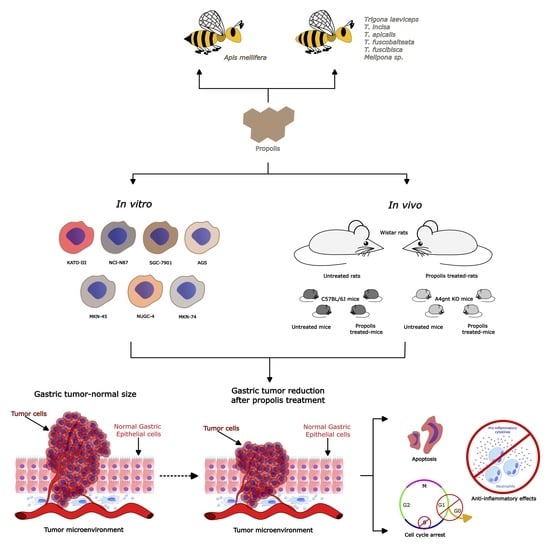
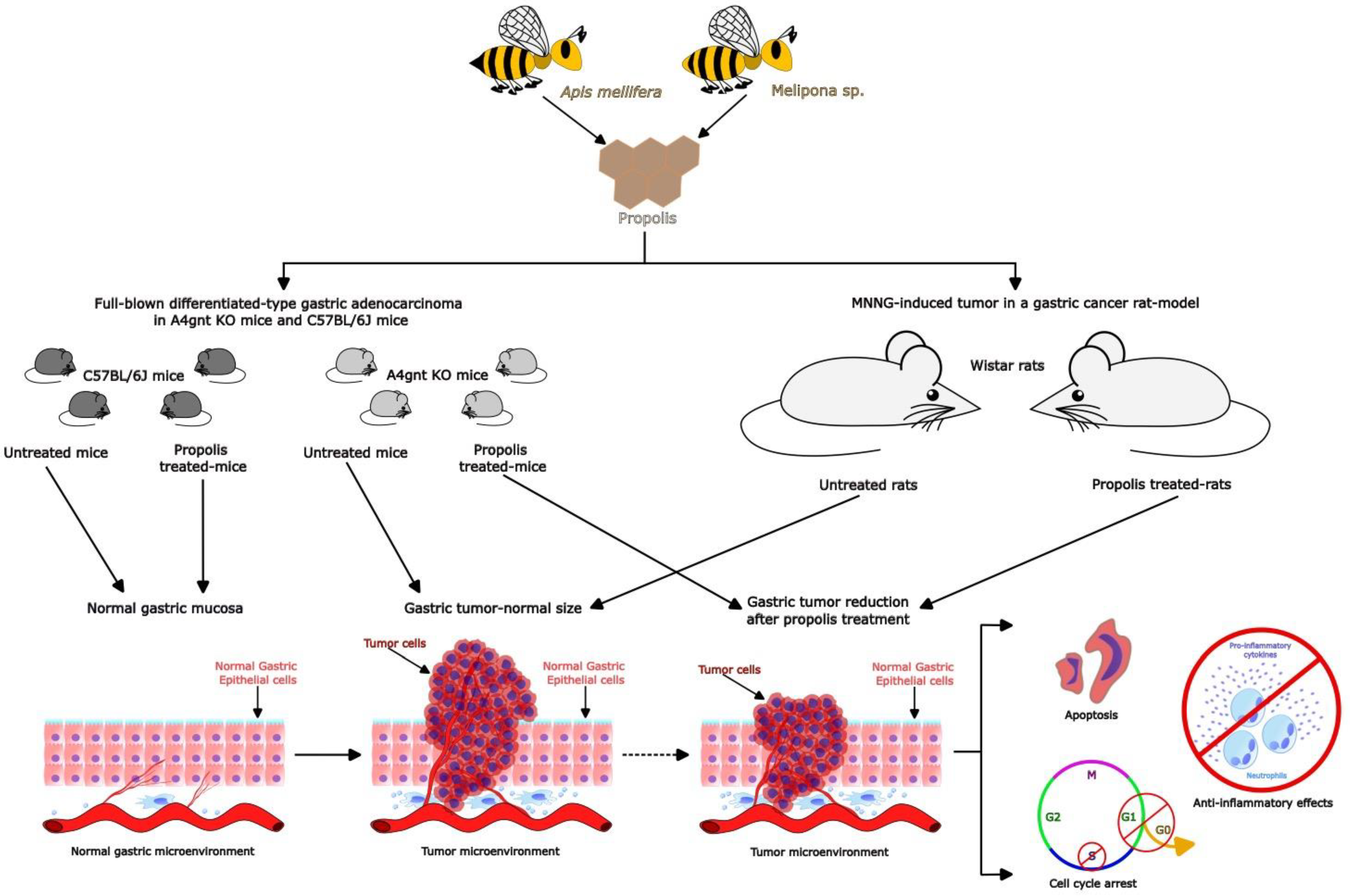
The Role of Propolis as a Natural Product with Potential Gastric Cancer Treatment Properties: A Systematic Review
Abstract
1. Introduction
1.1. Gastric Cancer
1.2. Propolis
2. Materials and Methods
3. Results
3.1. Selected Papers and Characteristics of Studies
3.2. Benefits of Propolis for Gastric Cancer in Cell and Animal Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Volume 3, p. 2019. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Mulligan, R.; Rember, R. Histogenesis and biologic behavior of gastric carcinoma: Study of one hundred thirty-eight cases. AMA Arch. Pathol. 1954, 58, 1–25. [Google Scholar]
- Teglbjaerg, P.S.; Vetner, M. Gastric Carcinoma: 2 An analysis of Morphological and Prognostic Parameters correlated to the Classification Proposed by Masson, Rember and Mulligan. Acta Pathol. Microbiol. Scand. Sect. A Pathol. 1977, 85, 528–534. [Google Scholar] [CrossRef]
- Leteurtre, E.; Zerimech, F.; Piessen, G.; Wacrenier, A.; Leroy, X.; Copin, M.-C.; Mariette, C.; Aubert, J.-P.; Porchet, N.; Buisine, M.-P. Relationships between mucinous gastric carcinoma, MUC2 expression and survival. World J. Gastroenterol. WJG 2006, 12, 3324. [Google Scholar] [CrossRef] [PubMed]
- Espejo Romero, H.; Navarrete Siancas, J. Clasificación de los adenocarcinomas de estómago. Rev. Gastroenterol. Perú 2003, 23, 199–212. [Google Scholar] [PubMed]
- Berlth, F.; Bollschweiler, E.; Drebber, U.; Hoelscher, A.H.; Moenig, S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. WJG 2014, 20, 5679. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239. [Google Scholar] [CrossRef]
- Aversa, J.G.; Song, M.; Hu, N.; Goldstein, A.M.; Hewitt, S.M.; Gulley, M.L.; Dawsey, S.; Camargo, M.C.; Taylor, P.R.; Rabkin, C.S. Low epstein–barr virus prevalence in cardia gastric cancer among a high-incidence Chinese population. Dig. Dis. Sci. 2021, 66, 1220–1226. [Google Scholar] [CrossRef]
- Schneider, S.; Carra, G.; Sahin, U.; Hoy, B.; Rieder, G.; Wessler, S. Complex cellular responses of Helicobacter pylori-colonized gastric adenocarcinoma cells. Infect. Immun. 2011, 79, 2362–2371. [Google Scholar] [CrossRef]
- Aird, I.; Bentall, H.H.; Roberts, J.F. Relationship between cancer of stomach and the ABO blood groups. Br. Med. J. 1953, 1, 799. [Google Scholar] [CrossRef]
- Nomura, A.M.; Hankin, J.H.; Kolonel, L.N.; Wilkens, L.R.; Goodman, M.T.; Stemmermann, G.N. Case–control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control. 2003, 14, 547–558. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Huerta, J.; Bueno-de-Mesquita, H.B.; Siersema, P.; Skeie, G.; Weiderpass, E.; Engeset, D.; Ericson, U.; Ohlsson, B. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int. J. Cancer 2015, 137, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Cheung, K.-W.; Sze, D.M.-Y. The immunomodulatory and anticancer properties of propolis. Clin. Rev. Allergy Immunol. 2013, 44, 262–273. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT-Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A preliminary study of chemical profiles of honey, cerumen, and propolis of the African stingless bee Meliponula ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Al-Hariri, M. Immune’s-boosting agent: Immunomodulation potentials of propolis. J. Fam. Community Med. 2019, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.Y.; Ambrosio, C.M.; Miano, A.C.; Rosalen, P.L.; Gloria, E.M.; Alencar, S.M. Essential oils extracted from organic propolis residues: An exploratory analysis of their antibacterial and antioxidant properties and volatile profile. Molecules 2021, 26, 4694. [Google Scholar] [CrossRef] [PubMed]
- Turan, I.; Demir, S.; Misir, S.; Kilinc, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop. J. Pharm. Res. 2015, 14, 777–782. [Google Scholar] [CrossRef]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The ethanol extract of polish propolis exhibits anti-proliferative and/or pro-apoptotic effect on HCT 116 colon cancer and Me45 Malignant melanoma cells in vitro conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef]
- Li, H.; Kapur, A.; Yang, J.X.; Srivastava, S.; McLeod, D.G.; Paredes-Guzman, J.F.; Daugsch, A.; Park, Y.K.; Rhim, J.S. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int. J. Oncol. 2007, 31, 601–606. [Google Scholar] [CrossRef]
- Ribeiro, D.R.; Alves, A.V.F.; dos Santos, E.P.; Padilha, F.F.; Gomes, M.Z.; Rabelo, A.S.; Cardoso, J.C.; Massarioli, A.P.; de Alencar, S.M.; de Albuquerque-Júnior, R.L.C. Inhibition of DMBA-induced oral squamous cells carcinoma growth by brazilian red propolis in rodent model. Basic Clin. Pharmacol. Toxicol. 2015, 117, 85–95. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Ishii, N.; Fujioka, M.; Doi, K.; Gi, M.; Wanibuchi, H. Ethanol-extracted Brazilian propolis exerts protective effects on tumorigenesis in Wistar Hannover rats. PLoS ONE 2016, 11, e0158654. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic extract of polish propolis: Chemical composition and TRAIL-R2 death receptor targeting apoptotic activity against prostate cancer cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 757628. [Google Scholar] [CrossRef] [PubMed]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish propolis—Chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, P.A.; Garduño-Siciliano, L.; Domínguez-Verano, P.; Balderas-Cordero, D.; Gorgua-Jiménez, G.; Canales-Álvarez, O.; Canales-Martínez, M.M.; Rodríguez-Monroy, M.A. Propolis and its gastroprotective effects on nsaid-induced gastric ulcer disease: A systematic review. Nutrients 2021, 13, 3169. [Google Scholar] [CrossRef]
- Fazalda, A.; Quraisiah, A.; Nur Azlina, M.F. Antiulcer effect of honey in nonsteroidal anti-inflammatory drugs induced gastric ulcer model in rats: A systematic review. Evid.-Based Complement. Altern. Med. 2018, 2018, 7515692. [Google Scholar] [CrossRef]
- Silva, L.M.d.; Souza, P.d.; Jaouni, S.K.A.; Harakeh, S.; Golbabapour, S.; de Andrade, S.F. Propolis and its potential to treat gastrointestinal disorders. Evid.-Based Complement. Altern. Med. 2018, 2018, 2035820. [Google Scholar] [CrossRef]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 37. [Google Scholar] [CrossRef]
- Teerasripreecha, D.; Phuwapraisirisan, P.; Puthong, S.; Kimura, K.; Okuyama, M.; Mori, H.; Kimura, A.; Chanchao, C. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complement. Altern. Med. 2012, 12, 27. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Puthong, S.; Arung, E.T.; Chanchao, C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014, 4, 549–556. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Phuwapraisirisan, P.; Puthong, S.; Palaga, T.; Arung, E.T.; Chanchao, C. Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Anti-gastrointestinal cancer activity of cyclodextrin-encapsulated propolis. J. Funct. Foods 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Jiang, X.-S.; Xie, H.-Q.; Li, C.-G.; You, M.-M.; Zheng, Y.-F.; Li, G.Q.; Chen, X.; Zhang, C.-P.; Hu, F.-L. Chinese Propolis Inhibits the Proliferation of Human Gastric Cancer Cells by Inducing Apoptosis and Cell Cycle Arrest. Evid.-Based Complement. Altern. Med. 2020, 2020, 2743058. [Google Scholar] [CrossRef]
- Dinparast-DjadidPhD, N.; Frankland, A.C.H.; Saleh Azizian, M. Chemoprotection of MNNG-initiated gastric cancer in rats using Iranian propolis. Arch. Iran. Med. 2015, 18, 18. [Google Scholar]
- Desamero, M.J.; Kakuta, S.; Tang, Y.; Chambers, J.K.; Uchida, K.; Estacio, M.A.; Cervancia, C.; Kominami, Y.; Ushio, H.; Nakayama, J. Tumor-suppressing potential of stingless bee propolis in in vitro and in vivo models of differentiated-type gastric adenocarcinoma. Sci. Rep. 2019, 9, 19635. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on propolis from mediterranean countries: Chemical composition, biological activities and application fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical properties of propolis on diverse chronic diseases and its potential applications and health benefits. Nutrients 2020, 13, 78. [Google Scholar] [CrossRef]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental evidence for therapeutic potentials of propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef]
- Sanches, M.A.; Pereira, A.M.S.; Serrão, J.E. Pharmacological actions of extracts of propolis of stingless bees (Meliponini). J. Apic. Res. 2017, 56, 50–57. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless Bees (Meliponini): Senses and Behavior; Springer: Berlin/Heidelberg, Germany, 2016; Volume 202, pp. 597–601. [Google Scholar]
- Lavinas, F.C.; Macedo, E.H.B.; Sá, G.B.; Amaral, A.C.F.; Silva, J.R.; Azevedo, M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- Han, F.; Wallberg, A.; Webster, M.T. From where did the W estern honeybee (A pis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef]
- Wilson, M.B.; Spivak, M.; Hegeman, A.D.; Rendahl, A.; Cohen, J.D. Metabolomics reveals the origins of antimicrobial plant resins collected by honey bees. PLoS ONE 2013, 8, e77512. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.D.; Blüthgen, N. A sticky affair: Resin collection by Bornean stingless bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Leonhardt, S.; Blüthgen, N.; Schmitt, T. Smelling like resin: Terpenoids account for species-specific cuticular profiles in Southeast-Asian stingless bees. Insectes Sociaux 2009, 56, 157–170. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant sources responsible for the chemical composition and main bioactive properties of poplar-type propolis. Plants 2020, 10, 22. [Google Scholar] [CrossRef]
- Kumazawa, S.; Nakamura, J.; Murase, M.; Miyagawa, M.; Ahn, M.-R.; Fukumoto, S. Plant origin of Okinawan propolis: Honeybee behavior observation and phytochemical analysis. Naturwissenschaften 2008, 95, 781–786. [Google Scholar] [CrossRef]
- Inui, S.; Hosoya, T.; Kumazawa, S. Hawaiian propolis: Comparative analysis and botanical origin. Nat. Prod. Commun. 2014, 9, 1934578X1400900208. [Google Scholar] [CrossRef]
- King, D.I.; Hamid, K.; Tran, V.H.; Duke, R.K.; Duke, C.C. Kangaroo Island propolis types originating from two Lepidosperma species and Dodonaea humilis. Phytochemistry 2021, 188, 112800. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132. [Google Scholar] [CrossRef]
- Duke, C.C.; Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Plunkett, G.T.; King, D.I.; Hamid, K.; Wilson, K.L.; Barrett, R.L.; Bruhl, J.J. A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 2017, 134, 87–97. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J. Plant origin authentication of Sonoran Desert propolis: An antiproliferative propolis from a semi-arid region. Sci. Nat. 2019, 106, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus× canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, J.; Zheng, Y.; Zhang, Y.; Wu, Y.; Zhang, C.; Zheng, H.; Hu, F. A new propolis type from Changbai mountains in North-east China: Chemical composition, botanical origin and biological activity. Molecules 2019, 24, 1369. [Google Scholar] [CrossRef]
- Falcão, S.I.; Tomás, A.; Vale, N.; Gomes, P.; Freire, C.; Vilas-Boas, M. Phenolic quantification and botanical origin of Portuguese propolis. Ind. Crops Prod. 2013, 49, 805–812. [Google Scholar] [CrossRef]
- Bertrams, J.; Kunz, N.; Müller, M.; Kammerer, D.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–LTQ/Orbitrap/MS/MS) study of phenolic profile of Serbian poplar type propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef]
- Okińczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Żbikowska, B.; Krzyżanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of Polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Salas, A.; Mercado, M.I.; Zampini, I.C.; Ponessa, G.I.; Isla, M.I. Determination of botanical origin of propolis from Monte region of Argentina by histological and chemical methods. Nat. Prod. Commun. 2016, 11, 1934578X1601100518. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography–photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef] [PubMed]
- Mendonca-Melo, L.; Mota, E.; Lopez, B.; Sawaya, A.; Freitas, L.; Jain, S.; Batista, M.; Araujo, E. Chemical and genetic similarity between Dalbergia ecastaphyllum and red propolis from the Northeastern Brazil. J. Apic. Res. 2017, 56, 32–39. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid.-Based Complement. Altern. Med. 2008, 5, 754625. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2017, 31, 1318–1324. [Google Scholar] [CrossRef]
- Aldana-Mejia, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef]
- Silva, B.B.; Rosalen, P.L.; Cury, J.A.; Ikegaki, M.; Souza, V.C.; Esteves, A.; Alencar, S.M. Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid.-Based Complement. Altern. Med. 2008, 5, 380385. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.C.A.S.d.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera Lf: The botanical sources of isoflavonoids and benzophenones in Brazilian red propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Kumazawa, S.; Yoneda, M.; Shibata, I.; Kanaeda, J.; Hamasaka, T.; Nakayama, T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem. Pharm. Bull. 2003, 51, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.M.; De Souza, M.C.; Arruda, C.; Pereira, R.A.S.; Bastos, J.K. The role of Baccharis dracunculifolia and its chemical profile on green propolis production by Apis mellifera. J. Chem. Ecol. 2020, 46, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, É.W.; Negri, G.; Meira, R.M.; Salatino, A. Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evid.-Based Complement. Altern. Med. 2005, 2, 697212. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.; Fernandes-Silva, C.C.; Salatino, A.; Negri, G.; Message, D. New propolis type from north-east Brazil: Chemical composition, antioxidant activity and botanical origin. J. Sci. Food Agric. 2017, 97, 3552–3558. [Google Scholar] [CrossRef]
- Adelmann, J.; Passos, M.; Breyer, D.H.; dos Santos, M.H.R.; Lenz, C.; Leite, N.F.; Lanças, F.M.; Fontana, J.D. Exotic flora dependence of an unusual Brazilian propolis: The pinocembrin biomarker by capillary techniques. J. Pharm. Biomed. Anal. 2007, 43, 174–178. [Google Scholar] [CrossRef]
- Park, Y. Classification of Brazilian propolis by physicochemical method and biological activity. Mensagem Doce. 2000, 58, 2–7. [Google Scholar]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar] [CrossRef]
- Georgieva, K.; Popova, M.; Dimitrova, L.; Trusheva, B.; Thanh, L.N.; Phuong, D.T.L.; Lien, N.T.P.; Najdenski, H.; Bankova, V. Phytochemical analysis of Vietnamese propolis produced by the stingless bee Lisotrigona cacciae. PLoS ONE 2019, 14, e0216074. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, M.T.; Nguyen, N.T.; Awale, S. Chemical constituents of propolis from Vietnamese Trigona minor and their antiausterity activity against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2017, 80, 2345–2352. [Google Scholar] [CrossRef]
- Pujirahayu, N.; Suzuki, T.; Katayama, T. Cycloartane-type triterpenes and botanical origin of propolis of stingless Indonesian bee Tetragonula sapiens. Plants 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Sanpa, S.; Popova, M.; Bankova, V.; Tunkasiri, T.; Eitssayeam, S.; Chantawannakul, P. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE 2015, 10, e0126886. [Google Scholar] [CrossRef]
- Ishizu, E.; Honda, S.; Vongsak, B.; Kumazawa, S. Identification of plant origin of propolis from Thailand stingless bees by comparative analysis. Nat. Prod. Commun. 2018, 13, 1934578X1801300813. [Google Scholar] [CrossRef]
- Massaro, C.F.; Katouli, M.; Grkovic, T.; Vu, H.; Quinn, R.J.; Heard, T.A.; Carvalho, C.; Manley-Harris, M.; Wallace, H.M.; Brooks, P. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 2014, 95, 247–257. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Fernandes-Silva, C.C.; Salatino, A.; Negri, G. Antioxidant activity of a geopropolis from northeast Brazil: Chemical characterization and likely botanical origin. Evid.-Based Complement. Altern. Med. 2017, 2017, 4024721. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis extract and its bioactive compounds—From traditional to modern extraction technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, Z.M. Method for Extracting Propolis and Water Soluble Dry Propolis Powder. U.S. Patent 4,382,886, 10 May 1983. [Google Scholar]
- Cunha, I.; Sawaya, A.C.; Caetano, F.M.; Shimizu, M.T.; Marcucci, M.C.; Drezza, F.T.; Povia, G.S.; Carvalho, P.D.O. Factors that influence the yield and composition of Brazilian propolis extracts. J. Braz. Chem. Soc. 2004, 15, 964–970. [Google Scholar] [CrossRef]
- Jin, C.S. Rinse Create Matter. KR Patent 20030050938A, 25 June 2003. [Google Scholar]
- Ramanauskienė, K.; Inkėnienė, A.M.; Petrikaitė, V.; Briedis, V. Total phenolic content and antimicrobial activity of different lithuanian propolis solutions. Evid.-Based Complement. Altern. Med. 2013, 2013, 842985. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Bertelli, D.; Benvenuti, S. An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. J. Pharm. Biomed. Anal. 2013, 81, 126–132. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J. Food Sci. Technol. 2020, 57, 70–78. [Google Scholar] [CrossRef]
- Funari, C.S.; Sutton, A.T.; Lajarim Carneiro, R.; Fraige, K.; Cavalheiro, A.J.; de Silva Bolzani, V.; Hilder, E.F.; Arrua, R.D. Searching for Alternative Solvents for Extracting Green Propolis Type. In Proceedings of the 1st International Congress on Bioactive Compounds and 2nd International Workshop on Bioactive Compounds: Food Design and Health, 22–23 November 2018; Cazarin, C.B.B., Bicas, J.L., Marostica, M.R., Eds.; The Faculty of Food Engineering of the State University of Campinas—FEA/UNICAMP: Campinas, Brasil, 2018; Volume 94196. Available online: https://proceedings.science/icbc-2018/papers/searching-for-alternative-solvents-for-extracting-green-propolis-type (accessed on 26 December 2022).
- Pattiram, P.D.; Abas, F.; Suleiman, N.; Mohamad Azman, E.; Chong, G.H. Edible oils as a co-extractant for the supercritical carbon dioxide extraction of flavonoids from propolis. PLoS ONE 2022, 17, e0266673. [Google Scholar] [CrossRef]
- Idrus, N.F.M.; Yian, L.N.; Idham, Z.; Aris, N.A.; Putra, N.R.; Aziz, A.H.A.; Yunus, M.A.C. Mini review: Application of supercritical carbon dioxide in extraction of propolis extract. J. Malays. J. Fundam. Appl. Sci. 2018, 14, 387–396. [Google Scholar] [CrossRef]
- Lastriyanto, A.; Kartika, A.A. Innovation Of Propolis Extraction Machine Based On Vacuum Resistive Heating. In Proceedings of Journal of Physics: Conference Series, Malang, Indonesia, 25–26 August 2020; IOP Publishing Ltd.: Bristol, UK, 2020; p. 012012. [Google Scholar]
- De Groot, A.C.; Popova, M.P.; Bankova, V.S. An Update on the Constituents of Poplar-Type Propolis; Acdegroot Publishing: Wapserveen, The Netherlands, 2014. [Google Scholar]
- Bankova, V.; Popova, M. PHCOG REV.: Review Article Propolis of Stingless Bees: A Promising Source of Biologically Active Compounds. Pharmacogn. Rev. 2007, 1, 88–92. [Google Scholar]
- Coelho, G.R.; Mendonça, R.Z.; Vilar, K.D.S.; Figueiredo, C.A.; Badari, J.C.; Taniwaki, N.; Namiyama, G.; Oliveira, M.I.D.; Curti, S.P.; Evelyn Silva, P. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). Evid.-Based Complement. Altern. Med. 2015, 2015, 296086. [Google Scholar] [CrossRef]
- Coelho, G.R.; Figueiredo, C.A.; Negri, G.; Fernandes-Silva, C.C.; Villar, K.D.S.; Badari, J.C.; Oliveira, M.I.D.; Barbosa, T.F.; Taniwaki, N.N.; Namiyama, G.M. Antiviral activity of geopropolis extract from Scaptotrigona aff. postica against rubella virus. J. Food Res. 2018, 7, 91–106. [Google Scholar] [CrossRef]
- Cisilotto, J.; Sandjo, L.P.; Faqueti, L.G.; Fernandes, H.; Joppi, D.; Biavatti, M.W.; Creczynski-Pasa, T.B. Cytotoxicity mechanisms in melanoma cells and UPLC-QTOF/MS2 chemical characterization of two Brazilian stingless bee propolis: Uncommon presence of piperidinic alkaloids. J. Pharm. Biomed. Anal. 2018, 149, 502–511. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z. Indole alkaloids with potential anticancer activity. Curr. Top. Med. Chem. 2020, 20, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zuo, L.; Xu, H.; Li, C.; Qiao, G.; Guo, M.; Lin, X. Alkaloids as anticancer agents: A review of chinese patents in recent 5 years. Recent Pat. Anti-Cancer Drug Discov. 2020, 15, 2–13. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Farooq, U.; Khan, S. Alkaloids as cyclooxygenase inhibitors in anticancer drug discovery. Curr. Protein Pept. Sci. 2018, 19, 292–301. [Google Scholar] [CrossRef]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal Content in Honey, Propolis, Wax, and Bee Pollen and Implications for Metal Pollution Monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Hodel, K.V.; Machado, B.A.; Santos, N.R.; Costa, R.G.; Menezes-Filho, J.A.; Umsza-Guez, M.A. Metal content of nutritional and toxic value in different types of Brazilian propolis. Sci. World J. 2020, 2020, 4395496. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.d.O.; Barreto, L.M.R.C.; Gomes, S.M.A.; Kadri, S.M. Pesticidas na própolis do Estado de São Paulo, Brasil. Acta Scientiarum. Anim. Sci. 2012, 34, 433–436. [Google Scholar]
- de Oliveira Orsi, R.; Barros, D.C.B.; Silva, R.d.C.M.; de Queiroz, J.V.; de Paula Araújo, W.L.; Shinohara, A.J. Toxic metals in the crude propolis and its transfer rate to the ethanolic extract. Sociobiology 2018, 65, 640–644. [Google Scholar] [CrossRef]
- González-Martín, M.; Revilla, I.; Betances-Salcedo, E.; Vivar-Quintana, A. Pesticide residues and heavy metals in commercially processed propolis. Microchem. J. 2018, 143, 423–429. [Google Scholar] [CrossRef]
- Wang, L.; Miao, C.; He, Y.; Li, H.; Zhang, S.; Li, K.; Liu, H.; Li, W.; Zhao, J.; Xu, Y. The Influence of Heavy Metals on Gastric Tumorigenesis. J. Oncol. 2022, 2022, 6425133. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, N.; Li, X. Advances in understanding how heavy metal pollution triggers gastric cancer. BioMed Res. Int. 2016, 2016, 7825432. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lijinsky, W.; Heineman, E.; Markin, R.S.; Weisenburger, D.; Ward, M. Agricultural pesticide use and adenocarcinomas of the stomach and oesophagus. Occup. Environ. Med. 2004, 61, 743–749. [Google Scholar] [CrossRef]
- Mills, P.K.; Yang, R.C. Agricultural exposures and gastric cancer risk in Hispanic farm workers in California. Environ. Res. 2007, 104, 282–289. [Google Scholar] [CrossRef]
- Santiago, I.S.D.; Cândido, E.L.; Lopes, L.F.; de Medeiros Almeida, J.P.L.; da Silva, C.G.L. Exposure to pesticides and digestive system cancers: Systematic review and meta-analysis. Res. Soc. Dev. 2021, 10, e06101119163. [Google Scholar] [CrossRef]
- Yildirim, M.; Kaya, V.; Yildiz, M.; Demirpence, O.; Gunduz, S.; Dilli, U.D. Esophageal cancer, gastric cancer and the use of pesticides in the southwestern of Turkey. Asian Pac. J. Cancer Prev. 2014, 15, 2821–2823. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Wang, Q.; Zhou, J.; Xue, X.; Zhao, J. Determination of organochlorine pesticides in propolis by gas chromatography–electron capture detection using double column series solid-phase extraction. Anal. Bioanal. Chem. 2009, 393, 1073–1079. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Yu, M.; Xu, J.; Qiu, H.; Shen, L.; Hu, Q. The chemical constituents detection of Brazilian propolis and its safety evaluation. Food Sci. 2005, 26, 236–239. [Google Scholar]
- Yokozaki, H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol. Int. 2000, 50, 767–777. [Google Scholar] [CrossRef]
- Yashiro, M.; Matsuoka, T.; Ohira, M. The significance of scirrhous gastric cancer cell lines: The molecular characterization using cell lines and mouse models. Hum. Cell 2018, 31, 271–281. [Google Scholar] [CrossRef]
- Long-Bao, W.; Bo-Wen, Q.; Yan-Xing, X. Establishment of human gastric cancer cell line (SGC-7901) intraperitoneally transplantable in nude mice. In Recent Advances in Management of Digestive Cancers; Springer: Berlin/Heidelberg, Germany, 1993; pp. 416–418. [Google Scholar]
- Junnila, S.; Kokkola, A.; Karjalainen-Lindsberg, M.-L.; Puolakkainen, P.; Monni, O. Genome-wide gene copy number and expression analysis of primary gastric tumors and gastric cancer cell lines. BMC Cancer 2010, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Saraiva-Pava, K.; Navabi, N.; Skoog, E.C.; Lindén, S.K.; Oleastro, M.; Roxo-Rosa, M. New NCI-N87-derived human gastric epithelial line after human telomerase catalytic subunit over-expression. World J. Gastroenterol. WJG 2015, 21, 6526. [Google Scholar] [CrossRef]
- Lemieux, M.; Bouchard, F.; Gosselin, P.; Paquin, J.; Mateescu, M.A. The NCI-N87 cell line as a gastric epithelial barrier model for drug permeability assay. Biochem. Biophys. Res. Commun. 2011, 412, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kosova, F.; Kurt, F.; Olmez, E.; Tuğlu, I.; Arı, Z. Effects of caffeic acid phenethyl ester on matrix molecules and angiogenetic and anti-angiogenetic factors in gastric cancer cells cultured on different substrates. Biotech. Histochem. 2016, 91, 38–47. [Google Scholar] [CrossRef]
- Abdel-Latif, M.M.; Windle, H.J.; Homasany, B.S.E.; Sabra, K.; Kelleher, D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br. J. Pharmacol. 2005, 146, 1139–1147. [Google Scholar] [CrossRef]
- Toyoda, T.; Tsukamoto, T.; Takasu, S.; Shi, L.; Hirano, N.; Ban, H.; Kumagai, T.; Tatematsu, M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-κB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int. J. Cancer 2009, 125, 1786–1795. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Z.; Zhu, J.; Liang, Q.; Zhou, H.; Li, J.; Fan, X.; Zhao, Z.; Pan, H.; Fei, B. Systematic elucidation of the mechanism of quercetin against gastric cancer via network pharmacology approach. BioMed Res. Int. 2020, 2020, 3860213. [Google Scholar] [CrossRef]
- Shang, H.S.; Lu, H.F.; Lee, C.H.; Chiang, H.S.; Chu, Y.L.; Chen, A.; Lin, Y.F.; Chung, J.G. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 2018, 33, 1168–1181. [Google Scholar] [CrossRef]
- Abe, M.; Yamashita, S.; Kuramoto, T.; Hirayama, Y.; Tsukamoto, T.; Ohta, T.; Tatematsu, M.; Ohki, M.; Takato, T.; Sugimura, T. Global expression analysis of N-methyl-N’-nitro-N-nitrosoguanidine-induced rat stomach carcinomas using oligonucleotide microarrays. Carcinogenesis 2003, 24, 861–867. [Google Scholar] [CrossRef]
- Kodama, M.; Murakami, K.; Fujioka, T.; Nishizono, A. Animal models for the study of Helicobacter-induced gastric carcinoma. J. Infect. Chemother. 2004, 10, 316–325. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Mizoshita, T.; Tatematsu, M. Animal models of stomach carcinogenesis. Toxicol. Pathol. 2007, 35, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Wakazono, K.; Yamamoto, M.; Kitano, M.; Tatematsu, M.; Nagao, M.; Sugimura, T.; Ushijima, T. Rare mutations of p53, Ki-ras, and β-catenin genes and absence of K-sam and c-erbB-2 amplification in N-methyl-N’-N-nitrosoguanidine–induced rat stomach cancers. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 1999, 25, 42–47. [Google Scholar]
- Hippo, Y.; Taniguchi, H.; Tsutsumi, S.; Machida, N.; Chong, J.-M.; Fukayama, M.; Kodama, T.; Aburatani, H. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002, 62, 233–240. [Google Scholar]
- El-Rifai, W.e.; Frierson Jr, H.F.; Harper, J.C.; Powell, S.M.; Knuutila, S. Expression profiling of gastric adenocarcinoma using cDNA array. Int. J. Cancer 2001, 92, 832–838. [Google Scholar] [CrossRef]
- Ekambaram, G.; Rajendran, P.; Devaraja, R.; Muthuvel, R.; Sakthisekaran, D. Impact of naringenin on glycoprotein levels in N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Anti-Cancer Drugs 2008, 19, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, G.; Rajendran, P.; Magesh, V.; Sakthisekaran, D. Naringenin reduces tumor size and weight lost in N-methyl-N’-nitro-N-nitrosoguanidine–induced gastric carcinogenesis in rats. Nutr. Res. 2008, 28, 106–112. [Google Scholar] [CrossRef]
- Ganapathy, E.; Peramaiyan, R.; Rajasekaran, D.; Venkataraman, M.; Dhanapal, S. Modulatory effect of naringenin on N-methyl-N’-nitro-N-nitrosoguanidine-and saturated sodium chloride-induced gastric carcinogenesis in male wistar rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.-J.; Feng, Z.-Z.; Yu, D.-H. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. Vitr. 2012, 26, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Ekström, A.; Serafini, M.; Nyren, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef]
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016, 38, 619–626. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef]


| Propolis (Country) | Components Identified | Experimental Model and Protocol | Results Obtained | Ref. |
|---|---|---|---|---|
| Thailand (stingless bee) | -N.I. | Model: Gastric carcinoma KATO-III (ATTC No. HTB 103) cell line. Protocol: In vitro cytotoxic activity was assessed by the MTT method. Liver (CH-liver) and fibroblasts (HS-27) were used for a comparison. | -Ethanol extract IC50 of 22.98 μg/mL. -Hexane extract obtained from the ethanol extract showed a IC50 of <20 μg/mL. -Partitions obtained from the hexane extract with IC50 of <20 μg/mL:
| [39] |
| Thailand (A. mellifera) | -Cardanol -Cardol | Model: Gastric carcinoma KATO-III (ATCC No. HTB 103) cell line. Protocol: In vitro cytotoxic activity was assessed by the MTT method. A non-transformed human foreskin fibroblast cell line (Hs27, ATCC No. CRL 1634) was used for the comparison. Chemical analysis of the fractions by NMR and ESI-MS. | -Hexane extract IC50 of 42.5 ± 6.61 μg/mL. -Dichloromethane extract IC50 of 43.8 ± 6.5 μg/mL. -Fractions obtained from the hexane extract with cytotoxic activity:
| [40] |
| Indonesia (stingless bee) | -N.I. | Model: Gastric carcinoma KATO-III (ATCC No. HTB 103) cell line. Protocol: In vitro cytotoxic activity was assessed by the MTT method. | -Extracts obtained from different bee species, with IC50 of 20 μg/mL:
| [41] |
| Indonesia (stingless bee) | -5-pentadecyl resorcinol (Cardol isomer) -Terpenoid-like pattern | Model: Gastric carcinoma KATO-III (ATCC No. HTB103) cell line. Protocol: In vitro cytotoxic activity was assessed by the MTT method. A normal skin fibroblast cell line (CCD-986 sk, ATCC No. CRL1947) was used for comparison. Chemical analysis of the fractions by NMR and ESI-MS. | -Ethyl acetate extract (partition) IC50 of 8.06 ± 0.08 μg/mL. -Fractions obtained from ethyl acetate extract with cytotoxic activity:
| [42] |
| New Zealand | -CAPE -Pinobanksin -Pinobanksin-3-O-acetate -Pinocembrin -Chrysin -Galangin | Model: Human gastric cancer cells NCI-N87 (ATCC CRL-5822). Protocol: Production of different types of propolis-cyclodextrin complexes: CD1, CD2, CD3, CD4 and CD5. In vitro cytotoxic activity was assessed by the MTT method. Activated neutrophil anti-inflammatory assays. Lipid antioxidant assay. Positive control 5-FU tested at 15 ng/mL. Compounds reported in propolis were given by the manufacturer of this sample. | -Cytotoxic activity: Propolis complexes had moderate cytotoxic activity since CD3 inhibited NCI-N87 cells by 32.7%, CD4 by 24.6%, and CD5 by 21.8% at 200 μg/mL. Pinocembrin had 72.5% cytotoxic activity at 200 μg/mL. -Anti-inflammatory activity: At 50 μg/mL, New Zealand propolis (alone) inhibited TNF-α by 85% ± 1, CD1 by 93% ± 1, and CD2 by 97% ± 1. At 200 μg/mL, all three samples inhibited this cytokine by 100%. -Lipid antioxidant activity: The five propolis complexes and CAPE (also in the γ-CD complex) had moderate antioxidant activity. CAPE (alone) showed strong antioxidant activity. | [43] |
| China | -Caffeic acid -p-Coumaric acid -Ferulic acid -Isoferulic acid -3,4-Dimethoxycinnamic acid -Pinobanksin -Naringenin -Quercetin -Kaempferol -Apigenin -Pinocembrin -Benzyl caffeate -3-O-Acetyl pinobanksin -Chrysin -CAPE -Galangin -Benzyl p-coumarate | Model: Cell line SGC-7901 Protocol: Cell viability was measured through the CCK-8 assay, and the morphological changes were examined with a microscopical technique. Apoptosis, cell cycle arrest, ROS generation, and changes in the mitochondrial membrane permeability were detected by the Annexin V-FITC/PI, PI, DCFH-DA, and JC-1 flow cytometry protocols, respectively. Cytochrome C, Cleaved PARP, tubulin CDK2, CDC2, E2F1, P-Rb, Cyclin A2, Cyclin E, Bcl-2, Cleaved Caspase-3, Cleaved Caspase-8, P-53, Bid, Bax, and Cleaved Caspase-9 were analyzed by Western blot assay. Chemical analysis of propolis by HPLC. | -Ethanolic propolis extract displayed an IC50 of 66.64 µg/mL in SGC-7901 cells. Moreover, it induced shrinking, loosening, and a decrease in the number of cells in plates analyzed by microscopy. -Propolis induced ROS generation and a loss in mitochondrial membrane permeability in SGC-7901 cells. -Apoptosis induced in SGC-7901 cells by propolis was related to the upregulation of the proteins Bax and Bid, the down-regulation of Bcl-2, and the activation of Cleaved Caspase-8, Cleaved Caspase-9, Cleaved Caspase-3, Cleaved PARP, and P-53. -S-phase arrest induced by propolis in SGC-7901 cells was associated with the dose- and time-dependent up-regulation of P-Rb, CDC2, CDK2, Cyclin E, Cyclin A2, and E2F1 expression. | [44] |
| Iran | In both propolis: -Caffeic acid -Caffeic acid isoprenyl ester -Ferrulic acid -Isoferrulic acid -P-coumaric acid -Quercetin -Quercetin-3 methyl ether -Quercetin-7 methyl ether -Kaempferol -Pinobanksin -Pinobanksin 5,7-dimethyl ether -Pinobanksin 3 methyl ether -Pinobanksin -3-O-acetate -Pinobanksin-3-O-proprionate -Pinobanksin-3-O-butyrate -Pinobanksin-3-O-pentanoate -Luteolin-5-methyl ether | Model: MNNG-induced tumor in a gastric cancer model Protocol: 55 Wistar rats were divided in 3 experimental groups: Control (n = 15), Taleghan propolis (n = 20) and Hamadan propolis (n = 20). All groups were treated with 100 μg/mL of MNNG in drinking water ad libitum for 34 weeks. Propolis-treated groups (ethanolic extract [500 mg/mL]) began propolis consumption prior to two weeks of MNNG administration. Observations the tumor type and presence of metastases, incidence, number, and size of tumors were made. A histological analysis was performed by hematoxylin-eosin (H&E) staining. Additionally, β-catenin, Bax, and Bcl2 antibodies were determined by immunohistochemistry analysis. | -The incidence and number of tumors were significantly decreased by propolis with respect to the control group. -The expression of the nuclear/cytoplasm ratio, epithelial stratification, nuclear dispolarity, structural abnormality, and b-catechin and Bcl-2 protein were decreased in propolis groups with respect to the control group. -Propolis groups showed increased expression of the Bax protein with respect to the control group. -The evidence shows that Iranian propolis exerts inhibitory effects on cell proliferation and apoptosis induction against MNNG-initiated gastric cancer. | [45] |
| Philippine (stingless bee) | -Guaiol -Tibolone -Andrographolide -Gallic acid -β-Eudesmol -Danthron -Ginkgolide-B -Colchicine -Cinnamic acid -Protocatechuic acid -Ginkgolic acid -Rhodoxanthin -Pterostilbene -Rosmanol -Butylated hydroxytoluene | Model:
Protocol:
| -Propolis showed the following values of IC50 (µg/mL) for different gastric cancer cell lines at 24, 48, and 72 h: AGS (650, 188, 39); MKN45 (1156, 386, 318); NUGC4 (580, 376, 315) and MKN74 (1259, 955, 925). -Propolis modulates the cell cycle and apoptosis through the regulation of gene expression in each gastric-cancer cell line, as follows:
| [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Yañez, N.; Ruiz-Hurtado, P.A.; Rivera-Yañez, C.R.; Arciniega-Martínez, I.M.; Yepez-Ortega, M.; Mendoza-Arroyo, B.; Rebollar-Ruíz, X.A.; Méndez-Cruz, A.R.; Reséndiz-Albor, A.A.; Nieto-Yañez, O. The Role of Propolis as a Natural Product with Potential Gastric Cancer Treatment Properties: A Systematic Review. Foods 2023, 12, 415. https://doi.org/10.3390/foods12020415
Rivera-Yañez N, Ruiz-Hurtado PA, Rivera-Yañez CR, Arciniega-Martínez IM, Yepez-Ortega M, Mendoza-Arroyo B, Rebollar-Ruíz XA, Méndez-Cruz AR, Reséndiz-Albor AA, Nieto-Yañez O. The Role of Propolis as a Natural Product with Potential Gastric Cancer Treatment Properties: A Systematic Review. Foods. 2023; 12(2):415. https://doi.org/10.3390/foods12020415
Chicago/Turabian StyleRivera-Yañez, Nelly, Porfirio Alonso Ruiz-Hurtado, Claudia Rebeca Rivera-Yañez, Ivonne Maciel Arciniega-Martínez, Mariazell Yepez-Ortega, Belén Mendoza-Arroyo, Xóchitl Abril Rebollar-Ruíz, Adolfo René Méndez-Cruz, Aldo Arturo Reséndiz-Albor, and Oscar Nieto-Yañez. 2023. "The Role of Propolis as a Natural Product with Potential Gastric Cancer Treatment Properties: A Systematic Review" Foods 12, no. 2: 415. https://doi.org/10.3390/foods12020415
APA StyleRivera-Yañez, N., Ruiz-Hurtado, P. A., Rivera-Yañez, C. R., Arciniega-Martínez, I. M., Yepez-Ortega, M., Mendoza-Arroyo, B., Rebollar-Ruíz, X. A., Méndez-Cruz, A. R., Reséndiz-Albor, A. A., & Nieto-Yañez, O. (2023). The Role of Propolis as a Natural Product with Potential Gastric Cancer Treatment Properties: A Systematic Review. Foods, 12(2), 415. https://doi.org/10.3390/foods12020415