Effect of Beef Silver Skin (Epimysium) Levels on Meat Emulsion Stability, Quality Attributes, and Texture Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredient Preparation
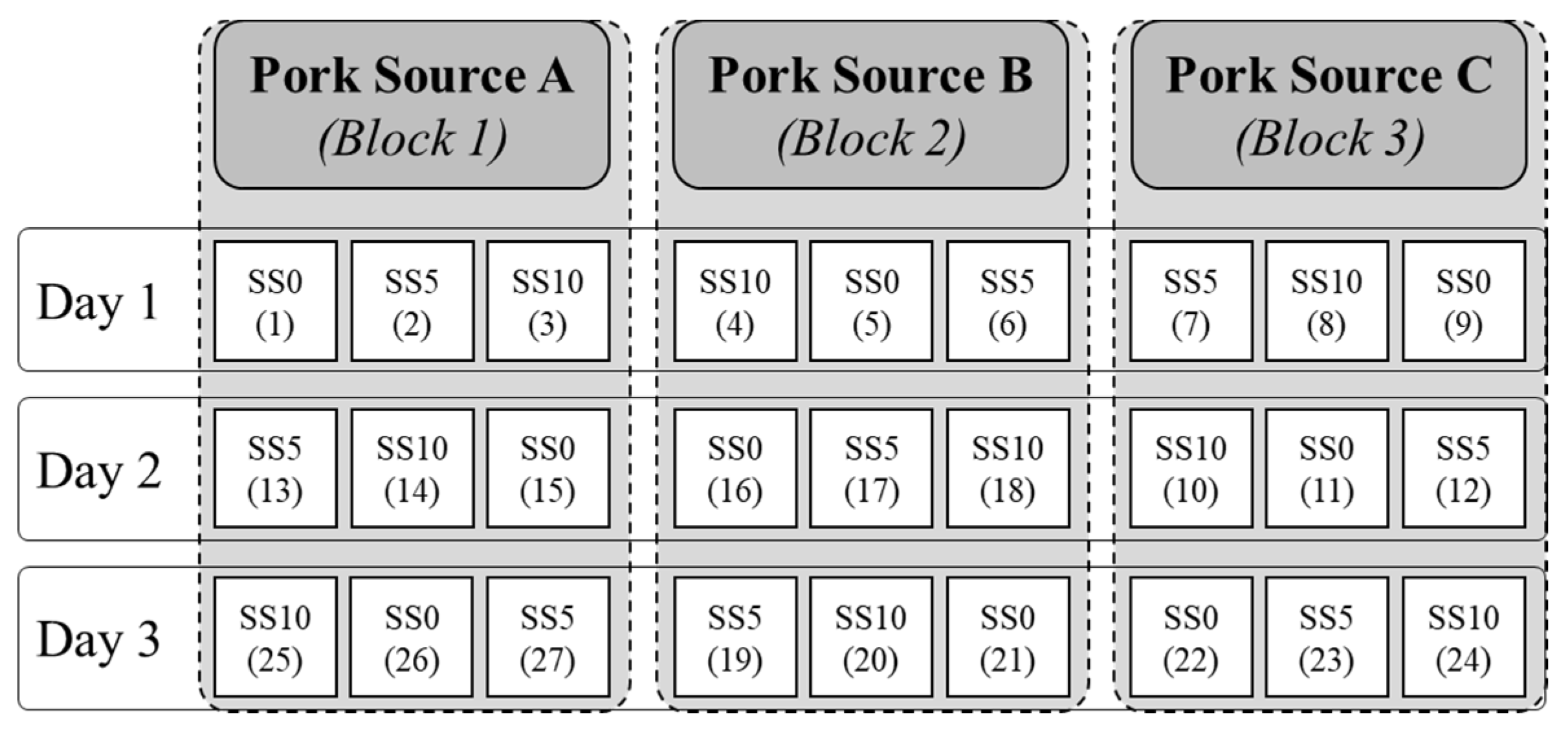
2.2. Experimental Design and Emulsion Preparation
2.3. pH
2.4. Emulsion Stability
2.5. Instrumental Colors
2.6. Water Activity
2.7. Collagen Analysis
2.8. Chemical Composition
2.9. Texture Profile Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Collagen Concentration
3.2. Physical Parameter and Emulsion Stability
3.3. Texture Profile Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC NHANES 2015–2016 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2015 (accessed on 8 August 2023).
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in Meat Consumption in the USA. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tarté, R. Meat-Derived Protein Ingredients. In Ingredients in Meat Products: Properties, Functionality and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 145–171. [Google Scholar]
- Gordon, A.; Barbut, S. Mechanisms of Meat Batter Stabilization: A Review. Crit. Rev. Food Sci. Nutr. 1992, 32, 299–332. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.; Barbut, S. Physicochemical Effects of the Lipid Phase and Protein Level on Meat Emulsion Stability, Texture, and Microstructure. J. Food Sci. 2010, 75, S108–S114. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.W. Emulsifier Applications in Meat Products. In Food Emulsifiers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 347–377. [Google Scholar]
- Pearson, A.M.; Albert, M.; Gillett, T.A. Processed Meats, 3rd ed.; Aspen Publishers: Boston, MA, USA, 1999; ISBN 0834213044. [Google Scholar]
- Sorapukdee, S.; Kongtasorn, C.; Benjakul, S.; Visessanguan, W. Influences of Muscle Composition and Structure of Pork from Different Breeds on Stability and Textural Properties of Cooked Meat Emulsion. Food Chem. 2013, 138, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Kim, T.K.; Jung, S.; Kim, Y.B.; Choi, Y.S. Quality of Reduced-Fat Meat Emulsion: Effect of Pre-Emulsified Duck Skin and Hydrocolloids. J. Food Sci. Technol. 2021, 58, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Petridis, D.; Raizi, P.; Ritzoulis, C. Influence of Citrus Fiber, Rice Bran and Collagen on the Texture and Organoleptic Properties of Low-Fat Frankfurters. J. Food Process. Preserv. 2014, 38, 1759–1771. [Google Scholar] [CrossRef]
- Neklyudov, A.D. Nutritive Fibers of Animal Origin: Collagen and Its Fractions as Essential Components of New and Useful Food Products. Appl. Biochem. Microbiol. 2003, 39, 229–238. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.d.L.S.; Fontes, P.R. Effects of the Addition of Mechanically Deboned Poultry Meat and Collagen Fibers on Quality Characteristics of Frankfurter-Type Sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef]
- Prabhu, G.A.; Doerscher, D.R.; Hull, D.H. Utilization of Pork Collagen Protein in Emulsified and Whole Muscle Meat Products. J. Food Sci. 2004, 69, C388–C392. [Google Scholar] [CrossRef]
- Sims, T.J.; Bailey, A.J. Connective Tissue. In Developments in Meat Science; Lawrie, R.A., Ed.; Applied Science Publications: London, UK, 1981; Volume 2, pp. 29–59. [Google Scholar]
- Purslow, P.P. Contribution of Collagen and Connective Tissue to Cooked Meat Toughness; Some Paradigms Reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Bergman, I.; Loxley, R. Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Cross, H.R.; Carpenter, Z.L.; Smith, G.C. Effects of intramuscular collagen and elastin on bovine muscle tenderness. J. Food Sci. 1973, 38, 998–1003. [Google Scholar] [CrossRef]
- Etherington, D.J.; Sims, T.J. Detection and Estimation of Collagen. J. Sci. Food Agric. 1981, 32, 539–546. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Bañón, S.; Díaz, P.; Nieto, G.; Castillo, M.; Álvarez, D. Modelling the Yield and Texture of Comminuted Pork Products Using Color and Temperature. Effect of Fat/Lean Ratio and Starch. Meat Sci. 2008, 80, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Masoodi, F.A.; Akhter, R.; Rather, J.A.; Gani, A.; Wani, S.M.; Malik, A.H. Application of Guar-Xanthan Gum Mixture as a Partial Fat Replacer in Meat Emulsions. J. Food Sci. Technol. 2016, 53, 2876–2886. [Google Scholar] [CrossRef] [PubMed]
- McKillop, K.; Harnly, J.; Pehrsson, P.; Fukagawa, N.; Finley, J. FoodData Central, USDA’s Updated Approach to Food Composition Data Systems. Curr. Dev. Nutr. 2021, 5, 596. [Google Scholar] [CrossRef]
- Office of the Federal Register, N.A. and R.A. 9 CFR 320.180 Frankfurter, Frank, Furter, Hotdog, Weiner, Vienna, Bologna, Garlic Bologna, Knockwurst, and Similar Products. 2011. Available online: govinfo.gov (accessed on 12 August 2023).
- Kristinsson, H.G.; Hultin, H.O. Role of PH and Ionic Strength on Water Relationships in Washed Minced Chicken-Breast Muscle Gels. J. Food Sci. 2003, 68, 917–922. [Google Scholar] [CrossRef]
- Eilert, S.J.; Mandigo, R.W. Procedure for Soluble Collagen in Thermally Processed Meat Products. J. Food Sci. 1993, 58, 948–949. [Google Scholar] [CrossRef]
- Ladwig, K.M.; Knipe, C.L.; Sebranek, J.G. Effects of Collagen and Alkaline Phosphate on Time of Chopping, Emulsion Stability and Protein Solubility of Fine-Cut Meat Systems. J. Food Sci. 1989, 54, 541–544. [Google Scholar] [CrossRef]
- Jones, K.W.; Mandigo, R.W. Effects of Chopping Temperature on the Microstructure of Meat Emulsions. J. Food Sci. 1982, 47, 2030–2035. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, D.; Zhu, S.; Nian, Y.; Xu, X.; Zhou, G.; Li, C. Overheating Induced Structural Changes of Type I Collagen and Impaired the Protein Digestibility. Food Res. Int. 2020, 134, 109225. [Google Scholar] [CrossRef]
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.A.; Arcanjo, N.M.O.; Silva, F.A.P.; Ferreira, V.C.S.; Barreto, M.D.S.; Araújo, Í.B.S. Quality Parameters of Frankfurter-Type Sausages with Partial Replacement of Fat by Hydrolyzed Collagen. LWT-Food Sci. Technol. 2017, 76, 320–325. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.d.S.; Miklos, R.; Lund, M.N. Green Tea Extract Impairs Meat Emulsion Properties by Disturbing Protein Disulfide Cross-Linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (g) | Treatments | ||
|---|---|---|---|
| SS-0 | SS-5 | SS-10 | |
| Ground pork | 366 | 344 | 322 |
| Ground beef | 184 | 172 | 160 |
| Fat trimming | 184 | 172 | 160 |
| Crushed ice | 184 | 184 | 184 |
| Silver skin | 0 | 46 | 92 |
| Cure salt 1 | 1.03 | 1.03 | 1.03 |
| Salt (sodium chloride) | 9.2 | 9.2 | 9.2 |
| Spice mix 2 | 14 | 14 | 14 |
| Item | Ground Beef | Silver Skin | Pork Source A | Pork Source B | Pork Source C | Beef Fat Trimmings |
|---|---|---|---|---|---|---|
| Moisture, % | 56.7 | 47.9 | 75.5 | 72.5 | 71.9 | 20.5 |
| Protein, % | 15.4 | 15.2 | 20.8 | 20.7 | 22.4 | 1.59 |
| Fat, % | 22.8 | 35.6 | 1.37 | 3.13 | 1.68 | 77.8 |
| Collagen, mg/g | 16.9 | 53.9 | 5.77 | 6.68 | 7.28 | 12.1 |
| pH | 5.86 | 5.96 | 5.85 | 5.71 | 5.80 | 5.89 |
| Silver Skin Level 1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Item | 0 | 5 | 10 | SEM 2 | Linear | Quadratic |
| Moisture, % | 65.0 | 64.9 | 65.1 | 0.349 | 0.880 | 0.679 |
| Protein, % | 12.4 | 12.4 | 12.3 | 0.088 | 0.497 | 0.140 |
| Fat, % | 19.4 | 19.8 | 19.2 | 0.512 | 0.856 | 0.389 |
| Total collagen, mg/g | 7.66 | 8.71 | 9.32 | 0.392 | 0.008 | 0.676 |
| Silver Skin Level 1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Item | 0 | 5 | 10 | SEM 2 | Linear | Quadratic |
| pH | 5.68 | 5.69 | 5.72 | 0.014 | 0.079 | 0.828 |
| Water activity | 0.984 | 0.987 | 0.988 | 0.002 | 0.133 | 0.623 |
| Emulsion temperature, °C | 7.57 | 8.93 | 8.96 | 0.60 | 0.019 | 0.165 |
| Total cook loss, % | 12.8 | 13.0 | 15.2 | 1.41 | 0.136 | 0.477 |
| Water loss, % | 11.5 | 11.6 | 12.9 | 1.17 | 0.225 | 0.584 |
| Fat loss, % | 1.39 | 1.92 | 2.27 | 0.28 | 0.026 | 0.770 |
| Raw emulsion color | ||||||
| L* | 63.4 | 64.0 | 63.8 | 0.72 | 0.671 | 0.553 |
| a* | 6.18 | 5.88 | 6.11 | 0.15 | 0.769 | 0.165 |
| b* | 17.7 | 17.5 | 17.6 | 0.16 | 0.746 | 0.302 |
| Cooked emulsion color | ||||||
| L* | 64.1 | 63.7 | 62.9 | 0.38 | 0.045 | 0.679 |
| a* | 7.80 | 7.57 | 8.04 | 0.28 | 0.368 | 0.132 |
| b* | 13.2 | 13.5 | 13.7 | 0.31 | 0.314 | 0.967 |
| Silver Skin Level 1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Item | 0 | 5 | 10 | SEM 2 | Linear | Quadratic |
| Hardness, N | 36.9 | 33.8 | 32.9 | 1.03 | 0.001 | 0.244 |
| Adhesiveness, N.mm | 0.873 | 0.598 | 0.641 | 0.050 | <0.001 | <0.001 |
| Cohesiveness, ratio | 0.240 | 0.248 | 0.232 | 0.009 | 0.171 | 0.024 |
| Springiness, ratio | 3.14 | 2.80 | 2.78 | 0.065 | <0.001 | 0.001 |
| Gumminess, N | 8.78 | 8.44 | 7.70 | 0.439 | <0.001 | 0.321 |
| Chewiness, N | 27.9 | 24.2 | 22.1 | 1.97 | <0.001 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawata, K.; Giotto, F.M.; de Mello, A.S.; Kingery, T.; Silva, L.H.P. Effect of Beef Silver Skin (Epimysium) Levels on Meat Emulsion Stability, Quality Attributes, and Texture Parameters. Foods 2023, 12, 3775. https://doi.org/10.3390/foods12203775
Kawata K, Giotto FM, de Mello AS, Kingery T, Silva LHP. Effect of Beef Silver Skin (Epimysium) Levels on Meat Emulsion Stability, Quality Attributes, and Texture Parameters. Foods. 2023; 12(20):3775. https://doi.org/10.3390/foods12203775
Chicago/Turabian StyleKawata, Kentaro, Francine M. Giotto, Amilton S. de Mello, Thomas Kingery, and Luiz H. P. Silva. 2023. "Effect of Beef Silver Skin (Epimysium) Levels on Meat Emulsion Stability, Quality Attributes, and Texture Parameters" Foods 12, no. 20: 3775. https://doi.org/10.3390/foods12203775






