Abstract
The milk fat globule membrane (MFGM) is a complex tri-layer membrane that wraps droplets of lipids in milk. In recent years, it has attracted widespread attention due to its excellent bioactive functions and nutritional value. MFGM contains a diverse array of bioactive lipids, including cholesterol, phospholipids, and sphingolipids, which play pivotal roles in mediating the bioactivity of the MFGM. We sequentially summarize the main lipid types in the MFGM in this comprehensive review and outline the characterization methods used to employ them. In this comprehensive review, we sequentially describe the types of major lipids found in the MFGM and outline the characterization methods employed to study them. Additionally, we compare the structural disparities among glycerophospholipids, sphingolipids, and gangliosides, while introducing the formation of lipid rafts facilitated by cholesterol. The focus of this review revolves around an extensive evaluation of the current research on lipid isolates from the MFGM, as well as products containing MFGM lipids, with respect to their impact on human health. Notably, we emphasize the clinical trials encompassing a large number of participants. The summarized bioactive functions of MFGM lipids encompass the regulation of human growth and development, influence on intestinal health, inhibition of cholesterol absorption, enhancement of exercise capacity, and anticancer effects. By offering a comprehensive overview, the aim of this review is to provide valuable insights into the diverse biologically active functions exhibited by lipids in the MFGM.
1. Introduction
Milk fat, a highly regarded and widely consumed nutrient, not only serves as a vital energy source for the human body, but also constitutes a significant reservoir of essential fatty acids and vitamins. Approximately 98% of the fat in milk exists as milk fat globules, which are mainly composed of triacylglycerols inside and surrounded by the milk fat globule membrane (MFGM) [1,2]. After secretion from the endoplasmic reticulum, milk fat globules are coated with a monolayer of phospholipids to form cytoplasmic lipid droplets (CLDs), a membrane derived from the endoplasmic reticulum. Subsequently, during secretion out of the cell, droplets bind to the apical plasma membrane, the outer bilayer membrane of the MFGM that contains a variety of polar lipids and proteins [3,4]. MFGMs with a thickness of 10–20 nm have a mass of 2–6% of the milk fat globules and act as a natural emulsifier, preventing fat from coalescing in the presence of enzymes [5,6].
This three-layer membrane with biologically active functions is mainly composed of proteins, enzymes, triacylglycerol, and polar lipids (cholesterol, sphingolipids, etc.) [7,8], and the composition of the MFGM in different species is different (Table 1). The nutrients in the MFGM, especially sphingolipids, phospholipids and proteins, make it an excellent source for the development of nutraceuticals, especially infant-development supplements [9,10]. The diverse bioactive functions of the MFGM have led to the emergence of various MFGM-related industrial products. Examples include Lacprodan MFGM 10 and Lacprodan PL 20, which serve as supplements for phospholipids [11]. The emulsifying properties of the MFGM help enhance the texture and flavor in various food products. For instance, the incorporation of MFGMs in the bread production process serves to retain moisture in bread crumbs, thereby impeding bread aging and hardening. Adding 4% MFGM-enrichment products combined with homogenization during the yogurt production process can increase the interaction between the MFGM and protein during the production process and improve the texture of yogurt [12].
Both fresh milk and by-products derived from dairy processing serve as effective sources of MFGMs [13]. The process of isolating an MFGM from milk typically involves several steps: the separation of milk fat globules, cream washing, MFGM release, and MFGM collection [6]. During the permeation process, the addition of reverse osmosis water helps maintain a constant feed rate after filtration to eliminate whey and casein. Finally, acidification is employed to isolate the MFGM [14]. MFGM separation can also be conducted by performing ceramic dia-microfiltration with a pore size of 1.4 μm on preheated whole raw milk, which can achieve the best results of a 2.5% low-fat penetration and 97% high protein penetration. Fractions of buttermilk and butter serum are separated from the filtered material, the pH is adjusted to 4.8, and an MFGM is obtained by centrifugation [15]. This method has simpler steps and better industrial adaptability. Industrial processes typically utilize by-product milk (e.g., buttermilk, cheese whey, etc.) to separate MFGMs. For instance, the removal of casein from buttermilk can be achieved through rennet-induced coagulation, followed by filtration to eliminate whey proteins. The MFGM is subsequently collected through diafiltration steps [16]. It must be noted that these by-products often undergo high-temperature heat treatment, leading to protein denaturation on the MFGM or reactions with whey protein or sugars, which can alter the emulsifying properties of the MFGM [17].
Dietary supplementation with MFGM lipids has demonstrated numerous beneficial bioactive functions. For instance, the addition of MFGM to milk powder has been found to mitigate the impact of phytosterols on infant nutrition by competing with cholesterol for absorption [18]. Furthermore, the intake of milk-derived phospholipids has been demonstrated to suppress the endocrine stress response in individuals exposed to high-intensity stress, with phospholipid-supplemented subjects exhibiting a faster recovery rate [19]. Furthermore, in a mouse study, ganglioside-rich MFGM isolates showed the potential to reduce carrageenan-induced paw edema, indicating their anti-inflammatory properties [20].
We used Google Scholar and Web of Science search engines to summarize reviews and articles between 2003 and 2023, using phospholipids, sphingomyelin, gangliosides, and MFGM as keywords. We chose to focus on the clinical trials focusing on the dietary supplementation of MFGM-enriched or isolated lipids. The purpose of this review is to offer an insightful understanding of the nutritional value of lipids in MFGMs, offering valuable insights into both daily dietary considerations and production practices.


Table 1.
Content of MFGM components in bovine milk [21,22,23,24].
Table 1.
Content of MFGM components in bovine milk [21,22,23,24].
| Component | MFGM | Whole Milk |
|---|---|---|
| Protein | 25–60% | 1–4% |
| Cholesterol | 2% | 80% |
| PL | 15–30% | 60–70% |
| PC (% PL in MFGM) | 27.4 | 32.7 |
| PE (% PL in MFGM) | 33.0 | 28.5 |
| PS/PI (% PL in MFGM) | 17.8 | 14.1 |
| SM (% PL in MFGM) | 18.8 | 23.0 |
PL: phospholipids; PC: phatidylcholine; PE: phosphatidylethanolamine; PS: phosphatidylserine; PI: phosphatidylinositol; SM: sphingomyelin.
2. Lipids in the MFGM
2.1. Composition and Distribution of Lipids in the MFGM
As the predominant components of monolayers, the primary lipids present in the tri-layer membrane of milk fat globules include phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), sphingomyelin (SM), cholesterol, and gangliosides [25,26,27,28]. The surface layer of the bilayer primarily consists of glycolipids, cerebrosides, and gangliosides, while the inner layer of the bilayer mainly contains PE, PI, and PS. Phospholipids are the predominant components of the monolayers [29].
Structurally, SM consists of long chains of bases, such as sphingosine, which form the backbone of the molecule [30]. On the other hand, glycerophospholipids are composed of phosphoric acid, glycerol, fatty acids, hydroxyl compounds, and fatty acids [31]. The presence of glycerophospholipids with relatively high unsaturation levels contributes to the improved fluidity of MFGMs [32]. Gangliosides, on the other hand, are formed through glycosidic linkages between ceramides and residues of sialic acid [33].
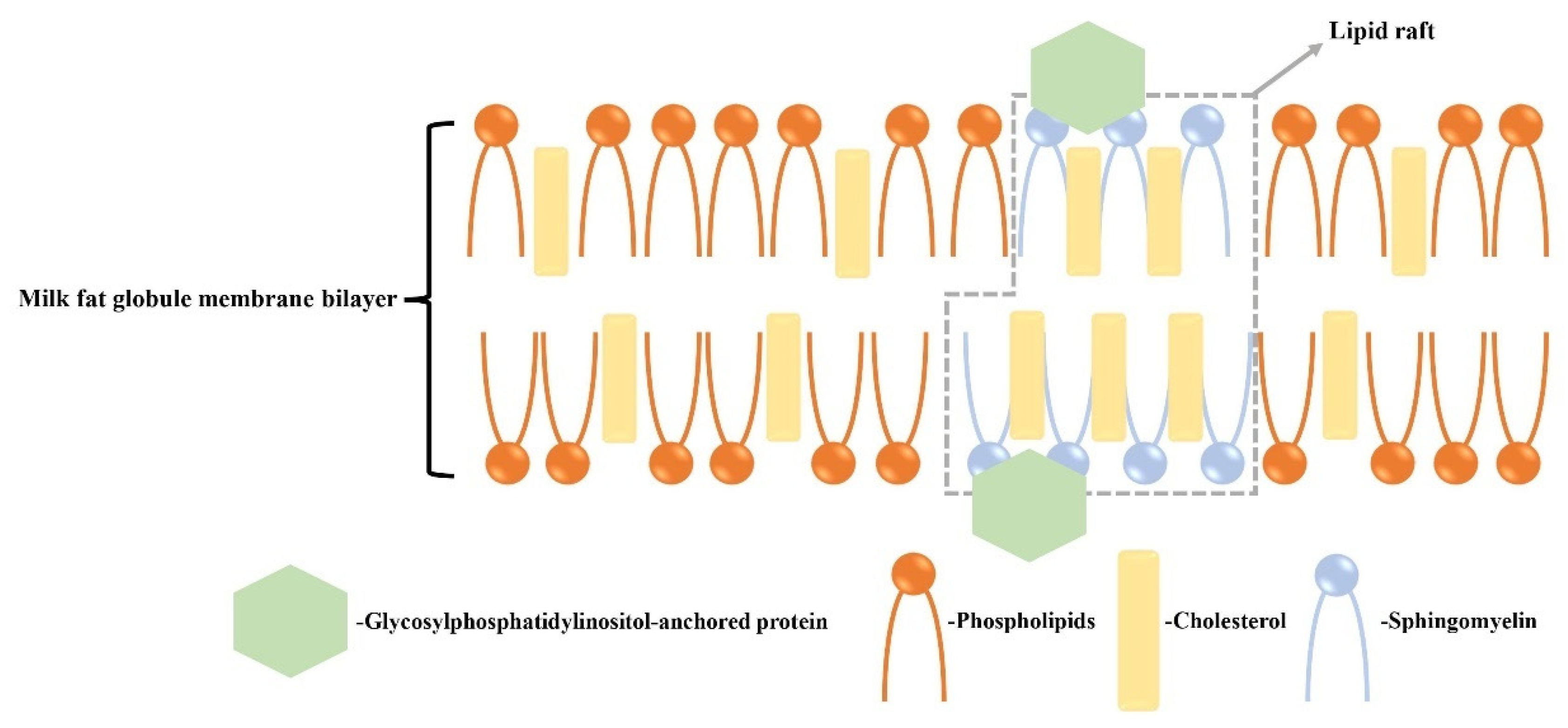
Cholesterol is found primarily in the outer bilayer of MFGMs and forms rigidly ordered domains known as lipid rafts when it binds with SM [34,35] (Figure 1). In contrast, the disordered phase of the membrane mainly consists of phospholipids. Lipid rafts have the ability to bind to proteins and can induce signaling processes. The structural role of lipids in MFGMs can be characterized by high-throughput synchrotron radiation X-ray diffraction (SR-XRD) and differential scanning calorimetry (DSC) [36]. Previous research has shown that the cholesterol enhances the order of the milk SM bilayer membrane, with the ordered phase being achieved at a 33 mol% cholesterol content. The ratio of cholesterol/SM can influence the interfacial properties of MFGMs, thereby impacting the functional properties of milk fat globules and their digestion mechanisms [37]. For the above-mentioned lipids, clinical trials have proven their beneficial effects on physical function improvement, development, and anticancer roles (Table 2).
Figure 1.
Structure of lipid rafts of MFGMs on cholesterol. Cholesterol in the MFGM binds to sphingolipids in the outer bilayer to form rigidly ordered domains, i.e., lipid rafts, and the disordered phase consists of phospholipids. The lipid rafts bind to proteins and can act as an induced signal [34,35].

Table 2.
Bioactive functions of lipids in the milk fat globule membrane.
2.2. Differences of Fatty Acid Composition in the Composition of Milk Fat Globules and MFGMs
The fatty acid compositions of MFGMs and MFGs (milk fat globules) were significantly different (Table 3), which was due to the fact that the main lipids in the MFG were triglycerides, cholesterol, and retinol esters [57,58], which led to the differences of their bioactive functions. Saturated fatty acids accounted for 55.2–67.0% and unsaturated fatty acids accounted for 33.0–44.8% in the MFGM; saturated fatty acids accounted for 66.3–73.0% and unsaturated fatty acids accounted for 27.0–33.7% in the MFGM [59,60,61]. There are many studies on the relative quantification of fatty acids in the MFGM. However, there are currently few studies on the absolute quantification of all fatty acids in the MFGM. This is because the fatty acid content in the MFGM is too low and requires higher throughput and sensitive technology for detection.

Table 3.
Differences in relative content fatty acid composition between the MFG and MFGM [27,59,60,61,62,63].
3. Characterization of Lipids in the MFGM
Liquid chromatography with an evaporative light-scattering detector (ELSD) is a frequently employed method for quantifying and characterizing lipids [64]. In the study by Zou et al., this technique was employed to determine the concentrations and relative proportions of PC, PI, PS, PE, and SM in the MFGMs of colostrum, mature milk, and transitional milk from cows. The findings indicated that the concentration of polar lipids in the total lipids reached its peak during the transitional stage of lactation. The relative content of the SM did not exhibit significant changes; however, the level of phosphatidylcholine in mature milk was higher compared to the other two stages [65]. This study highlighted that the level of polar lipids in mature milk was significantly higher than that in colostrum, which was in accord with the previous research [66]. In a previous comparative analysis, the lipid profile of the MFGM levels in buffalo and cow milk products were examined using HPLC-ELSD [60]. The results indicated that the percentage of phosphatidylcholine in buffalo milk was higher than that in cow milk, while the percentage of SM was lower when compared to cow milk.
Lipidomic, in combination with mass spectrometry, offers a powerful approach for identifying various lipid species based on different phospholipid classes [67]. In the study by George et al., the relationship between growth characteristics and lipids in the MFGM was explored, and the concentration and intake of lipids from different MFGMs were compared. Through LC-MS analysis, this research identified a total of 166 MFGM lipid species originating from 10 fractions. The study further demonstrated that infants exhibited variations in the content and intake of different lipids that existed in MFGMs, and the intake of MFGM lipids was positively correlated with infant development [68]. Similarly, Brink et al. utilized UPLC-MS to identify 338 MFGM lipid species derived from 10 fractions, thereby completing the lipid characterization of different commercial MFGM materials. In a study by Ali et al., UPLC-ESI-Q-TOF-MS was employed to identify 100 MFGM lipids derived from 7 fractions [69,70]. With the development of lipid analysis technology, MFGM lipid differences among different species have been widely characterized (Table 4), which is helpful for conducting better research on the nutritional value of milk and dairy products [37,71,72,73,74].

Table 4.
Relative proportion of lipids in MFGMs in different species [37,71,72,73,74].
4. Various Factors Alter the Lipid Composition of MFGMs
The size of the MFG can have a significant impact on the composition of the MFGM. Due to the different surface areas of MFGs, the volume ratio changes, and therefore the proportion of polar lipids also changes. Furthermore, diet and lactation change the size of the MFG, which has a significant effect on the composition of MFGMs [59,75,76,77]. The proportion of PI in small MFGs (3.32 ± 1.21 µm) is significantly lower than that in large MFGs (7.61 ± 0.90 µm), while the proportion of PE is significantly greater than that in large MFGs [78]. The size of the MFG at different positions in the cream fraction is classified. The upper milk fat globule is named F1, which is the largest, and the lower milk fat globule is the smallest, named F6. The results of the HPLC-ELSD analysis show that there are significant differences in the ratios of PI and PC between F1 and F6 fractions with significant differences in the MFG size, while there are no significant differences among F4, F5, and F6, and the changed composition of protein and fat produce this result [79]. In these groups of MFGs, the relative content of SM does not change significantly. For fat globule size-induced changes in the MFGM composition, this may be due to the difference in the curvature between MFGs of different sizes, resulting in different levels of dynamic processes at the molecular level, or it may be due to the rearrangement of the apical plasma membrane composition after secretion [80]. Changes in MFGM composition can affect its function during processing or digestion [73]. Studying the relationship between MFG size and MFGM composition contributes to further research on MFG secretion and allows for the production of products with MFGM compositions designed to meet specific needs.
5. Separation of Lipids in MFGMs
Extracts containing MFGMs are mainly divided into MFGM-enriched ingredients and phospholipid extracts. The former is widely used in nutrition, while the latter is mainly used in beauty products [81]. The Folch and Mojonnier methods are general lipid-extraction methods. The Folch method extracts lipids from milk using 20 volumes of 2:1 chloroform/methanol or 4 volumes of 1:1 chloroform/methanol, while the Mojonnier method extracts lipids using a mixture of ethanol, diethyl ether, and petroleum ether. After the milk fat is separated, solid phase extraction is usually used for fractionation [82,83]. Similarly, phospholipids can be extracted from dairy by-products using switchable solvents. Tertiary amines (CyNMe2) have been proven to extract PL from raw cream, buttermilk, concentrated buttermilk, and beta-serum, and CyNMe2 can extract most of PLs (mainly phosphatidylcholine and phosphatidylinositol) from buttermilk and beta-serum, which is higher than the extraction performance of the Folch and Mojonnier methods [84,85]. The combination of ultrafiltration and supercritical fluid extraction can obtain a fraction rich in globular membrane phospholipids of milk fat [86]. Lactose and ash are removed from whey buttermilk through a 10 kDa cutoff membrane. After spray drying, the powder is subjected to supercritical fluid extraction (CO2, 350 bar, 50 °C). Finally, a powder containing 21% lipid (61% PL) can be obtained. It is well known that lipid removal from milk is possible using organic solvents. The use of ethanol can effectively extract phospholipids from the by-product whey protein phospholipid concentrate. Using 70% aqueous ethanol at 70 °C can obtain a lipid concentrate with a higher PL content, while using 70% aqueous ethanol at 60 °C. Ethanol can obtain a lipid concentrate with a higher SM content [87]. The research on phospholipid extraction methods is relatively mature, most methods rely on organic solvents, and have high applicability; the extracted PL enrichment can be widely used in food emulsifiers.
6. Phospholipids
6.1. The Promoting Effect of Phospholipid Supplementation on Development
The addition of MFGMs to infant formula has become a widespread practice due to its role in promoting cognitive development [88]. The inclusion of sphingomyelin-enriched milk in the diet of premature infants has been investigated for its impact on cognitive development [89]. In a study involving 24 very-low-birth-weight babies, the infants were separated into two groups. The trial group accepted sphingomyelin-enriched milk, where sphingomyelin accounted for 20% of all phospholipids in the milk, while the control group received milk with only a 13% sphingomyelin content [38]. At eighteen months old, the babies in the group of sphingomyelin-enriched milk presented significantly higher scores in various developmental assessments, including the behavior rating scale of the BSID-II test, the Fagan test, visual evoked potentials (VEPs), and the sustained-attention test, compared to the control group. These findings suggest that infants receiving sphingomyelin-enriched milk exhibit improved neurobehavioral development.
Previous research examining the neurocognitive development and longitudinal trajectories of the brain in children who were fed different formulas for at least 3 months found significant developmental differences among the groups. Specifically, factors, such as long-chain sphingomyelin, iron, fatty acids, folic acid, and choline, were closely associated with early myelination trajectories [40]. Another study compared the impact of formula and conventional milk products on the central nervous system of 182 preschool children. The formula milk used in the trial group contained a 8–9-times-higher phospholipid concentration compared to the non-formula milk. The trial group received 200 mL of formula milk without phospholipids, while the control group received formula milk containing 500 mg of phospholipids. The children were evaluated using the Achenbach system of empirically based assessment. The results of the research indicate that formula milk with a higher phospholipid concentration has a more pronounced effect on children’s behavioral regulation [41]. These investigations emphasize the significance of specific nutrients, particularly phospholipids, in formula milk for promoting healthy brain and behavioral developments in children. The inclusion of appropriate levels of these nutrients in formula milk can positively impact neurodevelopment. This positive effect can be attributed to the role of sphingomyelin as an important component of the myelin sheath, as indicated by the previous research [39]. As a merely structural component of myelin, sphingomyelin promotes the proliferation, maturation, and differentiation of oligodendrocyte precursor cells (OPCs), as well as increased axonal myelination [90,91]. This explains why the dietary supplementation of MFGM-derived sphingolipids can increase cognitive and developmental functions in subjects. Considering the complexity of the nutritional needs of infants during their development, simple formula milk powder can no longer meet the market’s demands, and phospholipid supplementation is an effective means to enhance product competitiveness.
6.2. The Promoting Effect of Phospholipid Supplementation on Memory
Indeed, the supplementation of PS and sphingomyelin has been shown to enhance memory function [19]. In previous research involving 75 males aged 30 to 51 years old, different test groups were given milk containing a placebo, 0.5%, and 1% phospholipids (phospholipid content adjusted using Lacprodan PL 20) over a period of 42 days. The stress-protective effects of the phospholipids were evaluated using the Trier social stress test. It was concluded that high doses of phospholipids could inhibit the activity and responsiveness of the hypothalamic–pituitary–adrenal axis (HPAA) and result in a blunted psychological stress response. The age of the subjects and the duration of phospholipid supplementation may influence these effects [42]. In a study involving piglets, Lacprodan PL-20 (0%, 0.8%, 2.5% v/v) was added to the diets of three groups of piglets. The development of the piglets was observed from days 2 to 28 postpartum. The piglets’ performance in the T-maze was measured on day 14, and brain MRI data were obtained on day 28 postpartum. The piglets supplemented with 0.8% and 2.5% gangliosides showed better performances in the maze, had higher brain weights, and exhibited more white and gray matter. This suggests that gangliosides enhance spatial learning in newborn piglets and influence brain development [43]. Similar findings were observed in mouse experiments, where the supplementation of phospholipids from the MFGM improved the memory of mice [92]. These investigations emphasize the potential cognitive benefits of phospholipid supplementation, including an improved capacity to memorize and spatial learning, in both human and animal testing methods. The specific effects may vary depending on factors, such as dosage, duration of supplementation, and the age or developmental stage of the subjects. A recent study demonstrated the promoting effect of sphingomyelin on hippocampal development. Sphingomyelin can enter the nucleus of hippocampal cells, causing it to overexpress the sphingomyelin phosphodiesterase 4 gene encoding a neutral sphingomyelinase, thereby promoting changes in the soma of hippocampal cells and the formation of synapses [93]. In addition, the dietary supplementation of MFGM can also change the lipid abundance in the hippocampus without changing the lipidome of other brain tissues [94], which may also be the reason why supplementation with MFGMs improves memory, and the specific reason needs further research.
6.3. The Promoting Effect of Phospholipid Supplementation on Exercise Performance
The supplementation of dietary phospholipids, such as PC and PS, has been shown to improve the exercise performance of humans [44,95]. The supplementation of phospholipids and sphingolipids, in combination with exercise, has been investigated for its potential to improve muscle movement and neuromuscular development, including the formation of neuromuscular junctions. In Yoshinaka’s study, 71 subjects were divided into two groups. One group ingested 1 g of MFGM placebo (167 mg/placebo), while the other group received a placebo in the form of whole milk powder. Tablets containing MFGMs were produced for the test group, and placebo tablets were composed of whole milk powder. Both groups engaged in low-intensity exercise, and the trial lasted for eight weeks. The test group demonstrated a better performance in foot tapping and opening and closing steps, which could be attributed to the presence of sphingomyelin, one of the components that promote the development of nerve and muscle fibers [45]. Kim’s research expanded on this by including a placebo plus exercise group and an MFGM plus exercise group. This design allowed for a more targeted study to determine whether supplementation with milk fat globules or daily low-intensity exercise alone could improve frailty in the subjects [46]. These works suggest that dietary supplementation with phospholipid and sphingolipid supplements, in conjunction with exercise, may have positive effects on neuromuscular development and muscle movement. The combination of the phospholipid supplementation of MFGM and exercise can promote the expression of docking protein-7 and myogenin mRNA, thereby promoting the formation of neuromuscular junction synapses [11]. In addition, previous research points out that the improvement in exercise performance caused by phospholipid supplementation is due to the fact that phospholipids stabilize the cell membrane of red blood cells, thereby improving the oxygen transport capacity of red blood cells [96].
6.4. MFGM Phospholipid Supplementation Helps Alleviate Alzheimer’s Disease
Alzheimer’s disease is a neurodegenerative disease caused by cognitive decline with age, usually in patients over 65 years old [97]. As an important component of the cell membrane, changes in phospholipids at the cellular level cause different pathogenic processes, which can be improved by the dietary supplementation of PL [98,99]. Phospholipid supplementation by the intake of MFGM-enriched substances can effectively improve age-induced cognitive decline. A study on aged Wistar rats showed that dietary supplementation with enriched MFGMs isolated from buttermilk enhanced the rat’s spatial working memory. This was caused by changes in the lipid composition of the synaptic membrane. The contents of PS, PE, and SM increased after supplementing phospholipids, and these lipids were involved in the decline in cognitive function [47]. Recent studies indicate that AD symptoms can be effectively alleviated by adding phospholipid-rich protein powder (PP) to the diet of triple-transgenic AD (3 × Tg-AD) mice. This is because protein powder avoids neuroinflammation through the peroxisome proliferator-activated receptor γ (PPAR γ)–nuclear factor-κB signaling pathway [48].
6.5. Phospholipids Have a Regulating Effect on Gut Health
The dietary supplementation of phospholipids has an immunomodulatory role in immune regulation, particularly in the regulation of gut microbial composition and inflammation [100]. Compared to drugs, dietary modifications are often more cost-effective and sustainable in preventing infant diseases [101,102]. An investigation involving 119 infants aged ≤14 days old divided them into three groups: those receiving standard infant formula, MFGM lipid-enriched formula (MFGM-L), and MFGM protein-enriched formula (MFGM-P). The research aimed to assess the prevalence of adverse reactions in infants. The data showed that infants fed with the MFGM-L formula had the lowest frequency of diarrhea among the three groups [49]. This finding is likely attributable to the modification of gut microbiome by phospholipids or to phospholipid-induced changes in the immune system [103]. The beneficial effects of phospholipids on the gut were further reflected in their interaction with microorganisms, such as Lactobacillus. The interaction between phospholipids in the MFGM and Lactobacillus has been shown to significantly influence Lactobacillus adhesion and enhance gut microbiota health [104]. MFGM phospholipids can reduce the adhesion of Lactobacillus to Caco-2/goblet cell co-cultures. The electronegativity of the bacterial cell surface can be increased by adsorption or incorporation, which further causes changes in adhesion, leading to an increase in the adhesion process. MFGM phospholipids can promote the function of probiotics, which can provide them with beneficial prospects in the dairy industry. Moreover, phospholipids in the MFGM show the ability to inhibit the growth of Helicobacter pylori and reduce the levels of E. coli and Salmonella enteritidis [105].
6.6. Phospholipids Regulate Cholesterol Metabolism
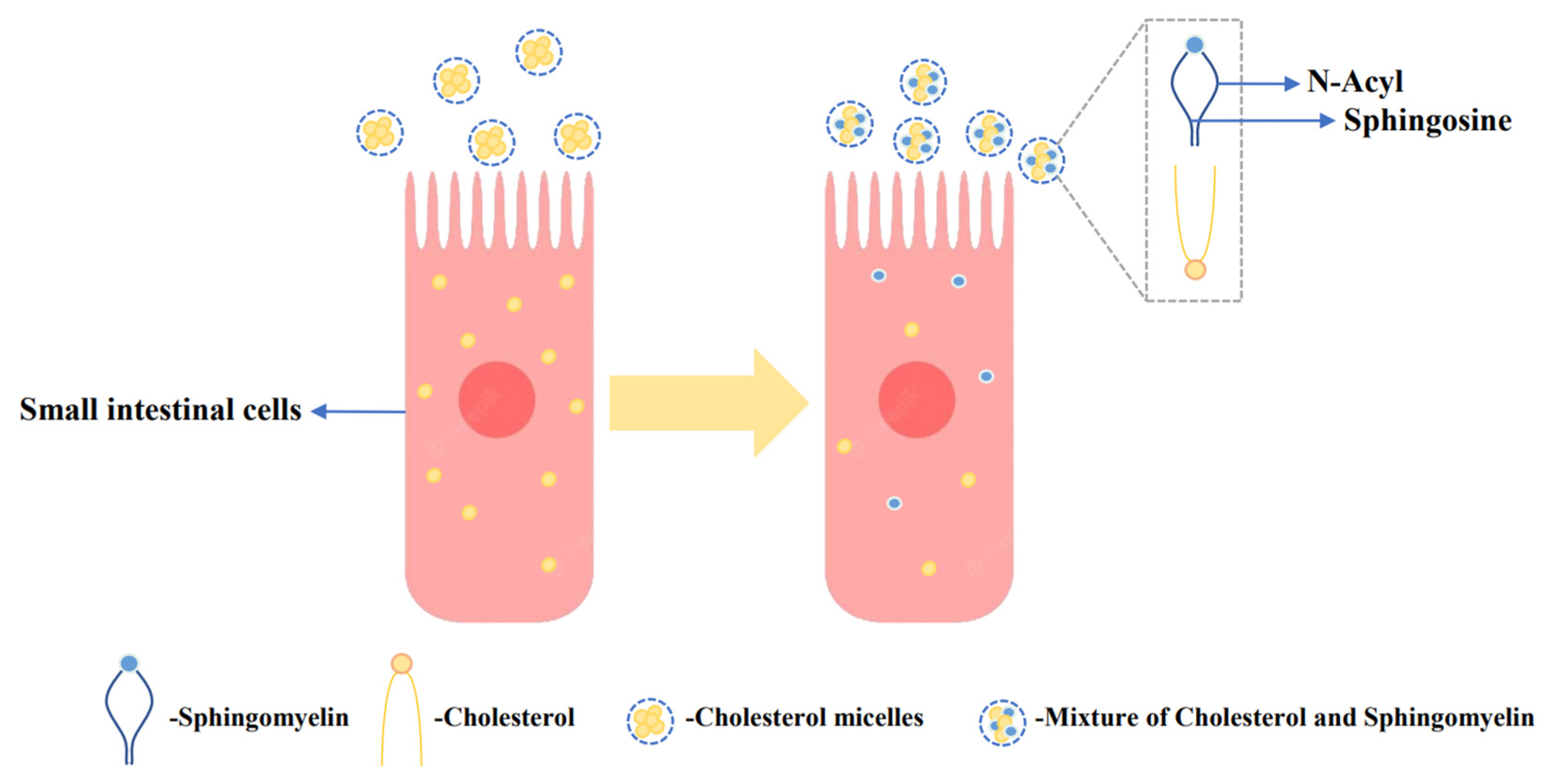
Increased serum cholesterol is a major contributor to cardiovascular disease (CVD), and supplementation with milk-derived lipids has been shown to modulate cholesterol levels [106]. Lipids present in the MFGM, particularly sphingomyelin, have been found to inhibit cholesterol absorption in the gut [107]. Dietary sphingomyelin has a substantial impact on plasma and tissue cholesterol levels. As shown in Figure 2, milk-derived sphingomyelin exhibits a stronger effect in inhibiting rat cholesterol absorption, and this can be attributed to the compatibility of sphingosine and N-acyl groups in the presence of cholesterol, promoting their mutual attraction [50]. In a study involving 34 subjects with low-density lipoprotein cholesterol (LDL-C) levels below 5.0 mmol/L, the effects of dietary supplementation with chocolate-flavor buttermilk or placebo were investigated. The placebo formulation matched the macro/micronutrient content of the buttermilk, except for the nutrients from MFGMs. The research found that dietary supplementation with buttermilk containing a high concentration of milk globular phospholipids effectively inhibited cholesterol absorption [51]. The strong affinity of milk-derived sphingomyelin for cholesterol can effectively lower the cholesterol thermodynamic activity and reduce the monomer content between cholesterol micelles, thereby inhibiting cholesterol absorption [108]. These works indicate that supplementation with milk-derived lipids, particularly sphingomyelin, can have a beneficial impact on cholesterol absorption and may help in managing serum cholesterol levels.
Figure 2.
Inhibitory effect of sphingomyelin of the MFGM on cholesterol. In the presence of cholesterol, the mutual matching of sphingomyelin and N-acyl groups promotes the mutual attraction of cholesterol and sphingomyelin, thereby inhibiting cholesterol absorption [50].
In a test performed by Rosqvist et al., a single-blind randomized controlled experiment was conducted over eight weeks on 57 overweight participants. The test group was supplemented with 40 g of whipping cream, which served as a source of MFGMs because of its enriched phospholipid level and relatively complete MFGM structure. The dietary phospholipid concentration in the experimental group was approximately 19-fold higher than that in the control group. The results indicated that blood lipids and the LDL-C of participants without the supplementation of MFGMs were significantly higher. The ingestion of MFGMs did not increase the cholesterol concentrations, potentially due to the influence of milk phospholipids on lipid metabolism and hepatic gene expression, affecting intra- and inter-organ lipid distributions [52]. Phospholipids have the ability to interfere with specific interactions in the gut, leading to the inhibition of cholesterol absorption without interfering with the gut microbiota [53]. This suggests that the mechanism of the phospholipid regulation of cholesterol absorption involves specific interactions in the gastrointestinal tract, which effectively reduce the uptake of cholesterol without disrupting the balance of gut microbial communities. The reason why phospholipid intake inhibits cholesterol absorption may be that phospholipids inhibit the expression of transporters related to cholesterol absorption, or reduce the solubility of cholesterol and increase the size of micelles and of bile acid binding capacity [109]. Many studies have proved the inhibitory effect of phospholipids on cholesterol absorption; however, further research is needed on the specific mechanism.
6.7. Anticancer Effects of Dietary Phospholipids
To assess the anticancer potential of sphingolipids, the AIN-76 feed was supplemented with anhydrous milk fat (AMF) and a combination of AMF and MFGM (1:1). A microscopic analysis revealed a significantly reduced number of colonic lesions in mice fed with MFGMs compared to those fed AIN-76 or anhydrous fat. This work proved that the anticancer function was attributed to sphingomyelin in MFGMs [54], which was supported by the subsequent research [110]. Buttermilk, obtained by extracting lipid components using food-grade solvents, has shown promising inhibitory effects on cancer cell activity. The presence of phospholipids in the extracts appeared to play a crucial role [111]. In one study, lipids from buttermilk powder were extracted by both food-grade ethanol and a non-food-grade solvent, such as a dichloromethane–methanol solution. The extracts were then fractionated using flash chromatography and the resulting solution’s inhibitory effects on nine human cancer cell lines were evaluated using an absorbance microplate reader [112]. The aforementioned findings suggest that sphingolipids, particularly those derived from MFGMs and buttermilk, can possess anticancer properties and exhibit inhibitory effects on colon cancer development and progression. Further research is needed on the mechanism by which phospholipids inhibit cancer. One review concludes that this may be because phospholipids are involved in the Kennedy pathway, and perturbations regulated by this pathway are related to a variety of diseases, including cancer [113].
7. Gangliosides
7.1. Gangliosides Promote Brain Development
The supplementation of gangliosides, a type of complex glycosphingolipids, has been found to promote neurodevelopment and cognitive function, potentially due to the presence of sialic acids in gangliosides, which play a role in synaptic growth and memory formation. When infants were fed formula with an increased ganglioside content, it was noted to have a favorable influence on their cognitive development [55]. In a study involving 60 infants aged 2–8 weeks old, one group was fed standard formula milk powder while the other group was fed formula milk powder enriched with compound milk fat to adjust the ganglioside content to 9 mg/100 g [56]. The cognitive development of the infants was assessed and the group receiving the ganglioside-enriched formula showed improved outcomes. Moreover, the prenatal supplementation of gangliosides by pregnant women was also found to promote brain development in their offspring [114]. This effect may be attributed to ganglioside supplementation enhancing brain region-specific increases in astrocytes, thereby increasing plasticity in the hippocampus, which is involved in learning and memory functions [115]. The abovementioned research underlines the benefits of ganglioside supplementation in promoting neurodevelopment, cognitive function, and brain plasticity in infants and offspring. At present, as an important part of the neuronal cell membrane, the supplementation of gangliosides promotes development to a certain extent; however, the physical fitness of the subjects needs to be considered.
7.2. Inhibitory Effect of Gangliosides on Intestinal Pathogenic Microorganisms
Indeed, dietary sphingolipids have been shown to modulate intestinal inflammation by influencing the gut microbiota [116]. Lee et al. studied the catabolism of gangliosides from milk-derived gangliosides using nano-HPLC Chip Q-TOF MS. Bifidobacterium infantis and B. bifidum significantly decreased the levels of degraded gangliosides GM3 and GD3, while B. longum subsp. longum and B. animalis subsp. lactis did not [117]. The sialic acid produced by the degradation of gangliosides leads to changes in glycolipid distribution in the intestine and exerts a prebiotic effect on the intestine, which may explain the improvement of intestinal health by gangliosides. In vitro experiments on Caco-2 cells show that gangliosides (GM3, GD3, and GM1) and sialic acid (Neu5Ac) can effectively prevent the adhesion of diarrheal pathogens [118]. They compete with pathogens to adhere to cells and can detach pathogens that adhere to cells. GM3 and GD3 are located on the apical and basolateral membranes of the Caco-2 cells, which may facilitate further studies on the competitive adhesion of gangliosides. MFGM gangliosides also protect tight-junction proteins [119]. By supplementing gangliosides, the level of the anti-inflammatory cytokine interleukin-10 can be increased, thereby preventing the decline in the level of intestinal tight-junction proteins and ensuring the good health of the intestinal tract. Similarly, gangliosides control the occurrence of necrotizing enterocolitis by regulating the levels of vasoactive mediators and pro-inflammatory factors [120].
8. Conclusions
This article presented an overview of the types and characterization methods of the main lipids from MFGMs. It also summarized the current works on the biological activities and functions of these lipids, including study designs, experimental results, and functional mechanisms. The supplementation of lipids derived from MFGMs in the diet was shown to promote development and improve immunity, making it a potential bridge between formula and breast milk products. The article emphasized the significance of studying MFGM lipids in the context of food nutrition and clinical applications. While there is existing research on the topic, the article suggests that there is still much to be explored, indicating potential future research directions. It was noted that the composition of the MFGM was influenced by external factors, such as processing methods and the stage of lactation. Therefore, accurately separating and purifying the MFGM and lipids in it is crucial for their commercial application. Overall, the article emphasizes the function of lipids in the MFGM in various aspects of human health and nutrition. It calls for further research to better understand their functions, explore their potential applications, and develop effective methods for their utilization in the food industry.
Author Contributions
J.P.: conceptualization, writing, original draft. M.C.: data curation, writing—review and editing. N.L.: investigation. Y.Y.: investigation, writing—review and editing. R.H.: investigation, data curation. S.Z.: writing—review and editing. N.Z.: conceptualization, writing—review and editing, validation, funding acquisition, supervision. Y.Z.: investigation, funding acquisition, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2022YFD1301004; the Agricultural Science and Technology Innovation Program, grant number ASTIP-IAS12; and the China Agriculture Research System of MOF and MARA, grant number CARS-36.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jukkola, A.; Rojas, O.J. Milk fat globules and associated membranes: Colloidal properties and processing effects. Adv. Colloid Interface Sci. 2017, 245, 92–101. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef]
- Park, Y.W. Bioactive Components in Milk and Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Huang, Q.X.; Yang, J.; Hu, M.; Lu, W.; Zhong, K.; Wang, Y.; Yang, G.; Loor, J.J.; Han, L. Milk fat globule membrane proteins are involved in controlling the size of milk fat globules during conjugated linoleic acid–induced milk fat depression. J. Dairy Sci. 2022, 105, 9179–9190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jiménez-Flores, R.; Everett, D.W. Bovine milk fat globule membrane proteins are affected by centrifugal washing processes. J. Agric. Food Chem. 2013, 61, 8403–8411. [Google Scholar] [CrossRef] [PubMed]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Lopez, C. Intracellular origin of milk fat globules, composition and structure of the milk fat globule membrane highlighting the specific role of sphingomyelin. In Advanced Dairy Chemistry, Volume 2: Lipids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 107–131. [Google Scholar] [CrossRef]
- Ali, F.; Wang, Z.-X. Effect of pasteurization on the enzymatic cross-linking of milk proteins by microbial transglutaminase in view of milk fat globule membrane isolation. Food Biosci. 2021, 43, 101100. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Z.; Liang, C.; Xie, S.; Wen, J.; Wang, H.; Wang, J.; Yang, Y.; Han, R. Developmental changes in proteins of casein micelles in goat milk using data-independent acquisition-based proteomics methods during the lactation cycle. J. Dairy Sci. 2023, 106, 47–60. [Google Scholar] [CrossRef]
- Yano, M.; Haramizu, S.; Ota, N.; Minegishi, Y.; Shimotoyodome, A. Continuous supplementation of milk fat globule membrane with habitual exercise from a young age improves motor coordination and skeletal muscle function in aged mice. J. Nutr. Sci. Vitaminol. 2019, 65, 405–413. [Google Scholar] [CrossRef]
- Ibitoye, J.O.; Ly-Nguyen, B.; Le, D.N.; Dewettinck, K.; Trzcinski, A.P.; Phan, T.T.Q. Quality of set yogurts made from raw milk and processed milk supplemented with enriched milk fat globule membrane in a two-stage homogenization process. Foods 2021, 10, 1534. [Google Scholar] [CrossRef]
- Qu, X.; Hu, H.; Wang, Y.; Cao, C.; Li, H.; Liu, X.; Yu, J. Proteomics analysis of milk fat globule membrane enriched materials derived from by-products during different stages of milk-fat processing. LWT 2019, 116, 108531. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, X.; Gao, Z.; Jiang, L.; Mu, Z. Advancement on Milk Fat Globule Membrane: Separation, Identification, and Functional Properties. Front. Nutr. 2021, 8, 807284. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.F.; Hogan, S.A.; Tobin, J.; Rasmussen, J.T.; Larsen, L.B.; Wiking, L. Microfiltration of raw milk for production of high-purity milk fat globule membrane material. J. Food Eng. 2020, 276, 109887. [Google Scholar] [CrossRef]
- Holzmüller, W.; Kulozik, U. Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. Int. Dairy J. 2016, 63, 88–91. [Google Scholar] [CrossRef]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Unraveling the complexity of milk fat globules to tailor bioinspired emulsions providing health benefits: The key role played by the biological membrane. Eur. J. Lipid Sci. Technol. 2019, 121, 1800201. [Google Scholar] [CrossRef]
- Claumarchirant, L.; Matencio, E.; Sanchez-Siles, L.M.; Alegría, A.; Lagarda, M.J. Sterol composition in infant formulas and estimated intake. J. Agric. Food Chem. 2015, 63, 7245–7251. [Google Scholar] [CrossRef]
- Hellhammer, J.; Waladkhani, A.R.; Hero, T.; Buss, C. Effects of milk phospholipid on memory and psychological stress response. Br. Food J. 2010, 57, 183–193. [Google Scholar] [CrossRef]
- Palmano, K.P.; MacGibbon, A.K.; Gunn, C.A.; Schollum, L.M. In vitro and in vivo anti-inflammatory activity of bovine milkfat globule (MFGM)-derived complex lipid fractions. Nutrients 2020, 12, 2089. [Google Scholar] [CrossRef]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical benefits of milk fat globule membranes for infants and children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional dynamics of the milk fat globule and its role in infant development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Lemaire, M.; Blat, S. Health benefits of dairy lipids and MFGM in infant formula. Oléagineux Corps Gras Lipides 2018, 25, D306. [Google Scholar] [CrossRef]
- Sánchez-Juanes, F.; Alonso, J.; Zancada, L.; Hueso, P. Distribution and fatty acid content of phospholipids from bovine milk and bovine milk fat globule membranes. Int. Dairy J. 2009, 19, 273–278. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Paboeuf, G.; Vie, V.; Lopez, C. Cholesterol strongly affects the organization of lipid monolayers studied as models of the milk fat globule membrane: Condensing effect and change in the lipid domain morphology. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2308–2316. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Pryce, J.; Rochfort, S. Comprehensive characterization of bovine milk lipids: Phospholipids, sphingolipids, glycolipids, and ceramides. J. Agric. Food Chem. 2020, 68, 6726–6738. [Google Scholar] [CrossRef]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Castro-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2020, 86, 103386. [Google Scholar] [CrossRef]
- Ferreiro, T.; Gayoso, L.; Rodríguez-Otero, J. Milk phospholipids: Organic milk and milk rich in conjugated linoleic acid compared with conventional milk. J. Dairy Sci. 2015, 98, 9–14. [Google Scholar] [CrossRef]
- Raza, G.S.; Herzig, K.-H.; Leppäluoto, J. Invited review: Milk fat globule membrane—A possible panacea for neurodevelopment, infections, cardiometabolic diseases, and frailty. J. Dairy Sci. 2021, 104, 7345–7363. [Google Scholar] [CrossRef]
- Alden, K.P.; Dhondt-Cordelier, S.; McDonald, K.L.; Reape, T.J.; Ng, C.K.-Y.; McCabe, P.F.; Leaver, C.J. Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants. Biochem. Biophys. Res. Commun. 2011, 410, 574–580. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Lopez, C. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Curr. Opin. Colloid Interface Sci. 2011, 16, 391–404. [Google Scholar] [CrossRef]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the brain: Physiology, pathophysiology and therapeutic applications. Front. Neurosci. 2020, 14, 1004. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Madec, M.-N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. Cholesterol decreases the size and the mechanical resistance to rupture of sphingomyelin rich domains, in lipid bilayers studied as a model of the milk fat globule membrane. Langmuir 2016, 32, 6757–6765. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Cheng, K.; Perez, J. Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chem. Phys. Lipids 2018, 215, 46–55. [Google Scholar] [CrossRef]
- Et-Thakafy, O.; Guyomarc’h, F.; Lopez, C. Lipid domains in the milk fat globule membrane: Dynamics investigated in situ in milk in relation to temperature and time. Food Chem. 2017, 220, 352–361. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef]
- Deoni, S.; Dean III, D.; Joelson, S.; O’Regan, J.; Schneider, N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage 2018, 178, 649–659. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.-J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef]
- Schubert, M.; Contreras, C.; Franz, N.; Hellhammer, J. Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr. Res. 2011, 31, 413–420. [Google Scholar] [CrossRef]
- Liu, H.; Radlowski, E.C.; Conrad, M.S.; Li, Y.; Dilger, R.N.; Johnson, R.W. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 2014, 144, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaka, Y.; Soga, S.; Ota, N.; Yokoyama, K.; Yamada, Y.; Kimura, M. Light rhythmic exercise with dietary milk fat globule membrane improves physical fitness in an elderly Japanese population: A double-blind randomized placebo-controlled trial. Biosci. Biotechnol. Biochem. 2018, 82, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Haramizu, S.; Mori, T.; Yano, M.; Ota, N.; Hashizume, K.; Otsuka, A.; Hase, T.; Shimotoyodome, A. Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice. Springerplus 2014, 3, 339. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suzuki, T.; Kim, M.; Kojima, N.; Ota, N.; Shimotoyodome, A.; Hase, T.; Hosoi, E.; Yoshida, H. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: A randomized double blind, placebo-controlled, follow-up trial. PLoS ONE 2015, 10, e0116256. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Calvo, M.V.; Piquera, D.; Montero, O.; Visioli, F.; Venero, C.; Fontecha, J. Milk fat globule membrane concentrate as a nutritional supplement prevents age-related cognitive decline in old rats: A lipidomic study of synaptosomes. Food Res. Int. 2023, 163, 112163. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.-H.; Huang, S.-L.; Yue, Z.-B.; Yin, X.-S.; Feng, Z.-Q.; Zhang, X.-G.; Song, G.-L. Whey protein powder with milk fat globule membrane attenuates Alzheimer’s disease pathology in 3×Tg-AD mice by modulating neuroinflammation through the peroxisome proliferator-activated receptor γ signaling pathway. J. Dairy Sci. 2023, 106, 5253–5265. [Google Scholar] [CrossRef]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clin. Med. Insights Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef]
- Conway, V.; Couture, P.; Richard, C.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1255–1262. [Google Scholar] [CrossRef]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary milk fat globule membrane reduces the incidence of aberrant crypt foci in Fischer-344 rats. J. Agric. Food Chem. 2010, 58, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krägeloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, A.J.; Roig-Sagués, A.X.; Zamora, A.; Ferragut, V. 12—High-Pressure Homogenization for Structure Modification. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 315–344. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Liu, Y.; Wang, L.; Li, X. Simulated in vitro infant gastrointestinal digestion of infant formulas containing different fat sources and human milk: Differences in lipid profiling and free fatty acid release. J. Agric. Food Chem. 2021, 69, 6799–6809. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Briard-Bion, V.; Menard, O.; Rousseau, F.; Pradel, P.; Besle, J.-M. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. J. Agric. Food Chem. 2008, 56, 5226–5236. [Google Scholar] [CrossRef]
- Ménard, O.; Ahmad, S.; Rousseau, F.; Briard-Bion, V.; Gaucheron, F.; Lopez, C. Buffalo vs. cow milk fat globules: Size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Food Chem. 2010, 120, 544–551. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Ménard, O. Polar lipids, sphingomyelin and long-chain unsaturated fatty acids from the milk fat globule membrane are increased in milks produced by cows fed fresh pasture based diet during spring. Food Res. Int. 2014, 58, 59–68. [Google Scholar] [CrossRef]
- Fauquant, C.; Briard, V.; Leconte, N.; Michalski, M.C. Differently sized native milk fat globules separated by microfiltration: Fatty acid composition of the milk fat globule membrane and triglyceride core. Eur. J. Lipid Sci. Technol. 2005, 107, 80–86. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K. Protein and lipid composition of bovine milk-fat-globule membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Tavazzi, I.; Fontannaz, P.; Lee, L.Y.; Giuffrida, F. Quantification of glycerophospholipids and sphingomyelin in human milk and infant formula by high performance liquid chromatography coupled with mass spectrometer detector. J. Chromatogr. B 2018, 1072, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-Q.; Guo, Z.; Huang, J.-H.; Jin, Q.-Z.; Cheong, L.-Z.; Wang, X.-G.; Xu, X.-B. Human milk fat globules from different stages of lactation: A lipid composition analysis and microstructure characterization. J. Agric. Food Chem. 2012, 60, 7158–7167. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Guo, Z.; Jin, Q.; Huang, J.; Cheong, L.; Xu, X.; Wang, X. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chem. 2015, 185, 362–370. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Yu, J.; Yuan, T.; Zhao, P.; Tao, G.; Wei, W.; Wang, X. Comprehensive lipidomic analysis of milk polar lipids using ultraperformance supercritical fluid chromatography-mass spectrometry. Food Chem. 2022, 393, 133336. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.; Selvalatchmanan, J.; Torta, F.; Bendt, A.K.; Wenk, M.R.; Murray, K.; Wlodek, M.E.; Geddes, D.T. Healthy Breastfeeding Infants Consume Different Quantities of Milk Fat Globule Membrane Lipids. Nutrients 2021, 13, 2951. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Wei, W.; Abed, S.M.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Impact of technological processes on buffalo and bovine milk fat crystallization behavior and milk fat globule membrane phospholipids profile. Lwt 2018, 90, 424–432. [Google Scholar] [CrossRef]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, G.; Xiang, J.; Zou, X.; Jin, Q.; Wang, X. Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. Int. Dairy J. 2016, 56, 64–73. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Luo, J.; Huang, Z.; Liu, H.; Zhang, Y.; Ren, F. Yak milk fat globules from the Qinghai-Tibetan Plateau: Membrane lipid composition and morphological properties. Food Chem. 2018, 245, 731–737. [Google Scholar] [CrossRef]
- Agyare, A.N.; Liang, Q. Nutrition of yak milk fat–Focusing on milk fat globule membrane and fatty acids. J. Funct. Foods 2021, 83, 104404. [Google Scholar] [CrossRef]
- Thum, C.; Wall, C.; Day, L.; Szeto, I.M.; Li, F.; Yan, Y.; Barnett, M.P. Changes in human milk fat globule composition throughout lactation: A review. Front. Nutr. 2022, 9, 835856. [Google Scholar] [CrossRef] [PubMed]
- Mesilati-Stahy, R.; Argov-Argaman, N. The relationship between size and lipid composition of the bovine milk fat globule is modulated by lactation stage. Food Chem. 2014, 145, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Fauquant, C.; Briard-Bion, V.; Leconte, N.; Guichardant, M.; Michalski, M.C. Membrane phospholipids and sterols in microfiltered milk fat globules. Eur. J. Lipid Sci. Technol. 2007, 109, 1167–1173. [Google Scholar] [CrossRef]
- Lu, J.; Argov-Argaman, N.; Anggrek, J.; Boeren, S.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K.A. The protein and lipid composition of the membrane of milk fat globules depends on their size. J. Dairy Sci. 2016, 99, 4726–4738. [Google Scholar] [CrossRef] [PubMed]
- Mesilati-Stahy, R.; Mida, K.; Argov-Argaman, N. Size-dependent lipid content of bovine milk fat globule and membrane phospholipids. J. Agric. Food Chem. 2011, 59, 7427–7435. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B.; Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Size-Dependent Variations in Fatty Acid Composition and Lipid Content of Native Milk Fat Globules. In Effect of Milk Fat Globule Size on the Physical Functionality of Dairy Products; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–34. [Google Scholar] [CrossRef]
- Lopez, C. Chapter 20—Valorization of dairy by-products for functional and nutritional applications: Recent trends toward the milk fat globule membrane. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 415–423. [Google Scholar] [CrossRef]
- Pimentel, L.; Gomes, A.; Pintado, M.; Rodríguez-Alcalá, L.M. Isolation and analysis of phospholipids in dairy foods. J. Anal. Methods Chem. 2016, 2016, 9827369. [Google Scholar] [CrossRef]
- Gallier, S.; Gragson, D.; Cabral, C.; Jiménez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef]
- Cheng, S.; Rathnakumar, K.; Martínez-Monteagudo, S.I. Extraction of dairy phospholipids using switchable solvents: A feasibility study. Foods 2019, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Rathnakumar, K.; Ortega-Anaya, J.; Jimenez-Flores, R.; Martínez-Monteagudo, S.I. Understanding the switchable solvent extraction of phospholipids from dairy byproducts. Food Bioprod. Process. 2021, 126, 175–183. [Google Scholar] [CrossRef]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- Price, N.; Fei, T.; Clark, S.; Wang, T. Extraction of phospholipids from a dairy by-product (whey protein phospholipid concentrate) using ethanol. J. Dairy Sci. 2018, 101, 8778–8787. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Lönnerdal, B. The role of milk fat globule membranes in behavior and cognitive function using a suckling rat pup supplementation model. J. Nutr. Biochem. 2018, 58, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.M.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk fat globule membrane supplementation in formula-fed rat pups improves reflex development and may alter brain lipid composition. Sci. Rep. 2018, 8, 15277. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Hauser, J.; Oliveira, M.; Cazaubon, E.; Mottaz, S.C.; O’Neill, B.V.; Steiner, P.; Deoni, S.C. Sphingomyelin in brain and cognitive development: Preliminary data. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Goñi, F.M. Sphingomyelin: What is it good for? Biochem. Biophys. Res. Commun. 2022, 633, 23–25. [Google Scholar] [CrossRef]
- Guan, J.; MacGibbon, A.; Fong, B.; Zhang, R.; Liu, K.; Rowan, A.; McJarrow, P. Long-term supplementation with beta serum concentrate (BSC), a complex of milk lipids, during post-natal brain development improves memory in rats. Nutrients 2015, 7, 4526–4541. [Google Scholar] [CrossRef]
- Albi, E.; Arcuri, C.; Kobayashi, T.; Tomishige, N.; Dei Cas, M.; Paroni, R.; Signorelli, P.; Cerquiglini, L.; Troiani, S.; Gizzi, C. Sphingomyelin in human breast milk might be essential for the hippocampus maturation. Front. Biosci. 2022, 27, 247. [Google Scholar] [CrossRef]
- Fraser, K.; Ryan, L.; Dilger, R.N.; Dunstan, K.; Armstrong, K.; Peters, J.; Stirrat, H.; Haggerty, N.; MacGibbon, A.K.; Dekker, J. Impacts of formula supplemented with milk fat globule membrane on the neurolipidome of brain regions of piglets. Metabolites 2022, 12, 689. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr. 2007, 4, 5. [Google Scholar] [CrossRef]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Kosicek, M.; Hecimovic, S. Phospholipids and Alzheimer’s disease: Alterations, mechanisms and potential biomarkers. Int. J. Mol. Sci. 2013, 14, 1310–1322. [Google Scholar] [CrossRef]
- Schipper, L.; van Dijk, G.; Broersen, L.M.; Loos, M.; Bartke, N.; Scheurink, A.J.W.; van der Beek, E.M. A Postnatal Diet Containing Phospholipids, Processed to Yield Large, Phospholipid-Coated Lipid Droplets, Affects Specific Cognitive Behaviors in Healthy Male Mice123. J. Nutr. 2016, 146, 1155–1161. [Google Scholar] [CrossRef]
- Lee, H.; Slupsky, C.M.; Heckmann, A.B.; Christensen, B.; Peng, Y.; Li, X.; Hernell, O.; Lönnerdal, B.; Li, Z. Milk fat globule membrane as a modulator of infant metabolism and gut microbiota: A formula supplement narrowing the metabolic differences between breastfed and formula-fed infants. Mol. Nutr. Food Res. 2021, 65, 2000603. [Google Scholar] [CrossRef]
- Herrmann, F.; Nieto-Ruiz, A.; Sepúlveda-Valbuena, N.; Miranda, M.T.; Diéguez, E.; Jiménez, J.; De-Castellar, R.; García-Ricobaraza, M.; García-Santos, J.A.; Bermúdez, M.G. Infant formula enriched with milk fat globule membrane, long-chain polyunsaturated fatty acids, synbiotics, gangliosides, nucleotides and sialic acid reduces infections during the first 18 months of life: The COGNIS study. J. Funct. Foods 2021, 83, 104529. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast milk lipids and fatty acids in regulating neonatal intestinal development and protecting against intestinal injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef]
- Ortega-Anaya, J.; Marciniak, A.; Jiménez-Flores, R. Milk fat globule membrane phospholipids modify adhesion of Lactobacillus to mucus-producing Caco-2/Goblet cells by altering the cell envelope. Food Res. Int. 2021, 146, 110471. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, G.; Czeczot, H.; Bielecka, M. The anticarcinogenic potential of milk fat. Ann. Agric. Environ. Med. 2020, 27, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views Off. J. Gulf Heart Assoc. 2017, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Pokala, A.; Torres-Gonzalez, M.; Blesso, C.N. Cardiometabolic health benefits of dairy-milk polar lipids. Nutr. Rev. 2021, 79 (Suppl. S2), 16–35. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.H.; Milard, M.; Michalski, M.-C.; Blesso, C.N. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, G.; Ma, M.; Qiu, N.; Zhu, L.; Li, J. Egg-yolk sphingomyelin and phosphatidylcholine attenuate cholesterol absorption in Caco-2 cells. Lipids 2018, 53, 217–233. [Google Scholar] [CrossRef]
- Zanabria, R.; Tellez, A.M.; Griffiths, M.; Corredig, M. Milk fat globule membrane isolate induces apoptosis in HT-29 human colon cancer cells. Food Funct. 2013, 4, 222–230. [Google Scholar] [CrossRef]
- Castro-Gomez, P.; Rodriguez-Alcala, L.M.; Monteiro, K.M.; Ruiz, A.; Carvalho, J.E.; Fontecha, J. Antiproliferative activity of buttermilk lipid fractions isolated using food grade and non-food grade solvents on human cancer cell lines. Food Chem. 2016, 212, 695–702. [Google Scholar] [CrossRef]
- Castro-Gomez, M.P.; Rodriguez-Alcala, L.M.; Calvo, M.V.; Romero, J.; Mendiola, J.A.; Ibanez, E.; Fontecha, J. Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef]
- Stoica, C.; Ferreira, A.K.; Hannan, K.; Bakovic, M. Bilayer forming phospholipids as targets for cancer therapy. Int. J. Mol. Sci. 2022, 23, 5266. [Google Scholar] [CrossRef]
- Ryan, J.M.; Rice, G.E.; Mitchell, M.D. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef]
- Guillermo, R.B.; Yang, P.; Vickers, M.H.; McJarrow, P.; Guan, J. Supplementation with complex milk lipids during brain development promotes neuroplasticity without altering myelination or vascular density. Food Nutr. Res. 2015, 59, 25765. [Google Scholar] [CrossRef]
- Rohrhofer, J.; Zwirzitz, B.; Selberherr, E.; Untersmayr, E. The impact of dietary sphingolipids on intestinal microbiota and gastrointestinal immune homeostasis. Front. Immunol. 2021, 12, 1690. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Garrido, D.; Mills, D.A.; Barile, D. Hydrolysis of milk gangliosides by infant-gut associated bifidobacteria determined by microfluidic chips and high-resolution mass spectrometry. Electrophoresis 2014, 35, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, J.; Barbera, R.; Matencio, E.; Alegría, A.; Lagarda, M. Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: An in vitro study. Food Chem. 2013, 136, 726–734. [Google Scholar] [CrossRef]
- Park, E.J.; Thomson, A.B.; Clandinin, M.T. Protection of intestinal occludin tight junction protein by dietary gangliosides in lipopolysaccharide-induced acute inflammation. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, K.L.; Larsen, B.; Van Aerde, J.E.; Lees, G.; Evans, M.; Belosevic, M.; Field, C.; Thomson, A.; Clandinin, M.T. Gangliosides protect bowel in an infant model of necrotizing enterocolitis by suppressing proinflammatory signals. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 382–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).