The Impact of High-Intensity Ultrasound-Assisted Extraction on the Structural and Functional Properties of Hempseed Protein Isolate (HPI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HPI Based on AE-IEP and Ultrasonic-Assisted Extraction
2.3. Mechanistic Investigation on US-Assisted Hempseed Extraction
2.4. Ultrasound Calorimetry Power and Intensity Determination
2.5. Determination of Protein Concentration (Protein Assay)
2.6. Determination of Free -SH Group Content
2.7. Amino Acid Composition (HPLC)
2.8. Circular Dichroism (CD)
2.9. Intrinsic Fluorescence Emission
2.10. Identification of Protein Subunits (SDS-PAGE)
2.11. Surface Hydrophobicity (H0)
2.12. Protein Percentage Solubility
2.13. Protein Emulsifying Properties
2.14. Particle Size and Microstructure
2.15. Foaming Properties
2.16. Water Holding Capacity and Oil Holding Capacity
2.17. Statistical Analysis
3. Results
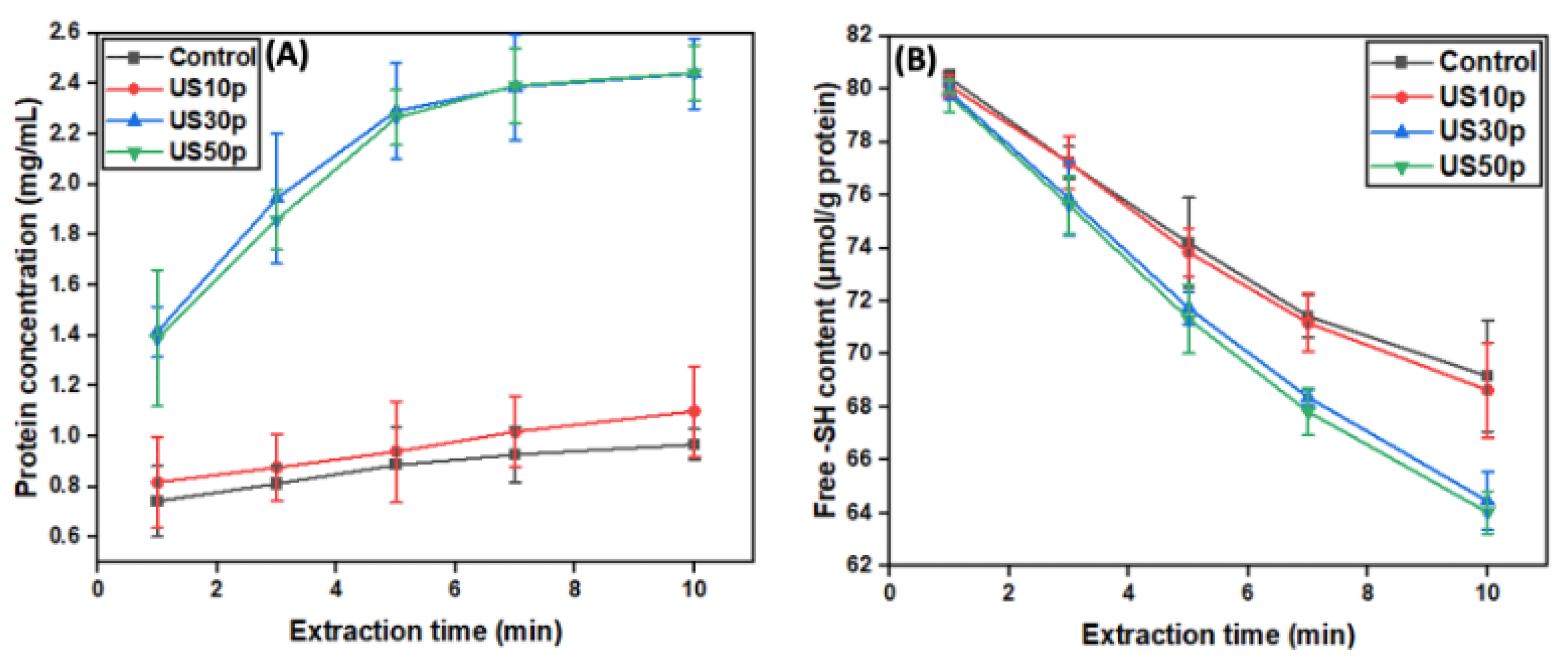
3.1. Mechanistic Investigation of US-Assisted Hempseed Protein Extraction
3.2. The Optimization of Ultrasound Parameter Selection
3.3. The Impact of Ultrasound Power on HPI Structural Properties
3.4. The Impact of Ultrasound on HPI Functional Properties
3.5. Proposed Mechanism of US-Assisted Hempseed Protein Extraction System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial hemp seed: From the field to value-added food ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef]
- Kim, J.-J.; Lee, M.-Y. Isolation and characterization of edestin from Cheungsam hempseed. J. Appl. Biol. Chem. 2011, 54, 84–88. [Google Scholar] [CrossRef] [Green Version]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Docimo, T.; Caruso, I.; Ponzoni, E.; Mattana, M.; Galasso, I. Molecular characterization of edestin gene family in Cannabis sativa L. Plant Physiol. Biochem. 2014, 84, 142–148. [Google Scholar] [CrossRef]
- Orio, L.P.; Boschin, G.; Recca, T.; Morelli, C.F.; Ragona, L.; Francescato, P.; Arnoldi, A.; Speranza, G. New ACE-inhibitory peptides from hemp seed (Cannabis sativa L.) proteins. J. Agric. Food Chem. 2017, 65, 10482–10488. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Lu, R.-R.; Qian, P.; Sun, Z.; Zhou, X.-H.; Chen, T.-P.; He, J.-F.; Zhang, H.; Wu, J. Hempseed protein derived antioxidative peptides: Purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010, 123, 1210–1218. [Google Scholar] [CrossRef]
- Raikos, V.; Duthie, G.; Ranawana, V. Denaturation and oxidative stability of hemp seed (Cannabis sativa L.) protein isolate as affected by heat treatment. Plant Foods Hum. Nutr. 2015, 70, 304–309. [Google Scholar] [CrossRef]
- Yin, S.W.; Tang, C.H.; Wen, Q.B.; Yang, X.Q. Functional and structural properties and in vitro digestibility of acylated hemp (Cannabis sativa L.) protein isolates. Int. J. Food Sci. Technol. 2009, 44, 2653–2661. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.L. Processing, nutrition, and functionality of hempseed protein: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 936–952. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M. Ultrasound-assisted processing: Science, technology and challenges for the plant-based protein industry. Ultrason. Sonochem. 2022, 84, 105955. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Farooq, M.A.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Manzoor, M.F. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef]
- Zisu, B.; Lee, J.; Chandrapala, J.; Bhaskaracharya, R.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effect of ultrasound on the physical and functional properties of reconstituted whey protein powders. J. Dairy Res. 2011, 78, 226. [Google Scholar] [CrossRef]
- Mohapatra, S.; Dandapat, S.; Thatoi, H. Physicochemical characterization, modelling and optimization of ultrasono-assisted acid pretreatment of two Pennisetum sp. using Taguchi and artificial neural networking for enhanced delignification. J. Environ. Manag. 2017, 187, 537–549. [Google Scholar] [CrossRef]
- Dabbour, M.; Jiang, H.; Mintah, B.K.; Wahia, H.; He, R. Ultrasonic-assisted protein extraction from sunflower meal: Kinetic modeling, functional, and structural traits. Innov. Food Sci. Emerg. Technol. 2021, 74, 102824. [Google Scholar] [CrossRef]
- Ghasemi Kia, A.; Ganjloo, A.; Bimakr, M. Optimization of Ultrasound-assisted Extraction of Fenugreek Seed Protein and Evaluation of Its Structural, Functional Properties and Antioxidant Activity. J. Food Sci. Technol. 2022, 19, 39–55. [Google Scholar]
- Hu, H.; Wu, J.; Li-Chan, E.C.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Karabulut, G.; Yemiş, O. Modification of hemp seed protein isolate (Cannabis sativa L.) by high-intensity ultrasound treatment. Part 1: Functional properties. Food Chem. 2022, 375, 131843. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Xue, F.; Adhikari, B. Application of ultrasound treatment to improve the technofunctional properties of hemp protein isolate. Future Foods 2022, 6, 100176. [Google Scholar] [CrossRef]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Popović, S.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques. Part I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uchida, T. Calorimetric method for measuring high ultrasonic power using water as a heating material. J. Phys. Conf. Ser. 2011, 279, 012012. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.; Nakai, S. Determination of SH-and SS-groups in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Martin, G.J.; Ashokkumar, M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 130057. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on the IgE binding capacity of Act d 2 allergen, secondary structure, and In-vitro digestibility of kiwifruit proteins. Ultrason. Sonochem. 2021, 71, 105409. [Google Scholar] [CrossRef]
- Wu, D.; Tang, L.; Duan, R.; Hu, X.; Geng, F.; Zhang, Y.; Peng, L.; Li, H. Interaction mechanisms and structure-affinity relationships between hyperoside and soybean β-conglycinin and glycinin. Food Chem. 2021, 347, 129052. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Y.; Xie, A.; Huang, H.; Hu, M.; Peng, J. Difference in an intermolecular disulfide-bond between two highly homologous serum proteins Leg1a and Leg1b implicates their functional differentiation. Biochem. Biophys. Res. Commun. 2021, 579, 81–88. [Google Scholar] [CrossRef]
- Gamlath, C.J.; Leong, T.S.; Ashokkumar, M.; Martin, G.J. Incorporating whey protein aggregates produced with heat and ultrasound treatment into rennet gels and model non-fat cheese systems. Food Hydrocoll. 2020, 109, 106103. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and functional properties of hemp seed protein products. J. Food Sci. 2014, 79, C1512–C1521. [Google Scholar] [CrossRef]
- Guo, Q.; Mu, T. Emulsifying properties of sweet potato protein: Effect of protein concentration and oil volume fraction. Food Hydrocoll. 2011, 25, 98–106. [Google Scholar] [CrossRef]
- Mettu, S.; Yao, S.; Sun, Q.; Lawson, S.R.; Scales, P.J.; Martin, G.J.O.; AshokKumar, M. Effect of bulk viscosity and emulsion droplet size on the separation efficiency of model mineral oil-in-water (O/W) emulsions under ultrasonic standing wave fields: A theoretical and experimental investigation. Ind. Eng. Chem. Res. 2020, 59, 7901–7912. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Ly, H.L.; Tran, T.M.C.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Application of ultrasound to protein extraction from defatted rice bran. Int. Food Res. J. 2018, 25, 695–701. [Google Scholar]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Zayas, J.F. Emulsifying properties of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 134–227. [Google Scholar]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Fu, Q. Optimization of ultrasound-assisted extraction process of perilla seed meal proteins. Food Sci. Biotechnol. 2012, 21, 1701–1706. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, P.; Dai, Y.; Luo, X.; Manickam, S.; Li, D.; Han, Y.; Show, P.L. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chem. Eng. J. 2022, 436, 135158. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Martin, G.J.; Ashokkumar, M. Turbulence-dependent reversible liquid-gel transition of micellar casein-stabilised emulsions. Food Hydrocoll. 2022, 131, 107819. [Google Scholar] [CrossRef]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.-B.; Rao, J.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Roman, L.; Swaraj, V.; Ragavan, K.; Vidal, N.P.; Dutcher, J.R.; Martinez, M.M. Hemp (Cannabis sativa L.) protein concentrates from wet and dry industrial fractionation: Molecular properties, nutritional composition, and anisotropic structuring. Food Hydrocoll. 2022, 131, 107755. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef]
- Jahan, K.; Ashfaq, A.; Islam, R.U.; Younis, K.; Yousuf, O. Optimization of ultrasound-assisted protein extraction from defatted mustard meal and determination of its physical, structural, and functional properties. J. Food Process. Preserv. 2022, 46, e16764. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, Z.; Zhang, C.; Hao, S.; Han, H.; Du, P.; Li, A.; Shao, H.; Li, C.; Liu, L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT 2021, 152, 112272. [Google Scholar] [CrossRef]
- Li, R.; Xiong, Y.L. Ultrasound-induced structural modification and thermal properties of oat protein. LWT 2021, 149, 111861. [Google Scholar] [CrossRef]
- Tamamizu-Kato, S.; Wong, J.Y.; Jairam, V.; Uchida, K.; Raussens, V.; Kato, H.; Ruysschaert, J.-M.; Narayanaswami, V. Modification by acrolein, a component of tobacco smoke and age-related oxidative stress, mediates functional impairment of human apolipoprotein E. Biochemistry 2007, 46, 8392–8400. [Google Scholar] [CrossRef]
- Wang, B.; Meng, T.; Ma, H.; Zhang, Y.; Li, Y.; Jin, J.; Ye, X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016, 32, 307–313. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: Effect of high intensity ultrasound. Food Hydrocoll. 2018, 81, 229–241. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Guo, X.; Lei, Y.; Yang, M. Effects of ultrasonic treatment on the structure, functional properties of chickpea protein isolate and its digestibility in vitro. Foods 2022, 11, 880. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Peng, Q.; Shan, Y.; Xue, F. Physicochemical properties of peanut protein isolate–glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Gao, K.; Rao, J.; Chen, B. Unraveling the mechanism by which high intensity ultrasound improves the solubility of commercial pea protein isolates. Food Hydrocoll. 2022, 25, 107823. [Google Scholar] [CrossRef]
- Yang, J.; Duan, Y.; Geng, F.; Cheng, C.; Wang, L.; Ye, J.; Zhang, H.; Peng, D.; Deng, Q. Ultrasonic-assisted pH shift-induced interfacial remodeling for enhancing the emulsifying and foaming properties of perilla protein isolate. Ultrason. Sonochem. 2022, 89, 106108. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, Y.; Li, J.; Wang, K.; Ma, C.; Sun, D.; Hussain, M.A.; Qayum, A.; Hou, J. Consecutive pH-shift and ultrasound treatment modify the physicochemical properties of whey protein isolate. Int. Dairy J. 2022, 127, 105211. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhu, C.; Liu, F.; Wang, S.; Liu, H.; Li, C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018, 98, 5690–5699. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Martínez, K.D.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Li, R.; Wang, Y.; Xiang, Q.; Li, K.; Bai, Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022, 124, 107351. [Google Scholar] [CrossRef]
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT 2022, 155, 112952. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Polyphenol removal from sunflower seed and kernel: Effect on functional and rheological properties of protein isolates. Food Hydrocoll. 2017, 63, 705–715. [Google Scholar] [CrossRef]
- Resendiz-Vazquez, J.; Ulloa, J.; Urías-Silvas, J.; Bautista-Rosales, P.; Ramírez-Ramírez, J.; Rosas-Ulloa, P.; González-Torres, L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017, 37, 436–444. [Google Scholar] [CrossRef]



| Function | Amount (μL) | Reagent |
|---|---|---|
| Draw | 2.5 | Borate buffer |
| Draw | 1.0 | Sample |
| Mix | 3.5 | - |
| Draw | 0.5 | OPA |
| Mix | 4.0 | - |
| Draw | 0.4 | FMOC |
| Mix | 4.4 | - |
| Draw | 32.0 | Injection diluent |
| Mix | 20.0 | - |
| Inject | 2.0 | - |
| Treatment | Extraction Yield (%) | EAI (m2/g) | ESI (min) | Particle Size of Extracted Protein Aggregates (μm) |
|---|---|---|---|---|
| Control | 17.6 ± 1.4 c | 6.6 ± 0.6 c | 20.6 ± 1.0 c | 45.7 ± 6.8 a |
| US30p, 1 min | 24.8 ± 3.6 b | 7.8 ± 1.2 b | 27.4 ± 3.3 b | 11.3 ± 2.1 b |
| US30p, 5 min | 35.1 ± 3.3 a | 8.7 ± 0.3 a | 31.6 ± 2.4 a | 5.4 ± 0.2 c |
| US30p, 10 min | 35.8 ± 2.5 a | 8.2 ± 0.7 b | 29.8 ± 1.6 ab | 7.2 ± 1.6 bc |
| Treatment | Ultrasound Power Intensity (W/cm2) | Extraction Yield (%) | Free -SH Content (μmol/g) | Particle Size of Extracted Protein Aggregates (μm) |
|---|---|---|---|---|
| Control | 0 | 17.5 ± 1.6 b | 19.1 ± 2.1 c | 45.7 ± 6.8 a |
| US10p, 5 min | 11.2 | 19.9 ± 2.4 b | 32.7 ± 2.8 b | 19.2 ± 2.3 b |
| US30p, 5 min | 26.8 | 35.1 ± 3.3 a | 48.0 ± 4.1 a | 5.4 ± 0.2 d |
| US50p, 5 min | 50.0 | 37.3 ± 2.1 a | 46.6 ± 5.3 a | 8.9 ± 0.4 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Li, W.; Wu, Y.; Martin, G.J.O.; Ashokkumar, M. The Impact of High-Intensity Ultrasound-Assisted Extraction on the Structural and Functional Properties of Hempseed Protein Isolate (HPI). Foods 2023, 12, 348. https://doi.org/10.3390/foods12020348
Yao S, Li W, Wu Y, Martin GJO, Ashokkumar M. The Impact of High-Intensity Ultrasound-Assisted Extraction on the Structural and Functional Properties of Hempseed Protein Isolate (HPI). Foods. 2023; 12(2):348. https://doi.org/10.3390/foods12020348
Chicago/Turabian StyleYao, Shunyu, Wu Li, Yue Wu, Gregory J. O. Martin, and Muthupandian Ashokkumar. 2023. "The Impact of High-Intensity Ultrasound-Assisted Extraction on the Structural and Functional Properties of Hempseed Protein Isolate (HPI)" Foods 12, no. 2: 348. https://doi.org/10.3390/foods12020348







