Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
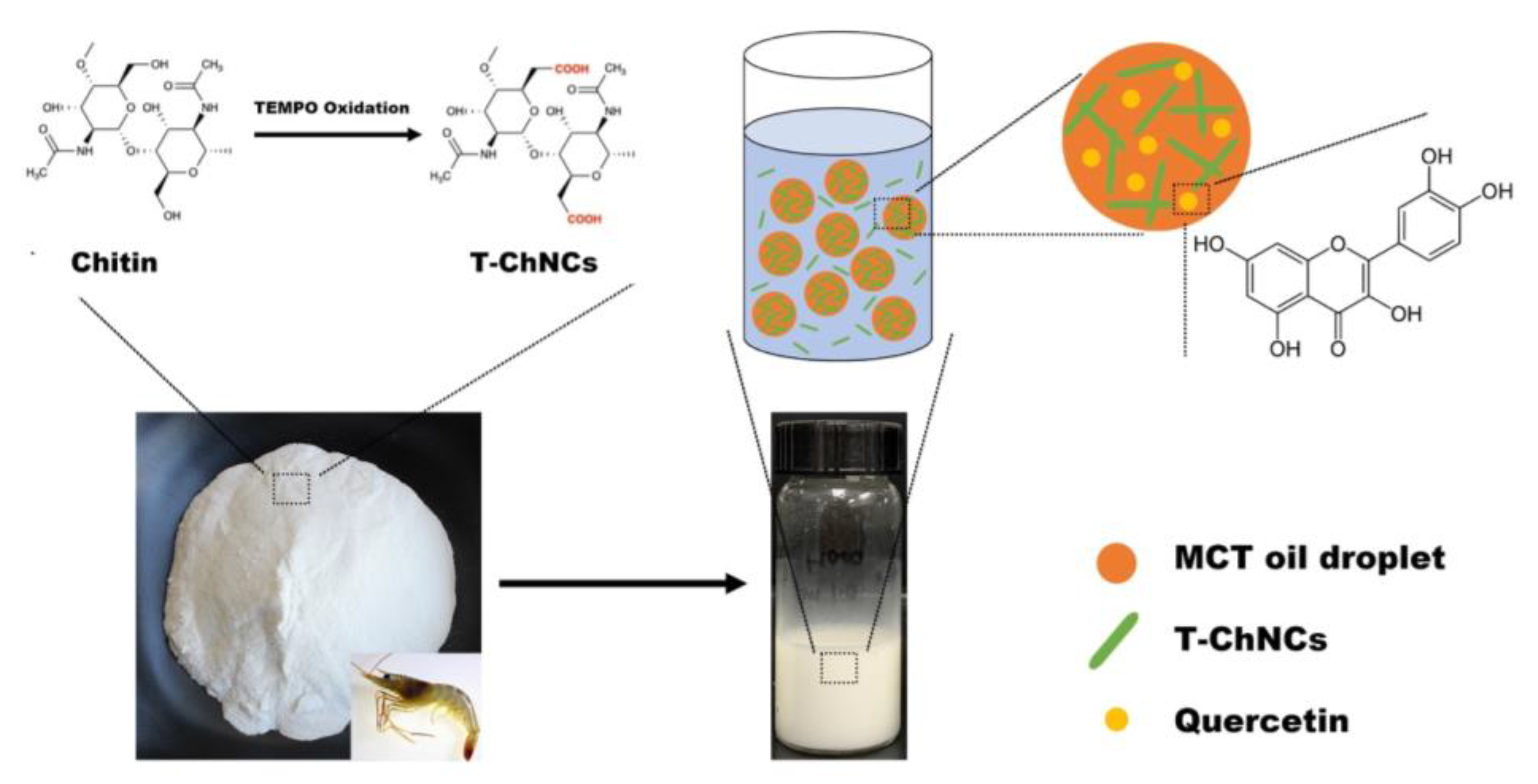
2.2. T-ChNCs Preparation
2.3. O/W Pickering Emulsion Preparation
2.4. Characterization of T-ChNCs and Pickering Emulsions
2.5. Emulsion Stability
2.6. Encapsulation Efficiency (EE)
2.7. Radical Scavenging Assay
2.8. In Vitro Release Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of T-ChNCs
3.2. Factors Affecting Pickering Emulsions Stabilized by T-ChNCs
3.3. Storage Stability
3.4. Microstructure of O/W Pickering Emulsions Stabilized with T-ChNCs
3.5. Encapsulation Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.R.; Heussen, P.C.; Hazekamp, J.; Drost, E.; Velikov, K.P. Quercetin loaded biopolymeric colloidal particles prepared by simultaneous precipitation of quercetin with hydrophobic protein in aqueous medium. Food Chem. 2012, 133, 423–429. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, Z.; Tsai, S.; Zhang, H.; Li, Y.; Yuan, F.; Wang, Q. Curcumin-Loaded Pickering Emulsion Formed by Ultrasound and Stabilized by Metal Organic Framework Optimization. Foods 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Gou, H.; Rao, H.; Zhao, G. Recent progress in nanoclay-based Pickering emulsion and applications. J. Environ. Chem. Eng. 2021, 9, 105941. [Google Scholar] [CrossRef]
- Griffith, C.; Daigle, H. Destabilizing Pickering emulsions using fumed silica particles with different wettabilities. J. Colloid Interface Sci. 2019, 547, 117–126. [Google Scholar] [CrossRef]
- Feldbaum, R.A.; Yaakov, N.; Mani, K.A.; Yossef, E.; Metbeev, S.; Zelinger, E.; Belausov, E.; Koltai, H.; Ment, D.; Mechrez, G. Single cell encapsulation in a Pickering emulsion stabilized by TiO2 nanoparticles provides protection against UV radiation for a biopesticide. Colloids Surf. B Biointerfaces 2021, 206, 111958. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, Z.; Lu, D.; Li, B.; Ren, L.; Di, W.; Yu, H.; He, J.; Sun, D. pH and magnetism dual-responsive Pickering emulsion stabilized by dynamic covalent Fe3O4 nanoparticles. Nanomaterials 2022, 12, 2587. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A.; Hejazi, S.H.; Gates, I. Dual stimuli-responsive Pickering emulsions from novel magnetic hydroxyapatite nanoparticles and their characterization using a microfluidic platform. Langmuir 2021, 37, 1353–1364. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Chen, Y. Fabrication of carbon nanotubes/polystyrene nanocomposites via Pickering emulsion polymerization. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 840–843. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Hua, N.Q.; Rusli, W.; Hadia, N.J.; Stubbs, L.P. Unique oil-in-brine Pickering emulsion using responsive antipolyelectrolyte functionalized latex: A versatile emulsion stabilizer. ACS Appl. Mater. Interfaces 2020, 12, 23443–23452. [Google Scholar] [CrossRef]
- Zheng, R.; Binks, B.P. Pickering emulsions stabilized by polystyrene particles possessing different surface groups. Langmuir 2022, 38, 1079–1089. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of food-grade Pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M.; Tabarestani, H.S. Production of d-limonene-loaded Pickering emulsions stabilized by chitosan nanoparticles. Food Chem. 2021, 354, 129591. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qiu, C.; Jin, Z.; Qin, Y.; Zhan, C.; Xu, X.; Wang, J. Pickering emulsions with enhanced storage stabilities by using hybrid β-cyclodextrin/short linear glucan nanoparticles as stabilizers. Carbohydr. Polym. 2020, 229, 115418. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huan, S.; Bai, L.; Ketola, A.; Shi, X.; Zhang, X.; Ketoja, J.A.; Rojas, O.J. High internal phase oil-in-water pickering emulsions stabilized by chitin nanofibrils: 3D structuring and solid foam. ACS Appl. Mater. Interfaces 2020, 12, 11240–11251. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Magdassi, S.; Neiroukh, Z. Chitin particles as O/W emulsion stabilizers. J. Dispers Sci. Technol. 1990, 11, 69–74. [Google Scholar] [CrossRef]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Perrin, E.; Bizot, H.; Cathala, B.; Capron, I. Chitin nanocrystals for Pickering high internal phase emulsions. Biomacromolecules 2014, 15, 3766–3771. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE 2019, 14, e0213189. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, A.; Chibowski, E. Zeta potential, effective diameter and multimodal size distribution in oil/water emulsion. Colloids Surf. A Physicochem. Eng. Asp. 1999, 159, 253–261. [Google Scholar] [CrossRef]
- Pedersen, J.S. Analysis of small-angle scattering data from colloids and polymer solutions: Modeling and least-squares fitting. Adv. Colloid Interface Sci. 1997, 70, 171–210. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohydr. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Development of stable curcumin nanoemulsions: Effects of emulsifier type and surfactant-to-oil ratios. J. Food Sci. Technol. 2018, 55, 3485–3497. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Y.; Briber, R.M. Efficient production of oligomeric chitin with narrow distributions of degree of polymerization using sonication-assisted phosphoric acid hydrolysis. Carbohydr. Polym. 2022, 276, 118736. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- Ye, W.; Liu, L.; Wang, Z.; Yu, J.; Fan, Y. Investigation of pretreatment methods for improving TEMPO-mediated oxidation and nanofibrillation efficiency of α-chitin. ACS Sustain. Chem. Eng. 2019, 7, 19463–19473. [Google Scholar] [CrossRef]
- Ifuku, S.; Hori, T.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of zwitterionically charged nanocrystals by surface TEMPO-mediated oxidation and partial deacetylation of α-chitin. Carbohydr. Polym. 2015, 122, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liu, K.; Zhan, C.; Geng, L.; Chu, B.; Hsiao, B.S. Characterization of nanocellulose using small-angle neutron, X-ray, and dynamic light scattering techniques. J. Phys. Chem. B 2017, 121, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, H.-L.; Hwang, D.S.; Lee, J.Y.; Cha, H.G.; Koo, J.M.; Hwang, S.Y.; Park, J.; Oh, D.X. Five different chitin nanomaterials from identical source with different advantageous functions and performances. Carbohydr. Polym. 2019, 205, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, C.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X.; Qu, S. In vitro digestion of oil-in-water emulsions stabilized by regenerated chitin. J. Agric. Food Chem. 2018, 66, 12344–12352. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Bian, W.; Feng, L.; Wu, Z.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsions with regenerated chitin nanofibers. Food Chem. 2015, 183, 115–121. [Google Scholar] [CrossRef]
- Wiącek, A.; Chibowski, E. Application of an extended DLVO theory for the calculation of the interactions between emulsified oil droplets in alcohol solutions. Colloids Surf. B Biointerfaces 1999, 14, 19–26. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, J.; Teng, Z.; Zhang, Y.; Bauchan, G.R.; Luo, Y.; Liu, D.; Wang, Q. Metal–organic framework-stabilized high internal phase Pickering emulsions based on computer simulation for curcumin encapsulation: Comprehensive characterization and stability mechanism. ACS Omega 2021, 6, 26556–26565. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT Food Sci. Technol. 2017, 84, 34–46. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Ma, P.; Taylor, K.S.-Y.; Tarwa, K.; Mao, Y.; Wang, Q. Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin. Foods 2023, 12, 367. https://doi.org/10.3390/foods12020367
Jia X, Ma P, Taylor KS-Y, Tarwa K, Mao Y, Wang Q. Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin. Foods. 2023; 12(2):367. https://doi.org/10.3390/foods12020367
Chicago/Turabian StyleJia, Xiaoxue, Peihua Ma, Kim Shi-Yun Taylor, Kevin Tarwa, Yimin Mao, and Qin Wang. 2023. "Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin" Foods 12, no. 2: 367. https://doi.org/10.3390/foods12020367
APA StyleJia, X., Ma, P., Taylor, K. S.-Y., Tarwa, K., Mao, Y., & Wang, Q. (2023). Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin. Foods, 12(2), 367. https://doi.org/10.3390/foods12020367







