Highland Barley Starch: Structures, Properties, and Applications
Abstract
1. Introduction
2. Isolation
2.1. Dry Extraction
2.2. Wet Extraction
2.3. Combined Wet and Dry Extraction
3. Chemical Composition
4. Structure
4.1. Molecular Structure
4.1.1. Molecular Weight Distribution
4.1.2. Chain Length Distribution
4.1.3. Particle Size Distribution
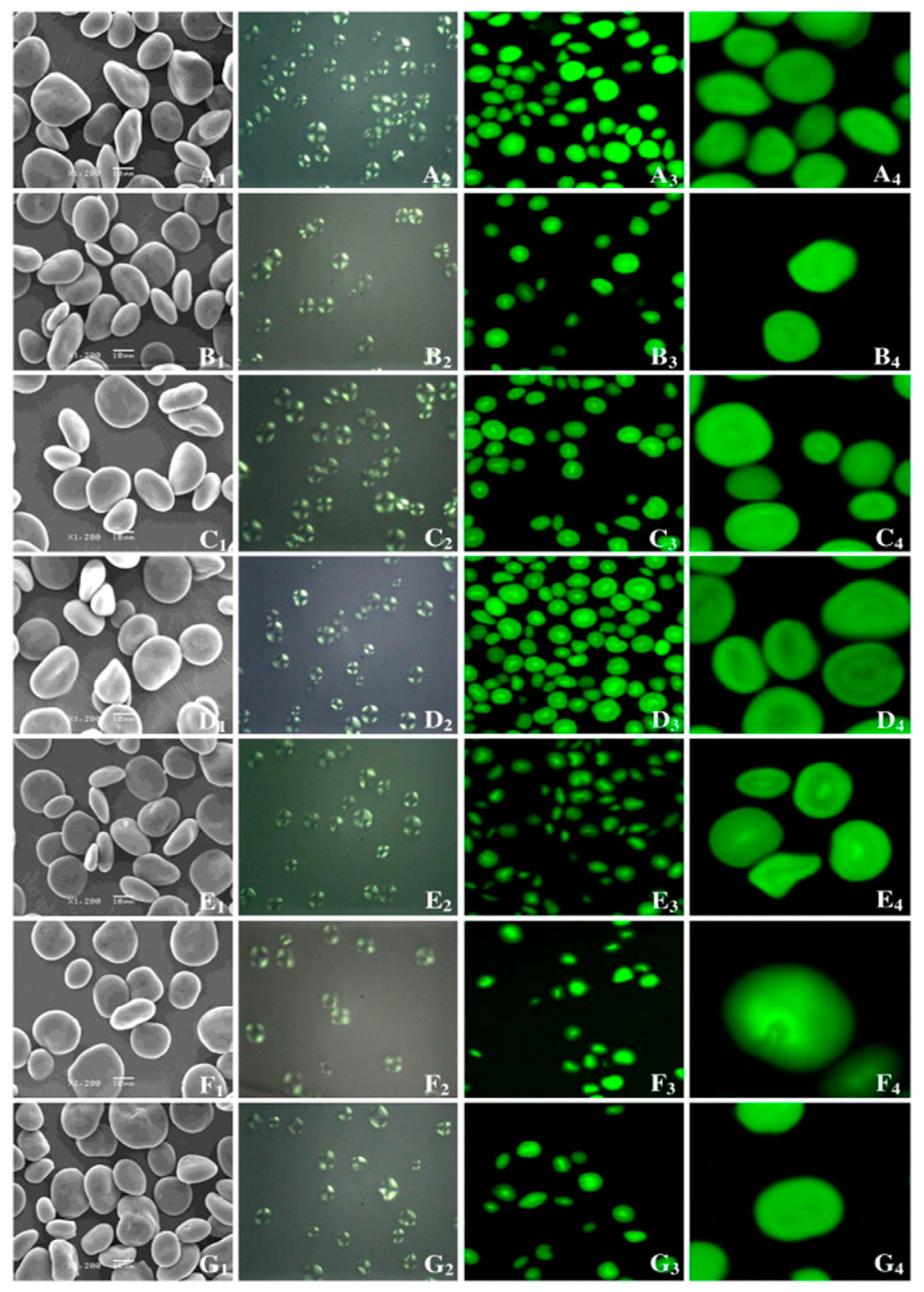
4.2. Particle Structure
4.3. Molecular Structure
4.3.1. Molecular Weight Distribution
4.3.2. Lamellar Structure
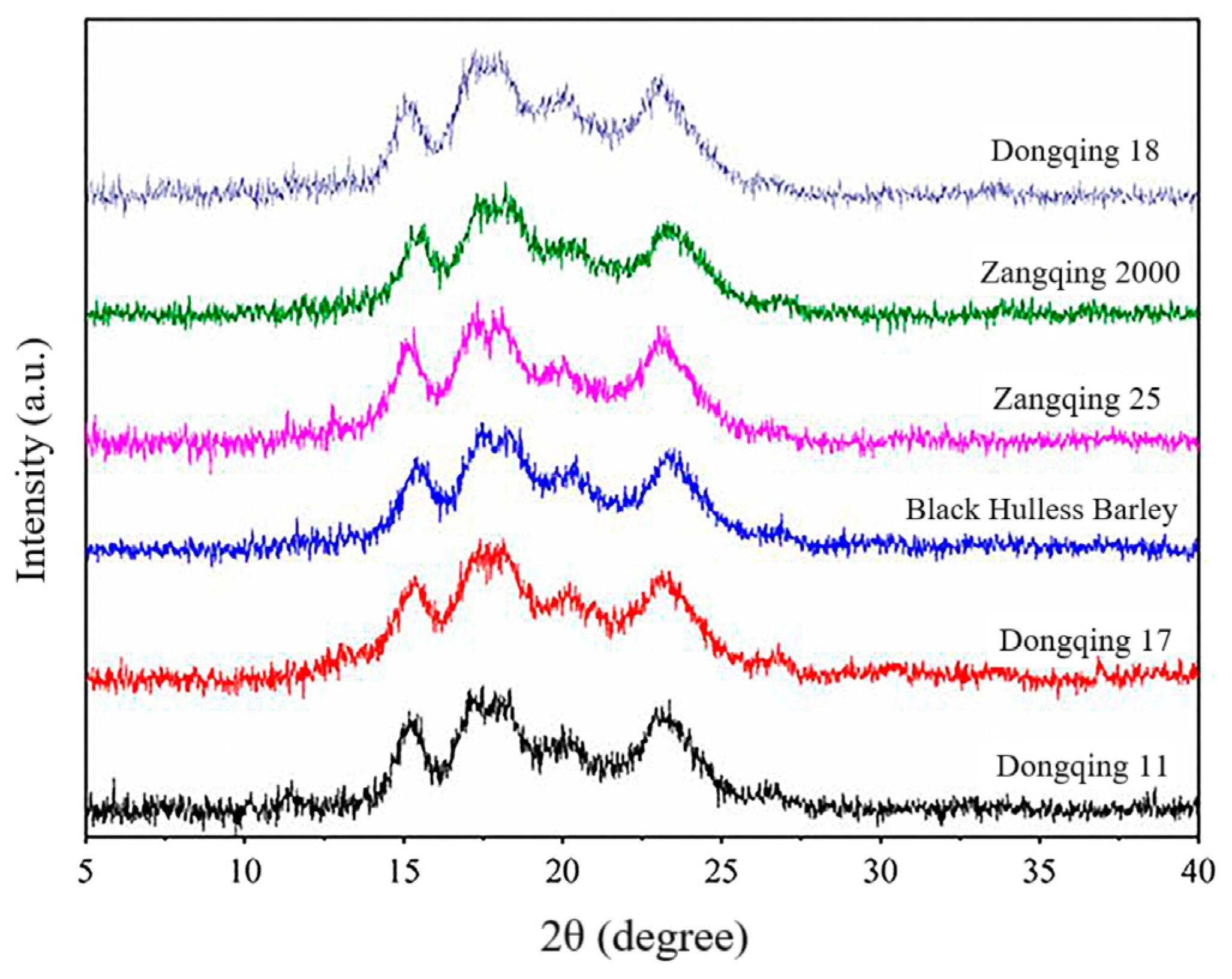
4.3.3. Ordered and Amorphous Structures
5. Physicochemical Properties
5.1. Gelatinization by Differential Scanning Calorimetry (DSC)
5.2. Swelling Power and Solubility
5.3. Rheological Properties
5.3.1. Pasting
5.3.2. Flow
5.4. Retrogradation
5.4.1. Freeze-Thaw Stability and Syneresis
5.4.2. Transparency of the Starch Paste
5.4.3. Gel Textural Properties
5.5. Digestibility
6. Applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, J.J.; Chen, X.; Deng, G.B.; Pan, Z.F.; Zhang, H.L.; Li, Q.; Yang, K.J.; Long, H.; Yu, M.Q. Dehydration induced transcriptomic responses in two tibetan hulless barley (Hordeum vulgare var. nudum) accessions distinguished by drought tolerance. BMC Genom. 2017, 18, 775. [Google Scholar] [CrossRef]
- Zhu, F.M.; Du, B.; Xu, B.J. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Tuersuntuoheti, T.; Wang, Z.H.; Duan, M.J.; Asimi, S.; Ren, X.; Wang, Z.Y.; Zheng, Y.Y.; Wu, Y.; Liang, S.; Zhang, M. Noodle processing, storage time and cooking affect the antioxidant activities and phenolic compounds content of Qingke barley noodles. Int. J. Food Sci. Technol. 2020, 55, 2730–2739. [Google Scholar] [CrossRef]
- Wu, D.Y.; Yu, L.W.; Guo, L.; Li, S.Q.; Yao, X.H.; Yao, Y.H.; Cao, X.Y.; Wu, K.L.; Gao, X. Effect of highland barley on rheological properties, textural properties and starch digestibility of chinese steamed bread. Foods 2022, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Q.; Pan, Z.F.; Li, Q.; Deng, G.B.; Long, H.; Tashi, N.; Zhao, Y.; Yu, M.Q. Nutritional components, in vitro digestibility, and textural properties of cookies made from whole hull-less barley. Cereal Chem. 2020, 97, 39–52. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of barley non-starchy polysaccharides on selected quality attributes of sponge cakes. Lwt-Food Sci. Technol. 2017, 85, 252–261. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.L.; Lang, F.F.; Zheng, Y.; Chen, F.S. Highland barley replaces sorghum as raw material to make shanxi aged vinegar. Appl. Sci. 2021, 11, 6039. [Google Scholar] [CrossRef]
- Zhang, K.Z.; Yang, J.G.; Qiao, Z.W.; Cao, X.Z.; Luo, Q.C.; Zhao, J.S.; Wang, F.Q.; Zhang, W.X. Assessment of beta-glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of china. Food Chem. 2019, 293, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.K.; Jaiswal, S.; Maley, J.; Baga, M.; Sammynaiken, R.; Rossnagel, B.G.; Chibbar, R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011, 59, 4743–4754. [Google Scholar] [CrossRef]
- Pekel, A.Y.; Calik, A.; Alatas, M.S.; Kuter, E.; Cengiz, O.; Omurtag, G.Z.; Inan, G. Evaluation of correlations between nutrients, fatty acids, heavy metals, and deoxynivalenol in corn (Zeamays L.). J. Appl. Poult. Res. 2019, 28, 94–107. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.H.; Wang, F.; Huang, J.H.; Wang, X.X.; Bao, Q.D. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A.; Safety, F. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Ai, Y.F.; Jane, J.L. Macronutrients in corn and human nutrition. Compr. Rev. Food Sci. Food Saf. 2016, 15, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; O’Regan, J.; Keny, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, T.; Liu, R.H. Bioactive compounds of highland barley and their health benefits. J. Cereal Sci. 2022, 103, 103366. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Starch digestibility and bioactivity of high altitude hulless barley. Food Chem. 2016, 194, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.G. Determination of nine mineral elements in hulless barley by ultraviolet spectrophotometry and flame atomic absorption spectrometry. Spectrosc. Spect. Anal. 2010, 30, 1126–1129. [Google Scholar]
- Huang, H.J.; Gao, X.L.; Li, Y.; Tian, P.J.; Nima, Y.Z.; Laba, Z.X.; Ci, Z.; Wei, X.H.; Qu, J.; Guan, W.X.; et al. Content analysis of vitamins, dietary fibers and amino acids in a wide collection of barley (Hordeum vulgare L.) from Tibet, china. Bioinformation 2020, 16, 314–321. [Google Scholar] [CrossRef]
- Martinez, M.; Motilva, M.J.; Lopez de las Hazas, M.C.; Romero, M.P.; Vaculova, K.; Ludwig, I.A. Phytochemical composition and beta-glucan content of barley genotypes from two different geographic origins for human health food production. Food Chem. 2018, 245, 61–70. [Google Scholar] [CrossRef]
- Shewry, P.R.; Van Schaik, F.; Ravel, C.; Charmet, G.; Mariann, R.; Zoltan, B.; Ward, J.L. Genotype and environment effects on the contents of vitamins b1, b2, b3, and b6 in wheat grain. J. Agric. Food Chem. 2011, 59, 10564–10571. [Google Scholar] [CrossRef]
- Liu, K.S.; Moreau, R.A. Concentrations of functional lipids in abraded fractions of hulless barley and effect of storage. J. Food Sci. 2008, 73, C569–C576. [Google Scholar] [CrossRef]
- Yeung, J.; Vasanthan, T. Pearling of hull-less barley: Product composition and gel color of pearled barley flours as affected by the degree of pearling. J. Agric. Food Chem. 2001, 49, 331–335. [Google Scholar] [CrossRef]
- Acar, O.; Izydorczyk, M.S.; Kletke, J.; Yazici, M.A.; Ozdemir, B.; Cakmak, I.; Koksel, H. Effects of roller and hammer milling on the yield and physicochemical properties of fibre-rich fractions from biofortified and non-biofortified hull-less barley. J. Cereal Sci. 2020, 92, 102907. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E.; Desjardins, R.G.; Rossnagel, B.G.; Lagasse, S.L.; Hatcher, D.W. Roller milling of canadian hull-less barley: Optimization of roller milling conditions and composition of mill streams. Cereal Chem. 2003, 80, 637–644. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Andersson, R.; Aman, P. Air classification of barley flours. Cereal Chem. 2000, 77, 463–467. [Google Scholar] [CrossRef]
- Silventoinen, P.; Sipponen, M.H.; Holopainen-Mantila, U.; Poutanen, K.; Sozer, N. Use of air classification technology to produce protein-enriched barley ingredients. J. Food Eng. 2018, 222, 169–177. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hicks, K.B.; Challa, R.K.; Wilson, J.; Kurantz, M.; Moreau, R.A. Fractionation of barley flour using elusieve processing: A combination of sieving and air classification. Trans. ASABE 2010, 53, 503–508. [Google Scholar] [CrossRef]
- Liu, K.S.; Barrows, F.T.; Obert, D. Dry fractionation methods to produce barley meals varying in protein, beta-glucan, and starch contents. J. Food Sci. 2009, 74, C487–C499. [Google Scholar] [CrossRef]
- Assatory, A.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Dry fractionation methods for plant protein, starch and fiber enrichment: A review. Trends Food Sci. Technol. 2019, 86, 340–351. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, M.Y.; Wang, H.; Hu, H.Y.; Liu, R.; Huang, Z.Q.; Chen, C.J.; Chen, D.; Feng, Z.F. Damaged starch derived carbon foam-supported heteropolyacid for catalytic conversion of cellulose: Improved catalytic performance and efficient reusability. Bioresour. Technol. 2019, 288, 121532. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.N.; Tadini, C.C.; Leon, A.E.; Ribotta, P.D. Use of alpha-amylase and amyloglucosidase combinations to minimize the bread quality problems caused by high levels of damaged starch. J. Food Sci. Tech. Mys. 2016, 53, 3675–3684. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.M.; Hong, J.; Zheng, X.L.; Bian, K.; Sun, Y.; Zhang, J. Effect of mechanically damaged starch on wheat flour, noodle and steamed bread making quality. Int. J. Food Sci. Technol. 2014, 49, 253–260. [Google Scholar] [CrossRef]
- Wang, S.J.; Yu, J.L.; Xin, Q.W.; Wang, S.; Copeland, L. Effects of starch damage and yeast fermentation on acrylamide formation in bread. Food Control. 2017, 73, 230–236. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Jiao, A.Q.; Zhao, S.N.; Liu, Q.; Fu, X.; Jin, Z.Y. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocoll. 2021, 117, 106698. [Google Scholar] [CrossRef]
- Nie, M.Z.; Piao, C.H.; Li, J.X.; He, Y.; Xi, H.H.; Chen, Z.Y.; Wang, L.L.; Liu, L.Y.; Huang, Y.T.; Wang, F.Z.; et al. Effects of different extraction methods on the gelatinization and retrogradation properties of highland barley starch. Molecules 2022, 27, 6524. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhu, Z.Y.; Li, J.Y.; Zhang, L.J. Optimization of neutral protease-assisted extraction of highland barley starch. Food Sci. 2016, 37, 31–36. [Google Scholar]
- Gao, J.; Vasanthan, T.; Hoover, R. Isolation and characterization of high-purity starch isolates from regular, waxy, and high-amylose hulless barley grains. Cereal Chem. 2009, 86, 157–163. [Google Scholar] [CrossRef]
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.; Ezernitskaja, M.; Yuryev, V.P. The relationship between thermodynamic and structural properties of low and high amylose maize starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Yangcheng, H.Y.; Gong, L.X.; Zhang, Y.; Jane, J.L. Pysicochemical properties of tibetan hull-less barley starch. Carbohydr. Polym. 2016, 137, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Xiao, X.L.; Zhang, W.H.; Zheng, J.M.; Luo, Q.G.; Ouyang, S.H.; Zhang, G.Q. Compositional, morphological, structural and physicochemical properties of starches from seven naked barley cultivars grown in china. Food Res. Int. 2014, 58, 7–14. [Google Scholar] [CrossRef]
- Storsley, J.M.; Izydorczyk, M.S.; You, S.; Biliaderis, C.G.; Rossnagel, B. Structure and physicochemical properties of beta-glucans and arabinoxylans isolated from hull-less barley. Food Hydrocoll. 2003, 17, 831–844. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.J.; Chen, L.; Li, X.X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Vasanthan, T.; Hoover, R.; Bressler, D. Amylolysis of amylopectin and amylose isolated from wheat, triticale, corn and barley starches. Food Hydrocoll. 2014, 35, 686–693. [Google Scholar] [CrossRef]
- Ma, Z.C.; Zhao, S.M.; Cheng, K.; Zhang, X.F.; Xu, X.J.; Zhang, L.N. Molecular weight and chain conformation of amylopectin from rice starch. J. Appl. Polym. Sci. 2007, 104, 3124–3128. [Google Scholar] [CrossRef]
- Czuchajowska, Z.; Klamczynski, A.; Paszczynska, B.; Baik, B.K. Structure and functionality of barley starches. Cereal Chem. 1998, 75, 747–754. [Google Scholar] [CrossRef]
- Song, Y.; Jane, J. Characterization of barley starches of waxy, normal, and high amylose varieties. Carbohydr. Polym. 2000, 41, 365–377. [Google Scholar] [CrossRef]
- Gilbert, R.G.; Wu, A.C.; Sullivan, M.A.; Sumarriva, G.E.; Ersch, N.; Hasjim, J. Improving human health through understanding the complex structure of glucose polymers. Anal. Bioanal. Chem. 2013, 405, 8969–8980. [Google Scholar] [CrossRef]
- SanGuan, Y.; Izydorczyk, M.S. Molecular characteristics of barley starches with variable amylose content. Carbohydr. Polym. 2002, 49, 33–42. [Google Scholar]
- Takeda, Y.; Takeda, C.; Mizukami, H.; Hanashiro, I. Structures of large, medium and small starch granules of barley grain. Carbohydr. Polym. 1999, 38, 109–114. [Google Scholar] [CrossRef]
- Servais, C.; Jones, R.; Roberts, I. The influence of particle size distribution on the processing of food. J. Food Eng. 2002, 51, 201–208. [Google Scholar] [CrossRef]
- Hegel, C.; Jones, C.; Cabrera, F.; Yanez, M.J.; Bucala, V. Particle size characterization: Comparison of laser difraction (ld) and scanning electron microscopy (sem). Acta Microsc. 2014, 23, 11–17. [Google Scholar]
- Li, L.; Liu, Z.D.; Wang, T.Q.; Wang, B.; Zhang, W.H.; Li, G.H.; Guo, Z.L.; Zhang, Y.X.; Xue, B.; Luo, Z. Starch isolated from different hulless barley cultivars differs in their chemical and structural characteristics. Food Sci. Nutr. 2019, 7, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Vasanthan, T.; Rossnagel, B.; Hoover, R. Starch from hull-less barley: Ii. Thermal, rheological and acid hydrolysis characteristics. Food Chem. 2001, 74, 407–415. [Google Scholar] [CrossRef]
- Kong, X.L.; Kasapis, S.; Zhu, P.; Sui, Z.Q.; Bao, J.S.; Corke, H. Physicochemical and structural characteristics of starches from chinese hull-less barley cultivars. Int. J. Food Sci. Technol. 2016, 51, 509–518. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying araylose content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- Tang, H.; Watanabe, K.; Mitsunaga, T. Structure and functionality of large, medium and small granule starches in normal and waxy barley endosperms. Carbohydr. Polym. 2002, 49, 217–224. [Google Scholar] [CrossRef]
- Wang, H.R.; Li, Y.; Wang, L.J.; Wang, L.L.; Li, Z.G.; Qiu, J. Multi-scale structure, rheological and digestive properties of starch isolated from highland barley kernels subjected to different thermal treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
- Lan, X.H.; Xie, S.C.; Wu, J.H.; Xie, F.; Liu, X.; Wang, Z.W. Thermal and enzymatic degradation induced ultrastructure changes in canna starch: Further insights into short-range and long-range structural orders. Food Hydrocoll. 2016, 58, 335–342. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Maurice, T.J.; Vose, J.R. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1680. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Page, C.M.; Maurice, T.J.; Juliano, B.O. Thermal characterization of rice starches: A polymeric approach to phase transitions of granular starch. J. Agr. Food. Chem. 1986, 34, 6–14. [Google Scholar] [CrossRef]
- Gonera, A.; Cornillon, P. Gelatinization of starch/gum/sugar systems studied by using dsc, nmr, and cslm. Starch-Starke 2002, 54, 508–516. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Morphological, thermal and rheological properties of starches separated from rice cultivar’s grown in india. Food Chem. 2003, 80, 99–108. [Google Scholar] [CrossRef]
- Ahmed, S.; Ru, W.D.; Han, H.X.; Cheng, L.R.; Bian, X.B.; Li, G.C.; Jin, L.P.; Wu, P.; Bao, J.S. Fine molecular structure and its effects on physicochemical properties of starches in potatoes grown in two locations. Food Hydrocoll. 2019, 97, 105172. [Google Scholar] [CrossRef]
- Konik, C.M.; Mikkelsen, L.M.; Moss, R.; Gore, P.J. Relationships between physical starch properties and yellow alkaline noodle quality. Starch-Starke 1994, 46, 292–299. [Google Scholar] [CrossRef]
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef]
- Li, J.H.; Vasanthan, T.; Rossnagel, B.; Hoover, R. Starch from hull-less barley: I. Granule morphology, composition and amylopectin structure. Food Chem. 2001, 74, 395–405. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Zhai, H.S.; Zhang, Z.H.; Xie, R.; Xiao, F.T.; Zeng, X.Q.; Zhang, Y.H.; Li, Z.Y.; Pan, Z.F. Extraction and characterization of waxy and normal barley β-glucans and their effects on waxy and normal barley starch pasting and degradation properties and mash filtration rate. Carbohydr. Polym. 2022, 302, 120405. [Google Scholar] [CrossRef]
- Chang, Y.N.; Lv, Y.D. Structure, functionality, and digestibility of acetylated hulless barley starch. Int. J. Food Prop. 2017, 20, 1818–1828. [Google Scholar] [CrossRef]
- Su, C.Y.; Saleh, A.S.M.; Zhang, B.; Feng, D.; Zhao, J.Y.; Guo, Y.; Zhao, J.; Li, W.H.; Yan, W.J. Effects of germination followed by hot air and infrared drying on properties of naked barley flour and starch. Int. J. Biol. Macromol. 2020, 165, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Donner, E.; Yin, Y.; Huang, R.L.; Fan, M.Z. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in china. Food Chem. 2006, 99, 470–477. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Ezekiel, R.; Guraya, H.S. Physicochemical, thermal and pasting properties of starches separated from different potato cultivars grown at different locations. Food Chem. 2007, 101, 643–651. [Google Scholar] [CrossRef]
- Pycia, K.; Galkowska, D.; Juszczak, L.; Fortuna, T.; Witczak, T. Physicochemical, thermal and rheological properties of starches isolated from malting barley varieties. J. Food Sci. Tech. Mys. 2015, 52, 4797–4807. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wang, Y.; Li, D.; Wang, L.J. The effect of dry heat parboiling processing on the short-range molecular order structure of highland barley. Lwt-Food. Sci. Technol. 2021, 140, 110797. [Google Scholar] [CrossRef]
- Bhatty, R.S. The potential of hull-less barley. Cereal Chem. 1999, 76, 589–599. [Google Scholar] [CrossRef]
- Zheng, G.H.; Han, H.L.; Bhatty, R.S. Physicochemical properties of zero amylose hull-less barley starch. Cereal Chem. 1998, 75, 520–524. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, J.; Liu, Y.L.; Gong, L.X. Studies on physicochemical properties of hull-less barley starch obtained by the alkali extraction method. J. Chin. Inst. Food Sci. Technol. 2016, 16, 75–80. [Google Scholar]
- Wang, S.J.; Li, C.L.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.J.; Xu, B. Highland barley starch (qingke): Structures, properties, modifications, and applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Gujral, H.S.; Sharma, P.; Kaur, H.; Singh, J. Physiochemical, pasting, and thermal properties of starch isolated from different barley cultivars. Int. J. Food Prop. 2013, 16, 1494–1506. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Wani, I.A.; Shah, A.; Masoodi, F.A.; Saxena, D.C. Production of resistant starch from rice by dual autoclaving-retrogradation treatment: Invitro digestibility, thermal and structural characterization. Food Hydrocoll. 2016, 56, 108–117. [Google Scholar] [CrossRef]
- Zheng, G.H.; Bhatty, R.S. Enzyme-assisted wet separation of starch from other seed components of hull-less barley. Cereal Chem. 1998, 75, 247–250. [Google Scholar] [CrossRef]
- Morikawa, K.; Nishinari, K. Effects of concentration dependence of retrogradation behaviour of dispersions for native and chemically modified potato starch. Food Hydrocoll. 2000, 14, 395–401. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Guo, T.L.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Shen, R.L.; Zhang, W.J.; Dong, J.L. Preparation, structural characteristics and digestibility of resistant starches from highland barley, oats and buckwheat starches. J. Food Nutr. Res.-Slov. 2016, 55, 303–312. [Google Scholar]
- Sasaki, T.; Kohyama, K.; Suzuki, Y.; Okamoto, K.; Noel, T.R.; Ring, S.G. Physicochemical characteristics of waxy rice starch influencing the in vitro digestibility of a starch gel. Food Chem. 2009, 116, 137–142. [Google Scholar] [CrossRef]
- MacGregor, A.W.; Bazin, S.L.; Izydorczyk, M.S. Gelatinisation characteristics and enzyme susceptibility of different types of barley starch in the temperature range 48-72 degrees c. J. Inst. Brew. 2002, 108, 43–47. [Google Scholar] [CrossRef]
- Hu, J.L.; Wu, Y.; Xie, H.F.; Shi, W.Y.; Chen, Z.Y.; Jiang, D.; Hu, H.; Zheng, X.W.; Xu, J.; Yang, Y.J.; et al. Purification, preliminary structural characterization, and in vitro inhibitory effect on digestive enzymes by beta-glucan from qingke (tibetan hulless barley). Adv. Polym. Technol. 2020, 2020, 2709536. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, K.Y.; Zhang, G.Y. Impact of native form oat beta-glucan on starch digestion and postprandial glycemia. J. Cereal Sci. 2017, 73, 84–90. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Zhai, H.S.; Zhang, Y.H.; Zeng, X.Q.; Tang, Y.W.; Tashi, N.; Pan, Z.F. Effects of the addition of waxy and normal hull-less barley flours on the farinograph and pasting properties of composite flours and on the nutritional value, textural qualities, and in vitro digestibility of resultant breads. J. Food Sci. 2020, 85, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.H.; Liu, Q.; Yada, R.Y. Methodologies for increasing the resistant starch content of food starches: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1219–1234. [Google Scholar] [CrossRef]
- Shand, P.J. Textural, water holding, and sensory properties of low-fat pork bologna with normal or waxy starch hull-less barley. J. Food Sci. 2000, 65, 101–107. [Google Scholar] [CrossRef]
- Lu, Y.D.; Chang, Y.N.; Dai, W.; Nie, J.R. Pasting and gel properties of hulless barley starch. Food Mach. 2016, 32, 33–38. [Google Scholar]
- Izydorczyk, M.S.; Lagasse, S.; Hatcher, D.W.; Dexter, J.E.; Rossnagel, B.G. The enrichment of asian noodles with fiber-rich fractions derived from roller milling of hull-less barley. J. Sci. Food Agric. 2005, 85, 2094–2104. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.H.; Peng, J.; Xue, B.; Liu, Z.D.; Luo, Z.; Lu, D.Z.; Zhao, X.R. A novel shell material-highland barley starch for microencapsulation of cinnamon essential oil with different preparation methods. Materials 2020, 13, 1192. [Google Scholar] [CrossRef]
- Mehfooz, T.; Ali, T.M.; Hasnain, A. Effect of cross-linking on characteristics of succinylated and oxidized barley starch. J. Food Meas. Charact. 2019, 13, 1058–1069. [Google Scholar] [CrossRef]
- Mello El Halal, S.L.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Villarreal Carreno, N.L.; Guerra Dias, A.R.; da Rosa Zavareze, E. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Summary of Research Results | References |
|---|---|---|
| Starch | HB had lower levels of starch (58.1–72.2%) than wheat (70–75%), corn (65–74%), and rice (~80%) | [9,10,11,12] |
| Protein | The protein content of HB was 8.20–20.80%, similar to wheat (8–20%), and higher than rice (6–7%) and corn (6–12%) | [13,14,15,16] |
| Lipid | The crude lipid content in HB was about 2.01–3.09%, which was higher than rice, but lower than corn, sorghum and oat | [16] |
| Fiber | HB contained 12.8–17.2% fibers, higher than most cereals, especially β-glucan | [17,18] |
| Mineral | The mineral content of HB was 1.46–2.20%, similar to normal staple foods, such as rice, wheat and corn | [19] |
| Vitamins | HB had about 39.0–379.7 mg/kg vitamin E, and 30.4–1327.4 mg/kg vitamin B, which was higher than the average of maize (3.9–36.3 mg/kg) and wheat (0.16–13.55 mg/kg) | [20,21,22] |
| Varieties | Amylose (%) | Protein (%) | Lipid (%) | Phosphorus (%) | Ash (%) | References |
|---|---|---|---|---|---|---|
| Zangqing 8 | 23.85 | 0.42 | 0.02 | - 1 | - | [43] |
| Xila 19 | 22.72 | - | 0.01 | - | - | |
| Kunlun 12 | 24.97 | 0.45 | 0.01 | - | - | |
| Kangqing 3 | 26.90 | - | 0.42 | 0.047 | - | [42] |
| Beiqing 7 | 24.80 | - | 0.45 | 0.048 | - | |
| CDC McGwire | - | 0.07 | 0.14 | 0.046 | 0.30 | [40] |
| CDC Freedom | - | 0.19 | 0.15 | 0.051 | 0.29 | |
| CDC Dawn | 25.80 | - | - | - | - | [44] |
| Falcon | 23.8 | - | - | - | - |
| Varieties | D(10) (μm) | D(50) (μm) | D(90) (μm) | References |
|---|---|---|---|---|
| Dongqing 11 | - 1 | 18.99 | - | [55] |
| Black HB | - | 22.51 | - | |
| Nakano blue 25 | - | 20.33 | - | |
| Dongqing 18 | - | 23.17 | - | |
| Beiqing 6 | 33.60 | 4.70 | 55.11 | [48] |
| Dongqing 11 | - | 13.10 | - | [42] |
| Varieties | Size (μm) | Shape | Reference |
|---|---|---|---|
| Beiqing 4, Beiqing 6, Beiqing 7, Kangqing 3, Kangqing 6, and Kangqing 7 | 2.0–13.8 | Lenticular, spherical | [42] |
| Zangqing 8, Zangqing 148, Beiqing 6, Zangqing 25, Kunlun 12, Zangqing 320, and Xila 19 | 10–30 | Oval, disk-like, and irregular | [43] |
| CDC Alamo, CDC Candle, CDC Dawn, and Phoenix | 6.2–9.8 | Lenticular, oval, and irregular | [56] |
| Dongqing 18, Zangqing 2000, Zangqing 25, Black HB, Dongqing 17, and Dongqing 11 | 18.99–23.17 | Oval, spherical, and polygonal | [55] |
| HRF, QK, YBL, and SX | 2–25 | Lenticular, oval, and disk-like | [57] |
| Varieties | Starch: Water Ratio (w:w) | Scanning Rate (°C/min) | To 1 (°C) | Tp 2 (°C) | Tc 3 (°C) | △H 4 (J/g) | References |
|---|---|---|---|---|---|---|---|
| Zangqing 8 | 3:12 | 10 | 57.30 | 60.56 | 69.88 | 8.93 | [43] |
| Zangqing 25 | 3:12 | 10 | 57.02 | 60.79 | 70.69 | 9.03 | |
| Zangqing 148 | 3:12 | 10 | 55.03 | 57.84 | 65.49 | 7.74 | |
| Zangqing 320 | 3:12 | 10 | 55.93 | 59.11 | 70.25 | 9.16 | |
| Beiqing 6 | 3:12 | 10 | 58.47 | 61.54 | 71.86 | 9.82 | |
| Kunlun 12 | 3:12 | 10 | 56.17 | 59.00 | 72.74 | 9.74 | |
| Xila 19 | 3:12 | 10 | 54.07 | 57.51 | 69.92 | 9.16 | |
| BQ 6 | - 5 | - | 54.1 | - | 63.6 | 10.5 | [42] |
| KQ 6 | - | - | 56.1 | - | 63.5 | 10.3 | |
| Dongqing 18 | 2:7 | 10 | 61.25 | 67.57 | 82.77 | 8.92 | [55] |
| Dongqing 11 | 2:7 | 10 | 58.73 | 65.12 | 83.36 | 9.84 | |
| Dongqing 17 | 2:7 | 10 | 57.81 | 64.36 | 84.70 | 10.66 | |
| Zangqing 2000 | 2:7 | 10 | 59.40 | 67.51 | 82.60 | 7.14 | |
| Zangqing 25 | 2:7 | 10 | 58.87 | 65.13 | 82.03 | 8.66 | |
| Black HB | 2:7 | 10 | 58.65 | 66.34 | 82.94 | 8.56 | |
| HRF (Qinghai) | 5:15 | 10 | 53.7 | 58.5 | 64.3 | 10.3 | [57] |
| SX (Shanxi) | 5:15 | 10 | 57.7 | 61.8 | 66.0 | 9.5 | |
| HB (Tibet) | 2:6 | 5 | 54.0 | 58.0 | 62.1 | 10.4 | |
| YX (Yunnan) | 2:6 | 5 | 53.4 | 57.7 | 61.9 | 9.7 | |
| Phoenix | 2:6 | 5 | 53.1 | 59.1 | 71.0 | 12.8 | [56] |
| CDC Dawn | 2:6 | 5 | 52.0 | 58.1 | 72.5 | 12.7 |
| Varieties | Parameters | Temperatures (°C) | References | ||||
|---|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | |||
| Zangqing 8 | SP 1 | 3.00 | 8.43 | 9.91 | 12.49 | 15.90 | [43] |
| S 2 | 1.21 | 4.26 | 3.68 | 7.62 | 18.63 | ||
| Beiqing 6 | SP | 2.55 | 8.40 | 10.91 | 12.33 | 17.35 | |
| S | 0.59 | 2.19 | 4.37 | 6.64 | 19.55 | ||
| Kunlun 12 | SP | 3.21 | 8.94 | 10.72 | 12.35 | 15.67 | |
| S | 0.59 | 2.00 | 2.53 | 5.06 | 17.42 | ||
| Xila 19 | SP | 3.74 | 8.51 | 9.44 | 11.31 | 13.33 | |
| S | 1.78 | 3.34 | 3.49 | 5.83 | 17.31 | ||
| Linzhou 148 | SP | 3.55 | 7.46 | 9.88 | 11.87 | 13.23 | |
| S | 2.62 | 5.77 | 9.49 | 15.15 | 15.71 | ||
| HB | SP | 3.59 | 5.53 | 6.73 | 11.15 | 18.56 | [71] |
| S | - 3 | - | - | - | - | ||
| Dulihuang | SP | 0.03 | 0.07 | 0.06 | 0.08 | 0.09 | [72] |
| S | 0.34 | 1.10 | 1.70 | 2.77 | 5.80 | ||
| Varieties | PV 1 (cP) | TV 2 (cP) | BD 3 (cP) | FV 4 (cP) | SB 5 (cP) | PT 6 (°C) | References |
|---|---|---|---|---|---|---|---|
| Zangqing 8 | 3012 | 2253 | 759 | 3094 | 841 | 53.83 | [43] |
| Zangqing 148 | 3351 | 2700 | 651 | 3665 | 964 | 57.25 | |
| Kunlun 12 | 3362 | 2636 | 726 | 3469 | 833 | 84.35 | |
| Xila 19 | 2977 | 2502 | 474 | 3231 | 729 | 50.33 | |
| Dongqing 18 | 264 | 237 | 27 | 406 | 169 | 93.80 | [55] |
| Dongqing 17 | 354 | 245 | 109 | 545 | 300 | 94.65 | |
| Dongqing 11 | 460 | 383 | 77 | 731 | 348 | 93.10 | |
| Zangqing 2000 | 380 | 305 | 75 | 638 | 333 | 95.50 | |
| Black HB | 523 | 357 | 166 | 831 | 474 | 93.10 | |
| HRF (Qinghai) | 206 | - 7 | 68 | - | 146 | 82.5 | [57] |
| SX (Shanxi) | 298 | - | 152 | - | 193 | 76.3 | |
| YX (Yunnan) | 294 | - | 135 | - | 171 | 79.1 | |
| HB (Tibet) | 234 | - | 83 | - | 154 | 83.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Ban, X. Highland Barley Starch: Structures, Properties, and Applications. Foods 2023, 12, 387. https://doi.org/10.3390/foods12020387
Xie J, Hong Y, Gu Z, Cheng L, Li Z, Li C, Ban X. Highland Barley Starch: Structures, Properties, and Applications. Foods. 2023; 12(2):387. https://doi.org/10.3390/foods12020387
Chicago/Turabian StyleXie, Jingjing, Yan Hong, Zhengbiao Gu, Li Cheng, Zhaofeng Li, Caiming Li, and Xiaofeng Ban. 2023. "Highland Barley Starch: Structures, Properties, and Applications" Foods 12, no. 2: 387. https://doi.org/10.3390/foods12020387
APA StyleXie, J., Hong, Y., Gu, Z., Cheng, L., Li, Z., Li, C., & Ban, X. (2023). Highland Barley Starch: Structures, Properties, and Applications. Foods, 12(2), 387. https://doi.org/10.3390/foods12020387






