2019, 54, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review on the health benefit of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Seymour, E.M.; Singer, A.A.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.R.; Taylor, B.C.R.; Thompkins, D. The effects of tart cherry juice with whey protein on the signs and symptoms of exercise-induced muscle damage following plyometric exercise. J. Funct. Foods 2017, 29, 185–192. [Google Scholar] [CrossRef]
- Altuntas, J.; Evrendilek, G.A.; Sangun, M.K.; Zhang, H.Q. Effects of pulsed electric field processing on the quality and microbial inactivation of sour cherry juice. Int. J. Food Sci. 2010, 45, 899–905. [Google Scholar] [CrossRef]
- Escribano-Bailon, M.T.; Santos-Buelga, C. Polyphenol extraction from foods. In Methods in Polyphenol Analysis; Santos-Buelga, C., Williamson, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 1–16. [Google Scholar]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. In Natural Products Isolation. Methods in Molecular Biology, 3rd ed.; Sarker, S., Nahar, L., Eds.; Humana Press: New York, NY, USA, 2012; Volume 864, pp. 427–464. [Google Scholar]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- AOAC Official Method 2005.02. Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. pH differential method first action 2005. In Official Methods of Analysis, of AOAC International, 18th ed.; Revision 2; AOAC International: Gaithersburg, MD, USA, 2005; Chapter 37. [Google Scholar]
- Bayindirli, A.; Alpas, H.; Bozoğlu, F.; Hızal, M. Efficiency of high pressure treatment on inactivation of pathogenic microorganisms and enzymes in apple, orange, apricot and sour cherry juices. Food Control 2006, 17, 52–58. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Cubeddu, A.; Fava, P.; Pulvirenti, A.; Haghighi, H.; Licciardello, F. Suitability assessment of PLA bottles for high-pressure processing of apple juice. Foods 2021, 10, 295. [Google Scholar] [CrossRef]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of high pressure processing on the microbial inactivation in fruit preparations and other vegetable based beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]
- Tauscher, B. Pasteurization of food by hydrostatic high pressure: Chemical aspects. Z. Lebensm. Unters. Forsch. 1995, 200, 3–13. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Zabetakis, I.; Leclerc, D.; Kajda, P. The effect of high hydrostatic pressure on the strawberry anthocyanins. J. Agric. Food Chem. 2000, 48, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effects of high hydrostatic pressure on b-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria x ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus idaeus). Food Chem. 2005, 90, 193–197. [Google Scholar] [CrossRef]
- Gimenez, J.; Kajda, P.; Margomenou, L.; Piggott, J.R.; Zabetakis, I. A study on the colour and sensory attributes of high hydrostatic-pressure jams as compared with traditional jams. J. Sci. Food Agric. 2001, 81, 1228–1234. [Google Scholar] [CrossRef]
- Kouniaki, S.; Kajda, P.; Zabetakis, I. The effect if high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants (Ribes nigrum). Flavour Fragr. J. 2004, 19, 281–286. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Viljevac Vuletić, M.; Dugalić, K.; Mihaljević, I.; Tomaš, V.; Vuković, D.; Zdunić, Z.; Puškar, B.; Jurković, Z. Season, location and cultivar influence on bioactive compounds of sour cherry fruits. Plant Soil Environ. 2017, 63, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Okur, İ.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the effect of different extraction techniques on sour cherry pomace phenolic content and antioxidant activity and determination of phenolic compounds by FTIR and HPLC. Waste Biomass Valor. 2019, 10, 3545–3555. [Google Scholar] [CrossRef]



| Time (Days) | Total Mesophilic Bacteria (CFU/g) | Yeasts and Molds (CFU/g) | |
|---|---|---|---|
| Untreated sour cherries | 0 | 2.4 × 105 | 2.1 × 101 |
| 0 | - | - | |
| 10 | - | - | |
| 20 | 15 | - | |
| HPP-treated sour cherries | 32 | - | - |
| 62 | - | - | |
| 94 | - | - | |
| 122 | - | - | |
| 150 | - | - |
| Time (Days) | TP (mg GAE/g) | TF (mg QE/g) | TA (mg CGE/g) | |
|---|---|---|---|---|
| Control (untreated) | 0 | 11.69 ± 0.24 a | 1.32 ± 0.05 e | 10.63 ± 1.93 a |
| 0 | 11.54 ± 0.09 a | 1.75 ± 0.02 d | 9.47 ± 0.07 ab | |
| 10 | 10.67± 0.72 ab | 1.90 ± 0.01 c | 9.38 ± 0.47 ab | |
| 20 | 10.38 ± 0.81 ab | 1.90 ± 0.05 c | 8.46 ± 0.09 abc | |
| Sour cherries HPP-treated | 32 | 10.11 ± 0.03 ab | 1.98 ± 0.01 c | 8.24 ± 0.07 abc |
| 62 | 9.78 ± 1.30 ab | 2.18 ± 0.01 b | 6.93 ± 0.32 bc | |
| 94 | 9.53 ± 0.15 bc | 2.39 ± 0.04 a | 6.84 ± 0.75 bc | |
| 122 | 8.63 ± 0.04 bc | 2.22 ± 0.00 b | 6.52 ± 0.01 c | |
| 150 | 7.42 ± 0.02 c | 1.93 ± 0.02 c | 3.29 ± 0.00 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenuta, M.C.; Artoni, E.; Fava, P.; Bignami, C.; Licciardello, F. Shelf Life Extension and Nutritional Quality Preservation of Sour Cherries through High Pressure Processing. Foods 2023, 12, 342. https://doi.org/10.3390/foods12020342
Tenuta MC, Artoni E, Fava P, Bignami C, Licciardello F. Shelf Life Extension and Nutritional Quality Preservation of Sour Cherries through High Pressure Processing. Foods. 2023; 12(2):342. https://doi.org/10.3390/foods12020342
Chicago/Turabian StyleTenuta, Maria Concetta, Elisa Artoni, Patrizia Fava, Cristina Bignami, and Fabio Licciardello. 2023. "Shelf Life Extension and Nutritional Quality Preservation of Sour Cherries through High Pressure Processing" Foods 12, no. 2: 342. https://doi.org/10.3390/foods12020342
APA StyleTenuta, M. C., Artoni, E., Fava, P., Bignami, C., & Licciardello, F. (2023). Shelf Life Extension and Nutritional Quality Preservation of Sour Cherries through High Pressure Processing. Foods, 12(2), 342. https://doi.org/10.3390/foods12020342