Indigenous Lactic Acid Bacteria Isolated from Raw Graviera Cheese and Evaluation of Their Most Important Technological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cheese Making
2.2. Samples
2.3. Isolation of Bacteria and Growth Conditions
2.4. Technological Characterization
2.5. Phenotypic Characterization of Isolates
2.6. Molecular Identification
2.6.1. DNA Extraction
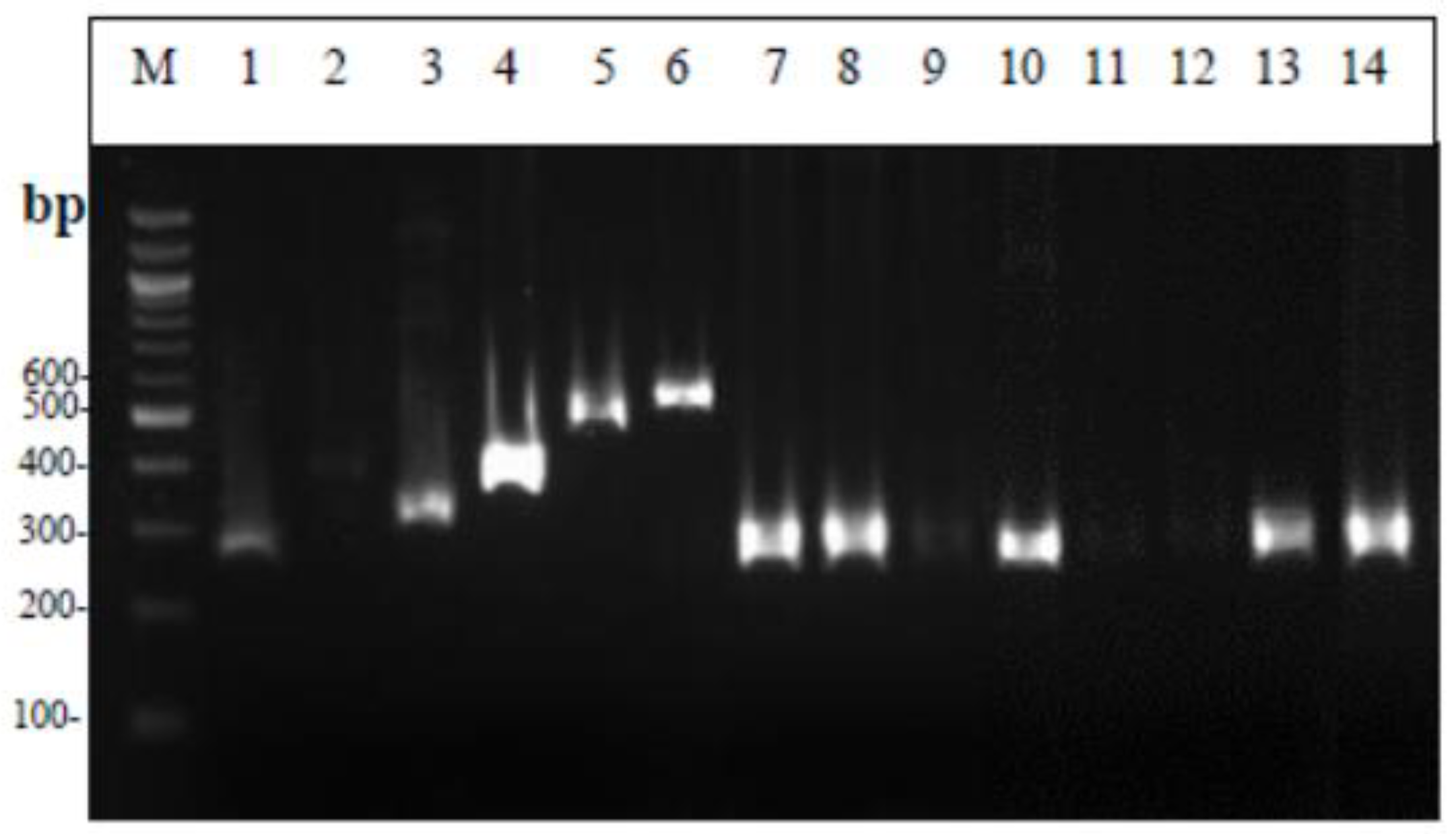
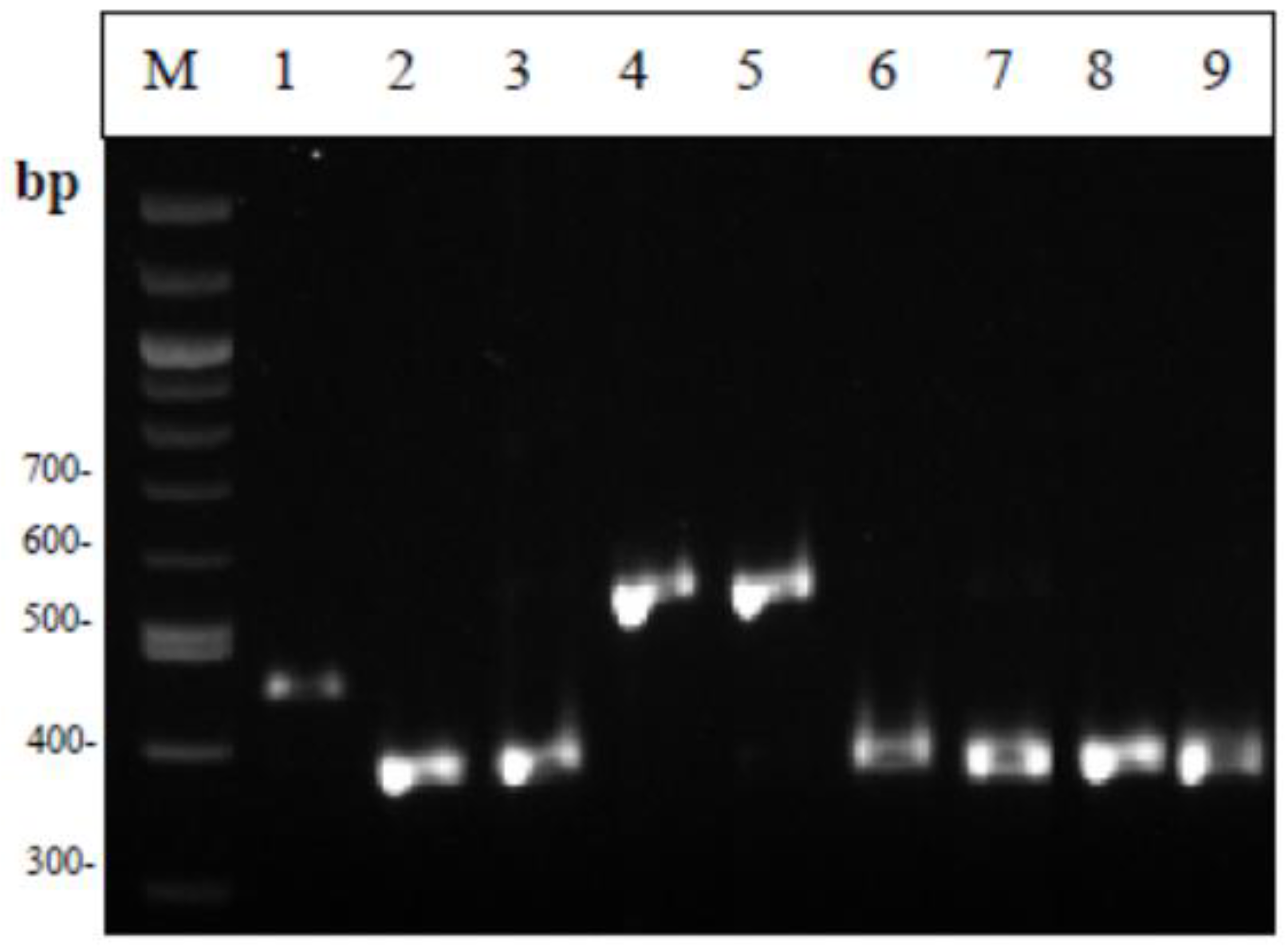
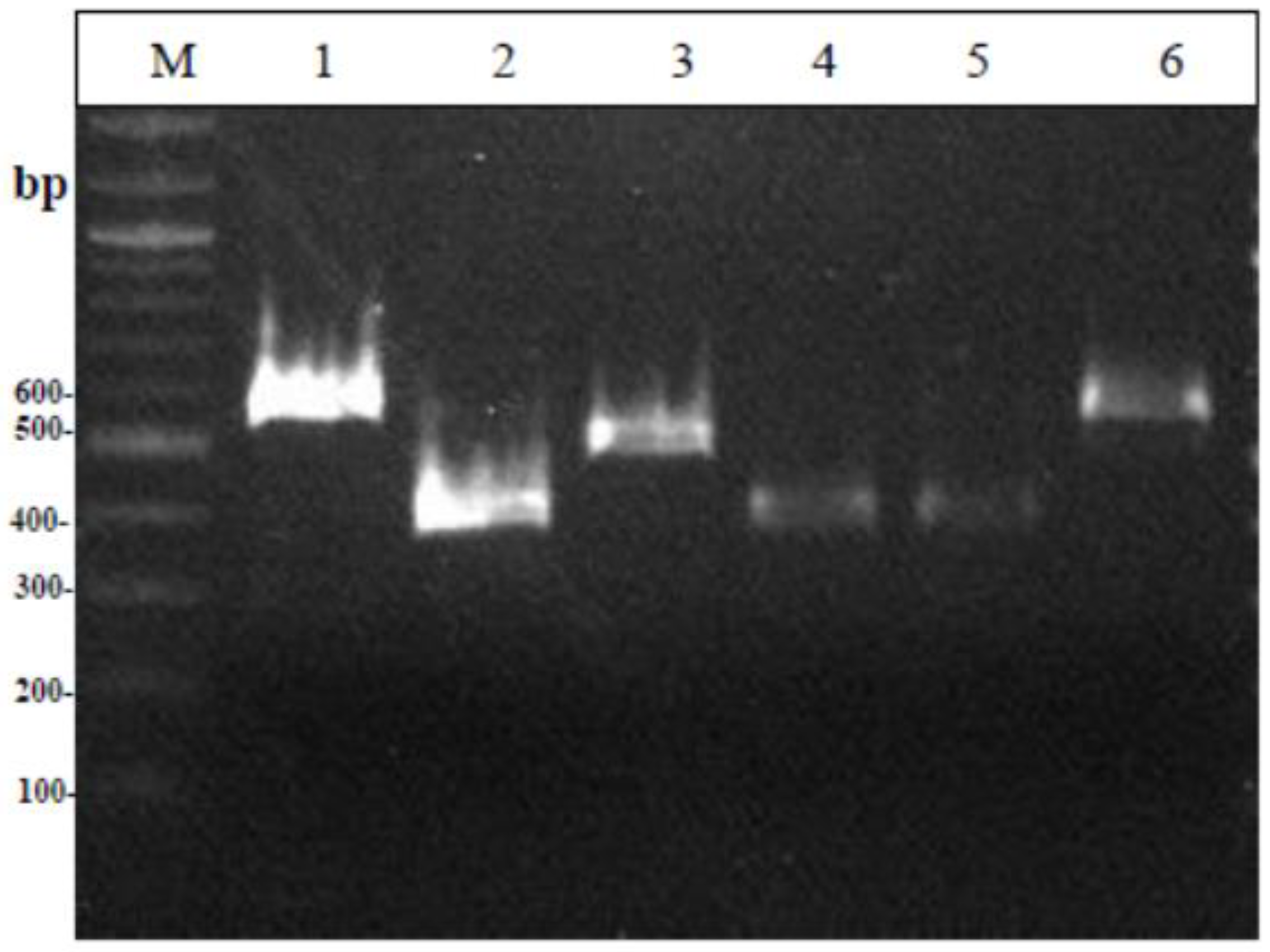
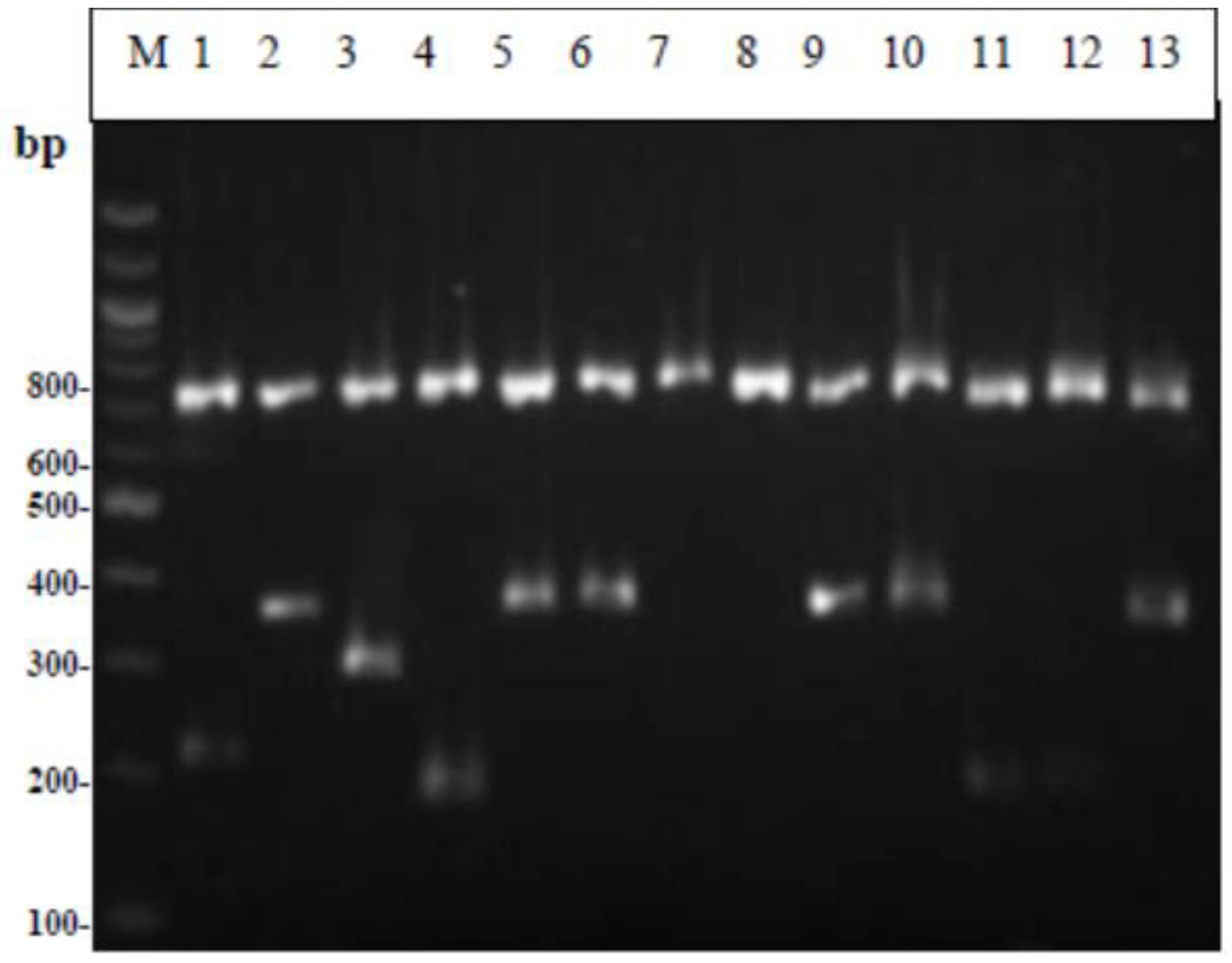
2.6.2. Multiplex PCR
2.6.3. Sequencing
3. Results
3.1. Technological Characterization
3.2. Phenotypic Identification
3.3. Molecular Identification
3.4. Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginzinger, W.; Jaros, D.; Lavanchy, P.; Rohm, H. Raw milk flora affects composition and quality of Bergkäse. 3. Physical and sensory properties, and conclusions. Le Lait 1999, 79, 411–421. [Google Scholar] [CrossRef]
- McSweeney, P.; Fox, P.; Lucey, J.; Jordan, K.N.; Cogan, T. Contribution of the indigenous microflora to the maturation of Cheddar cheese. Int. Dairy J. 1993, 3, 613–634. [Google Scholar] [CrossRef]
- Kilcawley, K.N. Cheese Flavour. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Grappin, R.; Beuvier, E. Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese. Int. Dairy J. 1997, 7, 751–761. [Google Scholar] [CrossRef]
- Wouters, J.; Ayad, E.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Gemechu, T. Review on lactic acid bacteria function in milk fermentation and preservation. Aust. J. Fr. Stud. 2015, 9, 170–175. [Google Scholar]
- Beresford, T.; Fitzsimons, N.; Brennan, N.; Cogan, T. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Martley, F.; Crow, V. Interactions between non-starter microorganisms during cheese manufacture and repening. Int. Dairy J. 1993, 3, 461–483. [Google Scholar] [CrossRef]
- Hansen, E. Commercial bacterial starter cultures for fermented foods of the future. Int. J. Food Microbiol. 2002, 78, 119–131. [Google Scholar] [CrossRef]
- Topisirovic, L.; Kojic, M.; Fira, D.; Golic, N.; Strahinic, I.; Lozo, J. Potential of lactic acid bacteria isolated from specific natural niches in food production and preservation. Int. J. Food Microbiol. 2006, 112, 230–235. [Google Scholar] [CrossRef]
- Van Hylckama Vlieg, J.; Rademaker, J.; Bachmann, H.; Molenaar, D.; Kelly, W.; Siezen, R. Natural diversity and adaptive responses of Lactococcus lactis. Curr. Opin. Biotechnol. 2006, 17, 183–190. [Google Scholar] [CrossRef]
- Centeno, J.; Menendez, S.; Hermida, M.; Rodrıguez-Otero, J. Effects of the addition of Enterococcus faecalis in Cebreiro cheese manufacture. Int. J. Food Microbiol. 1999, 48, 97–111. [Google Scholar] [CrossRef]
- Menéndez, S.; Centeno, J.; Godınez, R.; Rodrıguez-Otero, J. Effects of Lactobacillus strains on the ripening and organoleptic characteristics of Arzúa-Ulloa cheese. Int. J. Food Microbiol. 2000, 59, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.; Godınez, R.; Hermida, M.; Centeno, J.; Rodrıguez-Otero, J. Characteristics of “Tetilla” pasteurized milk cheese manufactured with the addition of autochthonous cultures. Food Microbiol. 2004, 21, 97–104. [Google Scholar] [CrossRef]
- Freni Tavaria, A.; Silva-Ferreira, C.; Malcata, F.X. Contribution of wild strains of lactic acid bacteria to the typical aroma of an artisanal cheese. In Developments in Food Science; Wender, M.A., Bredie, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 43, pp. 129–132. [Google Scholar]
- Durlu-Ozkaya, F.; Xanthopoulos, V.; Tunail, N.; Litopoulou-Tzanetaki, E. Technologically important properties of lactic acid bacteria isolates from Beyaz cheese made from raw ewes’ milk. J. Appl. Microbiol. 2001, 91, 861–870. [Google Scholar] [CrossRef]
- Georgieva, M.; Andonova, L.; Peikova, L.; Zlatkov, A. Probiotics–Health benefits, classification, quality assurance and quality control–Review. Pharmacia 2014, 61, 22–31. [Google Scholar]
- Floros, G.; Hatzikamari, M.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Probiotic and technological properties of facultatively heterofermentative lactobacilli from Greek traditional cheeses. Food Biotechnol. 2012, 26, 85–105. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.; Poltronieri, P.; Morea, M.; Baruzzi, F. Autochthonous and probiotic lactic acid bacteria employed for production of “advanced traditional cheeses”. Foods 2019, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, W.; Hertel, C. Genus Lactobacillus. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; The Firmicutes; Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 3. [Google Scholar]
- Holzapfel, W.H.; Biörkroth, J.A.; Dicks, L.M.T. Genus I. Leuconostoc. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; The Firmicutes; Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Tsirigoti, E.; Psomas, E.; Ekateriniadou, L.V.; Papadopoulos, A.I.; Boukouvala, E. Comparative qualitative and quantitative analysis of lactic acid bacteria by molecular methods in different Greek cheeses. J. Dairy Res. 2022, in press. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B. Use of a Genus- and Species-Specific Multiplex PCR for Identification of Enterococci. J. Clin. Microb. 2004, 42, 3558–3565. [Google Scholar] [CrossRef] [Green Version]
- Deasy, B.; Rea, M.; Fitzgerald, G.; Cogan, T.; Beresford, T. A rapid PCR based method to distinguish between Lactococcus and Enterococcus. Syst. Appl. Microbiol. 2000, 23, 510–522. [Google Scholar] [CrossRef]
- Hou, Q.; Bai, X.; Li, W.; Gao, X.; Zhang, F.; Sun, Z.; Zhang, H. Design of primers for evaluation of lactic acid bacteria populations in complex biological samples. Front. Microbiol. 2018, 9, 2045. [Google Scholar] [CrossRef]
- Sharpe, M. Identification of the lactic acid bacteria. In Identification Methods for Microbiologists; Skinner, F., Lovelock, W., Eds.; Academic Press: London, UK, 1979; pp. 233–259. [Google Scholar]
- Harrigan, W.; McCance, M. Laboratory Methods in Food and Dairy Microbiology. Academic Press Inc.: London, UK, 1976.
- Routray, W.; Mishra, H. Scientific and Technical Aspects of Yogurt Aroma and Taste: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Schillinger, U.; Lücke, F. Identification of lactobacilli from meat and meat products. Food Microbiol. 1987, 4, 199–208. [Google Scholar] [CrossRef]
- Devriese, L.; Pot, B.; Collins, M. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J. Appl. Bacteriol. 1993, 75, 399–408. [Google Scholar] [CrossRef]
- Giraffa, G. Overview of the Ecology and Biodiversity of the LAB. In Lactic Acid Bacteria; John Willey and Sons, Ltd.: Chichester, UK, 2014; Volume 9781444333, pp. 45–54. [Google Scholar]
- Facklam, R.R.; Collins, M.D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J. Clin. Microbiol. 1989, 27, 731–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathot, A.; Kihal, M.; Prevost, H.; Divies, C. Selective enumeration of Leuconostoc on vancomycin agar media. Int. Dairy J. 1994, 4, 459–469. [Google Scholar] [CrossRef]
- Björkroth, J.; Holzapfel, W. Leuconostoc, Oenococcus and Weissella. In The Prokaryotes: A Handbook on the Biology of Bacteria: Firmicutes, Cyanobacteria, 3rd ed.; Dworkin, M., Ed.; Springer-Verlag: New York, NY, USA, 2006; Volume 4, pp. 267–319. [Google Scholar]
- Funel, A. Leuconostocaceae Family. In Encyclopedia of Food Microbiology, 2nd ed.: Elsevier: Amsterdam, The Netherlands; Volume 2, pp. 455–465. [CrossRef]
- Holzapfel, W.; Wood, B. Introduction to the LAB. In Lactic Acid Bacteria; Holzapfel, W., Wood, B., Eds.; John Wiley & Sons: Chichester, UK, 2014; Volume 9781444333831, pp. 1–12. [Google Scholar]
- Collins, M.; Phillips, B.; Zanoni, P. Deoxyribonucleic Acid Homology Studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 1989, 39, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimons, N.; Cogan, T.; Condon, S.; Beresford, T. Phenotypic and genotypic characterization of non-starter lactic acid bacteria in mature cheddar cheese. Appl. Environ. Microbiol. 1999, 65, 3418–3426. [Google Scholar] [CrossRef] [Green Version]
- Vancanneyt, M.; Naser, S.; Engelbeen, K.; De Wachter, M.; Van der Meulen, R.; Cleenwerck, I.; Swings, J. Reclassification of Lactobacillus brevis strains LMG 11494 and LMG 11984 as Lactobacillus parabrevis sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 1553–1557. [Google Scholar] [CrossRef] [Green Version]
- Montel, M.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef]
- Tornadijo, M.; Fresno, J.; Bernardo, A.; Martin Sarmiento, R.; Carballo, J. Microbiological Changes throughout the Manufacturing and Ripening of a Spanish Goat’s Raw Milk Cheese (Armada Variety). Le Lait 1995, 75, 551–570. [Google Scholar] [CrossRef]
- Soldatou, H.; Psoni, L.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Populations, Types and Biochemical Activities of Aerobic Bacteria and Lactic Acid Bacteria from the Air of Cheese Factories. Int. J. Dairy Technol. 2006, 59, 200–208. [Google Scholar] [CrossRef]
- Picon, A.; Garde, S.; Ávila, M.; Nuñez, M. Microbiota Dynamics and Lactic Acid Bacteria Biodiversity in Raw Goat Milk Cheeses. Int. Dairy J. 2016, 59, 14–22. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. Microbiological Characteristics of Greek Traditional Cheeses. Small Rumin. Res. 2011, 101, 17–32. [Google Scholar] [CrossRef]
- Fortina, M.; Ricci, G.; Foschino, R.; Picozzi, C.; Dolci, P.; Zeppa, G.; Manachini, P. Phenotypic Typing, Technological Properties and Safety Aspects of Lactococcus Garvieae Strains from Dairy Environments. J. Appl. Microbiol. 2007, 103, 445–453. [Google Scholar] [CrossRef]
- Nikolaou, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Robinson, R. Changes in the microbiological and chemical characteristics of an artisanal, low-fat cheese made from raw ovine milk during ripening. Int. J. Dairy Technol. 2002, 55, 12–17. [Google Scholar] [CrossRef]
- Silvetti, T.; Capra, E.; Morandi, S.; Cremonesi, P.; Decimo, M.; Gavazzi, F.; Brasca, M. Microbial population profile during ripening of Protected Designation of Origin (PDO) Silter cheese, produced with and without autochthonous starter culture. LTW-Food Sci. Technol. 2017, 84, 821–831. [Google Scholar] [CrossRef]
- Veljovic, K.; Terzic-Vidojevic, A.; Vukasinovic, M.; Strahinic, I.; Begovic, J.; Lozo, J.; Topisirovic, L. Preliminary Characterization of Lactic Acid Bacteria Isolated from Zlatar Cheese. J. Appl. Microbiol. 2007, 103, 2142–2152. [Google Scholar] [CrossRef]
- Vandera, E.; Kakouri, A.; Koukkou, A.; Samelis, J. Major Ecological Shifts within the Dominant Nonstarter Lactic Acid Bacteria in Mature Greek Graviera Cheese as Affected by the Starter Culture Type. Int. J. Food Microbiol. 2019, 290, 15–26. [Google Scholar] [CrossRef]
- Prodromou, K.; Thasitou, P.; Haritonidou, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Microbiology of ‘Orinotyri’, a Ewe’s Milk Cheese from the Greek Mountains. Food Microbiol. 2001, 18, 319–328. [Google Scholar] [CrossRef]
- Franciosi, E.; Settanni, L.; Carlin, S.; Cavazza, A.; Poznanski, E. A factory-scale application of secondary adjunct cultures selected from lactic acid bacteria during Puzzone di Moena cheese ripening. J. Dairy Sci. 2008, 91, 2981–2991. [Google Scholar] [CrossRef] [Green Version]
- Mama, V.; Chatzikamari, M.; Lombardi, A.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Lactobacillus paracasei subsp. paracasei heterogeneity: The diversity among strains isolated from traditional Greek cheeses. Ital. J. Food Sci. 2002, 14, 351–362. [Google Scholar]
- Albenzio, M.; Corbo, M.; Rehman, S.; Fox, P.; De Angelis, M.; Corsetti, A.; Gobbetti, M. Microbiological and biochemical characteristics of Canestrato Pugliese cheese made from raw milk, pasteurized milk or by heating the curd in hot whey. Int. J. Food Microbiol. 2001, 67, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Ayad, E. Starter Culture Development for Improving Safety and Quality of Domiati Cheese. Food Microbiol. 2009, 26, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ayad, E.; Verheul, A.; Wouters, J.; Smit, G. Application of Wild Starter Cultures for Flavour Development in Pilot Plant Cheese Making. Int. Dairy J. 2000, 10, 169–179. [Google Scholar] [CrossRef]
- Ortigosa, M.; Torre, P.; Izco, J. Effect of Pasteurization of Ewe’s Milk and Use of a Native Starter Culture on the Volatile Components and Sensory Characteristics of Roncal Cheese. J. Dairy Sci. 2001, 84, 1320–1330. [Google Scholar] [CrossRef]
- Samelis, J.; Kakouri, A.; Pappa, E.; Bogovic Matijasic, B.; Georgalaki, M.; Tsakalidou, E.; Rogelj, I. Microbial Stability and Safety of Traditional Greek Graviera Cheese: Characterization of the Lactic Acid Bacterial Flora and Culture-Independent Detection of Bacteriocin Genes in the Ripened Cheeses and Their Microbial Consortia. J. Food Prot. 2010, 73, 1294–1303. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Bozoudi, D.; Pavlidou, S.; Kotzamanidis, C.; Hatzikamari, M.; Zdragas, A.; Litopoulou-Tzanetaki, E. Technological, phenotypic and genotypic characterization of lactobacilli from Graviera Kritis PDO Greek cheese, manufactured at two traditional dairies. LWT-Food Sci. Technol. 2016, 68, 681–689. [Google Scholar] [CrossRef]
- Sánchez, I.; Seseña, S.; Poveda, J.; Cabezas, L.; Palop, L. Phenotypic and Genotypic Characterization of Lactobacilli Isolated from Spanish Goat Cheeses. Int. J. Food Microbiol. 2005, 102, 355–362. [Google Scholar] [CrossRef]
- Crow, V.; Curry, B.; Hayes, M. The Ecology of Non-Starter Lactic Acid Bacteria (NSLAB) and Their Use as Adjuncts in New Zealand Cheddar. Int. Dairy J. 2001, 11, 275–283. [Google Scholar] [CrossRef]
- Psoni, L.; Kotzamanides, C.; Andrighetto, C.; Lombardi, A.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Genotypic and Phenotypic Heterogeneity in Enterococcus Isolates from Batzos, a Raw Goat Milk Cheese. Int. J. Food Microbiol. 2006, 109, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, O.; Akbulut, N. In vitro characterisation of probiotic properties of Enterococcus faecium and Enterococcus durans strains isolated from raw milk and traditional dairy products. Int. J. Dairy Technol. 2020, 73, 98–107. [Google Scholar] [CrossRef]
- Bintsis, T.; Vafopoulou-Mastrojiannaki, A.; Litopoulou-Tzanetaki, E.; Robinson, R. Protease, peptidase and esterase activities by lactobacilli and yeast isolates from Feta cheese brine. J. Appl. Microbiol. 2003, 95, 68–77. [Google Scholar] [CrossRef]
- El-Din, B.B.; El-Soda, M.; Ezzat, N. Proteolytic, lipolytic and autolytic activities of enterococci strains isolated from Egyptian dairy products. Le Lait 2002, 82, 289–304. [Google Scholar] [CrossRef]
- Vedamuthu, E. Starter cultures for yogurt and fermented milks. In Manufacturing Yogurt and Fermented Milks; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 89–116. [Google Scholar]
| Isolation Stage | Media-Strain | CLOT 32 °C | CLOT 42 °C | AROMA 32 °C | AROMA 42 °C |
|---|---|---|---|---|---|
| Raw milk | RO-1 | + | − | yes | yes |
| RO-2 | + | + | yes | no | |
| M17-1 | + | − | yes | yes | |
| MRS-1 | + | + | yes | yes | |
| MRS-2 | + | + | yes | yes | |
| RO-3 | + | + | yes | yes | |
| KAA-1 | + | + | yes | yes | |
| Cheese after salting | MRS-3 | + | + | yes | yes |
| RO-4 | + | + | yes | yes | |
| RO-5 | + | + | yes | yes | |
| MRS-4 | + | + | yes | yes | |
| MRS-5 | + | + | yes | yes | |
| M17-2 | + | + | yes | yes | |
| M17-3 | + | + | yes | yes | |
| KAA-2 | + | + | yes | yes | |
| KAA-3 | + | + | yes | yes | |
| Cheese (30 days) | MRS-6 | + | + | yes | yes |
| MRS-7 | + | + | yes | yes | |
| RO-6 | + | + | yes | yes | |
| M17-4 | + | − | yes | yes | |
| M17-5 | + | − | yes | no | |
| RO-7 | + | + | yes | yes | |
| MRS-8 | + | + | yes | yes | |
| MRS-9 | + | + | yes | yes | |
| M17-6 | + | + | yes | yes | |
| KAA-4 | + | + | yes | yes | |
| KAA-5 | + | + | yes | yes | |
| KAA-6 | + | + | yes | yes | |
| Cheese (60 days) | MRS-10 | + | + | yes | yes |
| RO-8 | + | + | yes | yes | |
| RO-9 | + | + | yes | yes | |
| MRS-11 | + | + | yes | yes | |
| RO-10 | − | + | no | yes | |
| RO-11 | + | + | yes | yes | |
| M17-7 | + | + | yes | yes | |
| KAA-7 | + | - | yes | yes | |
| Cheese (90 days) | MRS-12 | + | + | yes | yes |
| MRS-13 | + | + | yes | yes | |
| RO-12 | + | + | yes | yes | |
| RO-13 | − | − | yes | no | |
| KAA-8 | + | + | yes | yes | |
| KAA-9 | + | − | yes | yes |
| Growth Media | Strain | Species |
|---|---|---|
| MRS | 1 | Lc. garvieae |
| MRS | 2 | Lc. garvieae |
| MRS | 4 | Lc. lactis subsp. lactis |
| MRS | 5 | Lactococcus spp. |
| M17 | 1 | Lc. garvieae |
| M17 | 4 | Lc. lactis subsp. lactis |
| M17 | 2 | Streptococcus spp. |
| M17 | 7 | Streptococcus spp. |
| RO | 1 | Lu. pseudomesenteroides |
| RO | 2 | Lu. pseudomesenteroides |
| KAA | 1 | Ent. faecalis |
| KAA | 2 | Ent. faecalis |
| KAA | 3 | Ent. gallinarum |
| KAA | 4 | Ent. faecalis |
| KAA | 5 | Ent. faecalis |
| KAA | 6 | Ent. gallinarum |
| KAA | 7 | Ent. faecium |
| KAA | 8 | Ent. faecalis |
| KAA | 9 | Ent. faecium |
| Growth Media | Strain | Species |
|---|---|---|
| MRS | 3 | Lb. paracasei subsp. paracasei |
| MRS | 8 | Lb. brevis |
| MRS | 9 | Lb. brevis |
| MRS | 6 | Lb. rhamnosus |
| MRS | 7 | Lb. rhamnosus |
| MRS | 11 | Lb. paracasei subsp. paracasei |
| MRS | 10 | Lb. paracasei subsp. paracasei |
| MRS | 12 | Lb. paracasei subsp. paracasei |
| MRS | 13 | Lb. paracasei subsp. paracasei |
| Μ17 | 4 | Lb. paracasei subsp. paracasei |
| Μ17 | 5 | Lb. paracasei subsp. paracasei |
| Μ17 | 6 | Lb. kefiri |
| RO | 3 | Lb. paracasei subsp. paracasei |
| RO | 4 | Lb. paracasei subsp. paracasei |
| RO | 5 | Lb. paracasei subsp. paracasei |
| RO | 7 | Lb. rhamnosus |
| RO | 6 | Lb. rhamnosus |
| RO | 10 | Lb. paracasei subsp. paracasei |
| RO | 11 | Lb. paracasei subsp. paracasei |
| RO | 8 | Lb. rhamnosus |
| RO | 9 | Lb. rhamnosus |
| RO | 12 | Lb. paracasei subsp. paracasei |
| RO | 13 | Lb. paracasei subsp. paracasei |
| Raw Milk | (Sugar/Carbohydrate) Fermentation | Multiplex PCR |
|---|---|---|
| RO-1 | Leuconostoc pseudomesenteroides | Leuconostoc |
| RO-2 | Leuconostoc pseudomesenteroides | Leuconostoc |
| M17-1 | Lactococcus garvieae | |
| MRS-1 | Lactococcus garvieae | Lactococcus |
| MRS-2 | Lactococcus garvieae | |
| RO-3 | Lacticaseibacillus paracasei subsp. paracasei | |
| KAA-1 | Enterococcus faecalis | Enterococcus faecalis |
| AFTER SALTING | ||
| MRS-3 | Lacto Lacticaseibacillus paracasei subsp. paracasei | Lactobacillus |
| RO-4 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-5 | Lacticaseibacillus paracasei subsp. paracasei | |
| MRS-4 | Lactococcus lactis subsp. lactis | Lactococcus |
| MRS-5 | Lactococcus spp. | Lactococcus |
| M17-2 | Streptococcus spp. | Streptococcus + Leuconostoc |
| M17-3 | Lactococcus lactis subsp. lactis | Lactococcus |
| KAA-2 | Enterococcus faecalis | Enterococcus faecalis |
| KAA-3 | Enterococcus gallinarum | Enterococcus |
| CHEESE (30 DAYS) | ||
| MRS-6 | Lacticaseibacillus rhamnosus | Lactobacillus |
| MRS-7 | Lacticaseibacillus rhamnosus | Lactobacillus |
| RO-6 | Lacticaseibacillus rhamnosus | |
| M17-4 | Lacticaseibacillus paracasei subsp. paracasei | Lactobacillus |
| M17-5 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-7 | Lacticaseibacillus paracasei subsp. paracasei | |
| MRS-8 | Lacticaseibacillus parabrevis | Lactococcus |
| MRS-9 | Lacticaseibacillus parabrevis | Lactococcus |
| M17-6 | Lacticaseibacillus kefiri | Leuconostoc |
| KAA-4 | Enterococcus faecalis | Enterococcus faecalis |
| KAA-5 | Enterococcus faecalis | Enterococcus faecalis |
| KAA-6 | Enterococcus gallinarum | Enterococcus |
| CHEESE (60 DAYS) | ||
| MRS-10 | Lacticaseibacillus paracasei subsp. paracasei | Lactobacillus |
| RO-8 | Lacticaseibacillus rhamnosus | Lactobacillus |
| RO-9 | Lacticaseibacillus rhamnosus | |
| MRS-11 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-10 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-11 | Lacticaseibacillus paracasei subsp. paracasei | Lactobacillus |
| M17-7 | Streptococcus spp. | Streptococcus + Leuconostoc |
| KAA-7 | Enterococcus faecium | Enterococcus hirae |
| CHEESE (90 DAYS) | ||
| MRS-12 | Lacticaseibacillus paracasei subsp. paracasei | Lactobacillus |
| MRS-13 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-12 | Lacticaseibacillus paracasei subsp. paracasei | |
| RO-13 | Lacticaseibacillus paracasei subsp. paracasei | |
| KAA-8 | Enterococcus faecalis | Enterococcus faecalis |
| KAA-9 | Enterococcus faecium | Enterococcus hirae |
| Strain | Identification | E Value | Percentage | Accession No | |
|---|---|---|---|---|---|
| RO-1 | Leuconostoc pseudomesenteroides strain MC2/2W 16S ribosomal RNA gene, partial sequence | Leuconostoc pseudomesenteroides | 0.0 | 99.37% | MF103729.1 |
| MRS-5 | Lactococcus lactis subsp. Lactis KLDS 4.0325 chromosome, complete genome | Lactococcus lactis subsp. Lactis | 0.0 | 99.37% | CP006766.1 |
| M17-2 | Streptococcus lutetiensis gene for 16S ribosomal RNA, partial sequence, strain: C57 | Streptococcus lutetiensis | 0.0 | 99.23% | LC090604.1 |
| Μ17-3 | Lactococcus lactis strain 4598 16S ribosomal RNA gene, partial sequence | Lactococcus lactis subsp. Lactis | 0.0 | 96.91% | MT545096.1 |
| MRS-8 | Levilactobacillus parabrevis strain KM18 16S ribosomal RNA gene, partial sequence | Levilactobacillus parabrevis | 0.0 | 99.78% | MN473283.1 |
| MRS-9 | Levilactobacillus parabrevis strain KM18 16S ribosomal RNA gene, partial sequence | Levilactobacillus parabrevis | 0.0 | 98.56% | MN473283.1 |
| M17-6 | Lactobacillus otakiensis JCM 15,040 gene for 16S ribosomal RNA, partial sequence | Lentilactobacillus otakiensis | 0.0 | 99.54% | C480801.1 |
| Lactobacillus parabuchneri strain 2173 16S ribosomal RNA gene, partial sequence | Lentilactobacillus parabuchneri | 0.0 | 99.07% | EF535231.1 | |
| Lentilactobacillus kefiri strain DH5 chromosome, complete genome | Lentilactobacillus kefiri | 0.0 | 98.16% | CP029971.1 | |
| MRS-10 | Lacticaseibacillus rhamnosus strain 14,235 16S ribosomal RNA gene, partial sequence | Lacticaseibacillus rhamnosus | 0.0 | 96.15% | MW453828.1 |
| Lacticaseibacillus paracasei subsp. Paracasei strain MA51 16S ribosomal RNA gene, partial sequence | Lacticaseibacillus paracasei subsp. Paracasei | 0.0 | 95.40% | KY425781.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psomas, E.; Sakaridis, I.; Boukouvala, E.; Karatzia, M.-A.; Ekateriniadou, L.V.; Samouris, G. Indigenous Lactic Acid Bacteria Isolated from Raw Graviera Cheese and Evaluation of Their Most Important Technological Properties. Foods 2023, 12, 370. https://doi.org/10.3390/foods12020370
Psomas E, Sakaridis I, Boukouvala E, Karatzia M-A, Ekateriniadou LV, Samouris G. Indigenous Lactic Acid Bacteria Isolated from Raw Graviera Cheese and Evaluation of Their Most Important Technological Properties. Foods. 2023; 12(2):370. https://doi.org/10.3390/foods12020370
Chicago/Turabian StylePsomas, Evdoxios, Ioannis Sakaridis, Evridiki Boukouvala, Maria-Anastasia Karatzia, Loukia V. Ekateriniadou, and Georgios Samouris. 2023. "Indigenous Lactic Acid Bacteria Isolated from Raw Graviera Cheese and Evaluation of Their Most Important Technological Properties" Foods 12, no. 2: 370. https://doi.org/10.3390/foods12020370






