Visualizing the Distribution of Jujube Metabolites at Different Maturity Stages Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
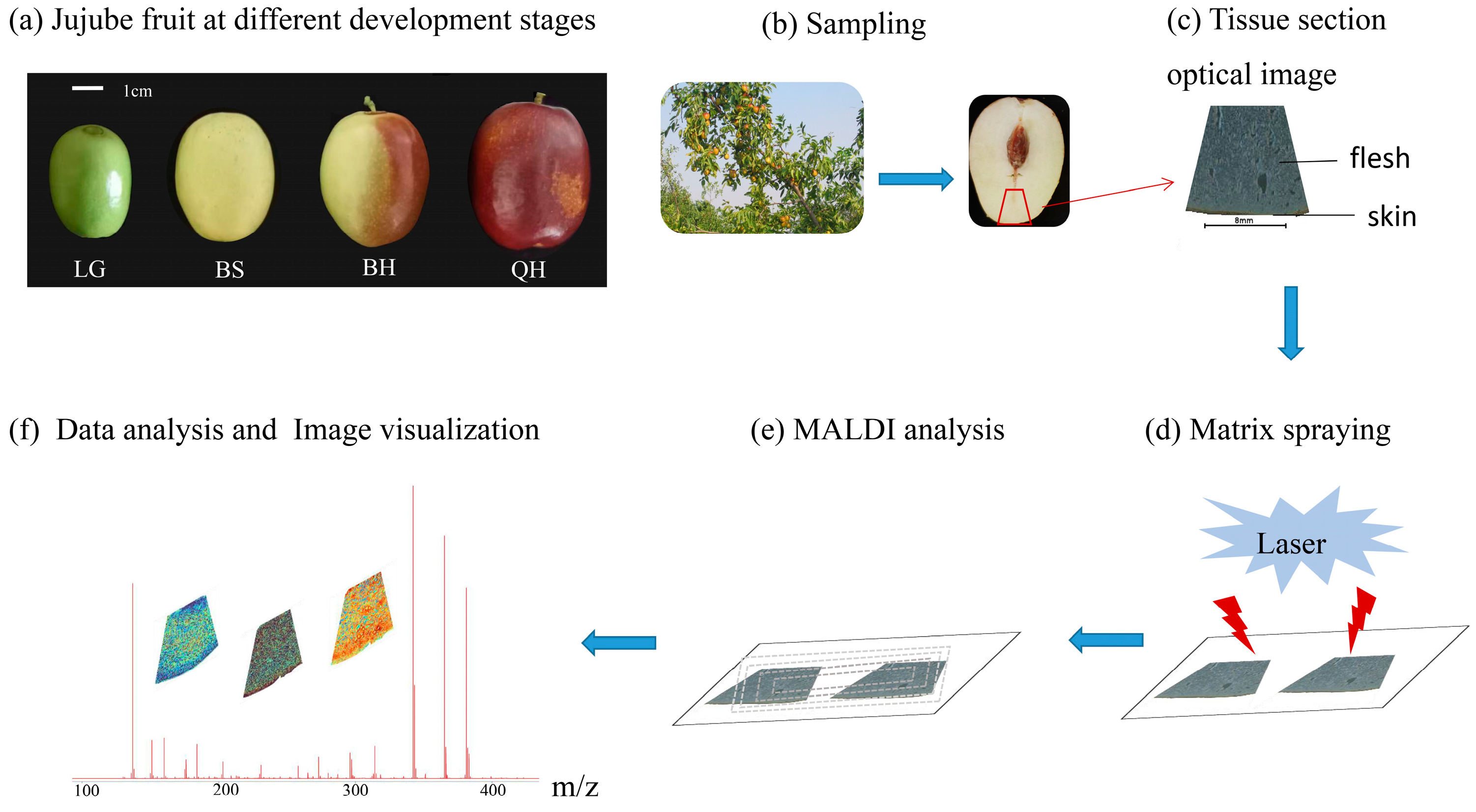
2.2. Plant Materials
2.3. Jujube Sample Preparation and Matrix Coating
2.4. Mass Spectrometry Imaging
2.5. Data Analysis
3. Results and Discussion
3.1. Matrix Selection
3.2. Identification of Metabolites in Jujube Samples
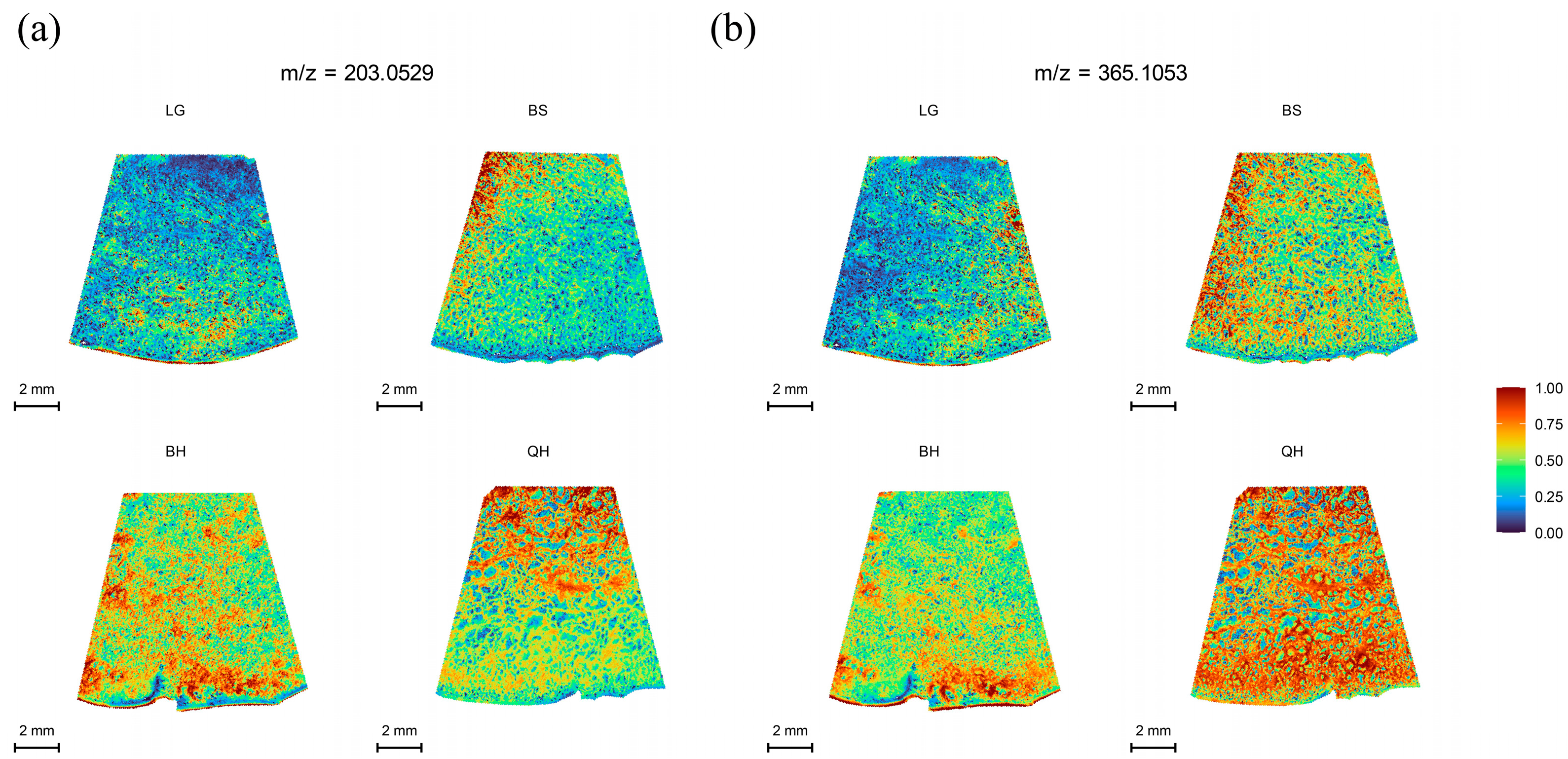
3.3. Spatial Distribution of Soluble Sugars
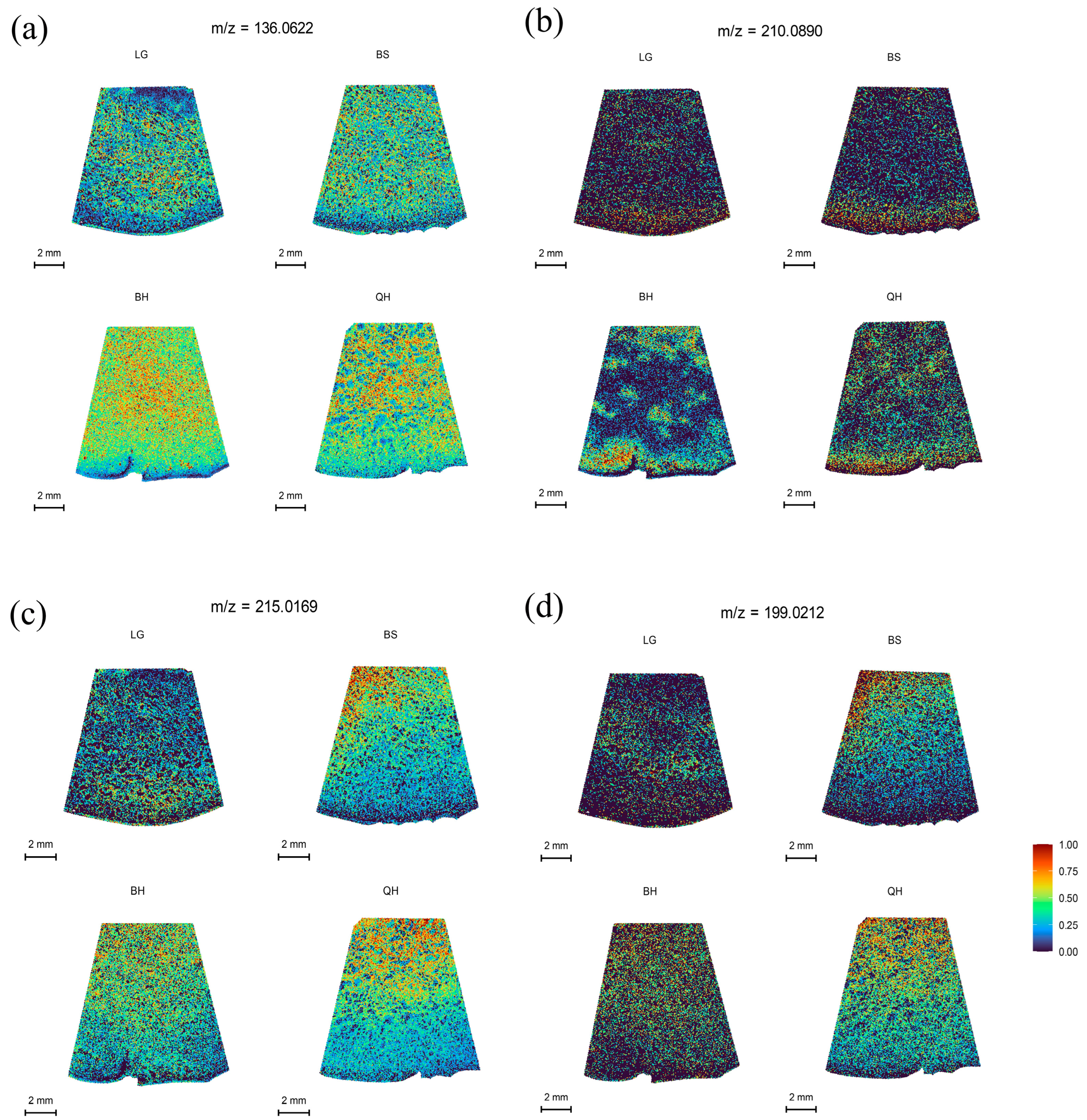
3.4. Spatial Distribution of Organic Acids
3.5. Spatial Distribution of Procyanidins and Flavonoid
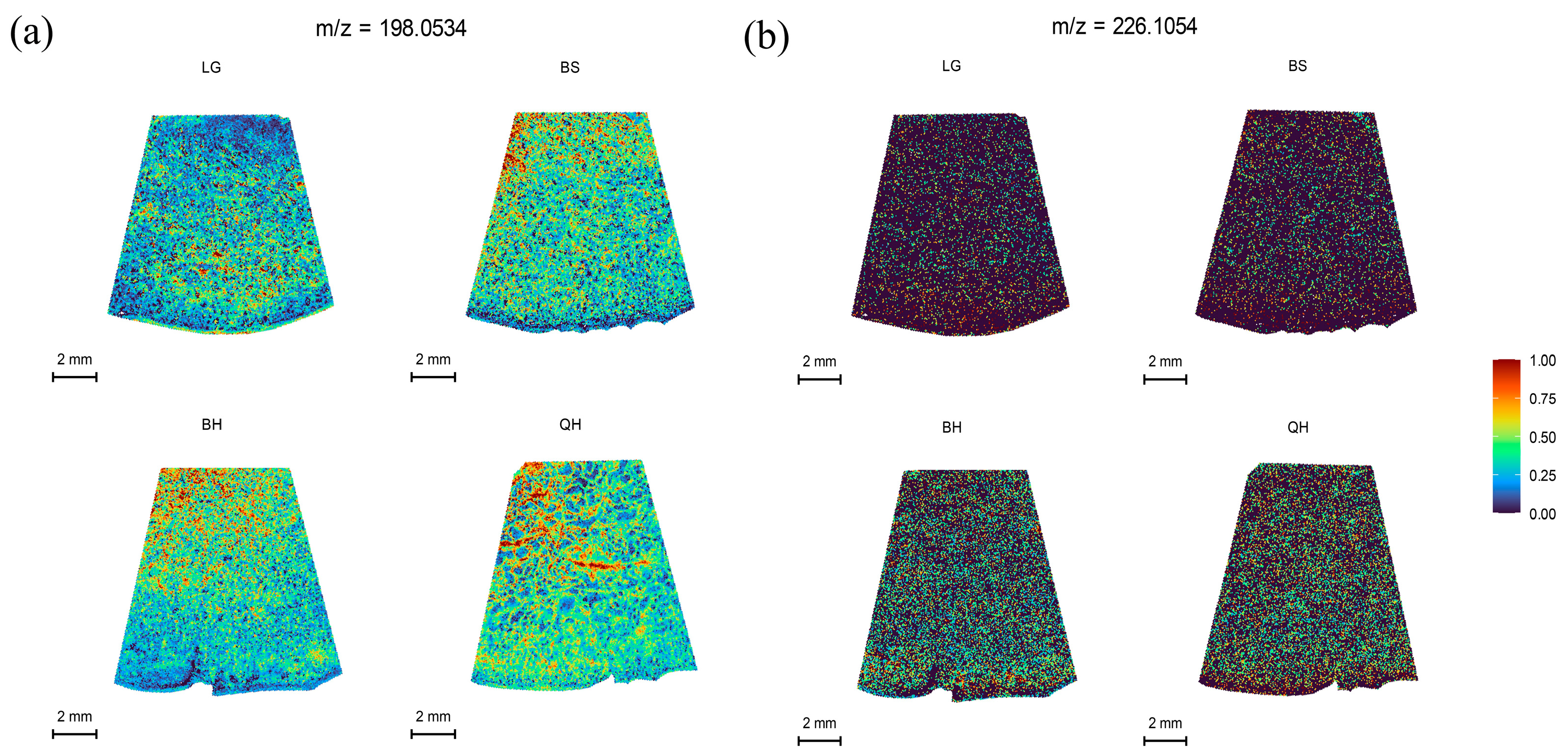
3.6. Spatial Distribution of Other Substances
3.7. Prospects for MALDI-MSI Technology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Wang, J.; Liu, P.; Zhao, J.; Zhao, Z.; Dai, L.; Li, X.; Liu, Z. Historical achievements and frontier advances in the production and research of Chinese jujube (Ziziphus jujuba) in China. Acta Hortic. Sin. 2015, 42, 1683. [Google Scholar]
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stănică, F.; Liu, Z.; Wang, L.; et al. The historical and current research progress on jujube–a superfruit for the future. Hortic. Res. 2020, 7, 119. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and antioxidant effects of vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxidative Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef]
- Yao, S. Past, present, and future of jujubes—Chinese dates in the United States. HortScience 2013, 48, 672–680. [Google Scholar] [CrossRef]
- Stănică, F.; Vasile, S. Chinese date-a new promising fruit plant for Romania southern areas. In XXVII International Horticultural Congress-IHC2006: International Symposium on Asian Plants with Unique Horticultural 769; International Society for Horticultural Science: Korbeek-Lo, Belgium, 2006. [Google Scholar]
- Stănică, F. Characterization of two Romanian local biotypes of Ziziphus jujuba. Acta Hortic. 2009, 840, 259–262. [Google Scholar] [CrossRef]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Wang, M. The Jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, J.; Liu, M.; Yang, L.; Tian, S. Variations of main nutrition components during fruit developing process of Ziziphus jujuba cv. Dongzao and Ziziphus jujuba cv. linyilizao. Chin. Agric. Sci. Bull. 2006, 22, 261–264. [Google Scholar]
- Lu, D.; Zhang, L.; Wu, Y.; Pan, Q.; Zhang, Y.; Liu, P. An integrated metabolome and transcriptome approach reveals the fruit flavor and regulatory network during jujube fruit development. Front. Plant Sci. 2022, 13, 952698. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Zaima, N. Application of mass spectrometry imaging for visualizing food components. Foods 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.T.; Su, H.; Chiang, Y.Y.; Shiea, J.; Yuan, S.S.F.; Hung, W.C.; Yeh, Y.T.; Hou, M.-F. Fine Needle Aspiration Combined with Matrix-assisted Laser Desorption Ionization Time-of-Flight/Mass Spectrometry to Characterize Lipid Biomarkers for Diagnosing Accuracy of Breast Cancer. Clin. Breast Cancer 2017, 17, 373–381.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hang, L.; Wang, T.; Leng, Y.; Zhang, H.; Meng, Y.; Yin, Z.; Hang, W. Nanoscale three-dimensional imaging of drug distributions in single cells via laser desorption post-ionization mass spectrometry. J. Am. Chem. Soc. 2021, 143, 21648–21656. [Google Scholar] [CrossRef]
- Wang, J.; Yang, E.; Chaurand, P.; Raghavan, V. Visualizing the distribution of strawberry plant metabolites at different maturity stages by MALDI-TOF imaging mass spectrometry. Food Chem. 2021, 345, 128838. [Google Scholar] [CrossRef]
- Lahaye, M.; Tabi, W.; Le Bot, L.; Delaire, M.; Orsel, M.; Campoy, J.A.; Garcia, J.Q.; Le Gall, S. Comparison of cell wall chemical evolution during the development of fruits of two contrasting quality from two members of the Rosaceae family: Apple and sweet cherry. Plant Physiol. Biochem. 2021, 168, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, C.; Zhao, H.; Zhou, G.; Qin, L.; Li, X.; Chen, L.; Wang, X.; Wan, Y. In-situ detection and imaging of Areca catechu fruit alkaloids by MALDI-MSI. Ind. Crop. Prod. 2022, 188, 115533. [Google Scholar] [CrossRef]
- Kentaro, H.; Takuya, H.; Hanako, S.; Yutaka, J.; Takashi, S. Visualization of soluble carbohydrate distribution in apple fruit flesh utilizing MALDI-TOF MS imaging. Plant Sci. 2019, 278, 107–112. [Google Scholar]
- Wang, X.; Chen, Y.; Liu, Y.; Ouyang, L.; Yao, R.; Wang, Z.; Kang, Y.; Yan, L.; Huai, D.; Jiang, H.; et al. Visualizing the distribution of lipids in peanut seeds by MALDI mass spectrometric imaging. Foods 2022, 11, 3888. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Lin, Y.; Li, L.; Liu, J.; Wang, Z.; Xiong, S.; Zhao, Z. A uniform 2,5-dihydroxybenzoic acid layer as a matrix for MALDI-FTICR MS-based lipidomics. Analyst 2014, 140, 1298–1305. [Google Scholar] [CrossRef]
- Jaskolla, T.W.; Lehmann, W.-D.; Karas, M. 4-Chloro-α-cyanocinnamic acid is an advanced, rationally designed MALDI matrix. Proc. Natl. Acad. Sci. USA 2008, 105, 12200–12205. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Zhou, D.; Li, Z. Electric field-assisted matrix coating method enhances the detection of small molecule metabolites for mass spectrometry imaging. Anal. Chem. 2015, 87, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Qin, L.; Zhang, Y.; Han, M.; Li, J.; Liu, Y.; Qiu, K.; Dai, X.; Li, Y.; Zeng, M.; et al. 3,4-Dimethoxycinnamic acid as a novel matrix for enhanced In Situ detection and imaging of low-molecular-weight compounds in biological tissues by MALDI-MSI. Anal. Chem. 2019, 91, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, W.; Xiong, C.; Zhou, X.; Xiong, S.; Nie, Z.; Mao, L.; Chen, Y.; Chang, H.-C. High-salt-tolerance matrix for facile detection of glucose in rat brain Microdialysates by MALDI mass spectrometry. Anal. Chem. 2012, 84, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.S.; da Silva, D.F.; Forim, M.R.; Fernandes, J.B.; Vieira, P.C.; Silva, D.B.; Lopes, N.P.; de Carvalho, S.A.; de Souza, A.A.; Machado, M.A.; et al. Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC-UV and MALDI imaging mass spectrometry. Phytochemistry 2015, 115, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.-Y.; Li, L.-X.; Jin, H.-Y.; Ni, J. Relative molecular weight determination of astragalus polysaccharides for injection. Acta Pharm. Sin. 2019, 348–353. [Google Scholar]
- Korte, A.R.; Lee, Y.J. MALDI-MS analysis and imaging of small molecule metabolites with 1,5-diaminonaphthalene (DAN). J. Mass Spectrom. 2014, 49, 737–741. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Nishio, S.; Saito, T.; Terakami, S.; Takada, N.; Kato, H.; Itai, A.; Yamamoto, T. Identification of QTLs associated with conversion of sucrose to hexose in mature fruit of Japanese pear. Plant Mol. Biol. Rep. 2018, 36, 643–652. [Google Scholar] [CrossRef]
- Wang, C.; Fang, J.; Wang, T.; Tan, H. The sugar metabolism in fruits. Acta Agric. Zhejiangensis 2009, 21, 529–534. [Google Scholar]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Enomoto, H.; Sato, K.; Miyamoto, K.; Ohtsuka, A.; Yamane, H. Distribution Analysis of anthocyanins, sugars, and organic acids in strawberry fruits using matrix-assisted laser desorption/ionization-imaging mass spectrometry. J. Agric. Food Chem. 2018, 66, 4958–4965. [Google Scholar] [CrossRef]
- Zhao, A.; Xue, X.; Wang, Y.; Sui, C.; Ren, H.; Li, D. The Sugars and Organic Acids Composition in Fruits of Different Chinese Jujube Cultivars of Different Development Stages. Acta Hortic. Sin. 2016, 43, 1175–1185. [Google Scholar]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Shi, Y. Visualizing the spatial distribution of endogenous molecules in wolfberry fruit at different development stages by matrix-assisted laser desorption/ionization mass spectrometry imaging. Talanta 2021, 234, 122687. [Google Scholar] [CrossRef]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.Y.; Guo, L.-X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent advances in the regulation of citric acid metabolism in Citrus Fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- de Jesús Ornelas-Paz, J.; Yahia, E.M.; Ramírez-Bustamante, N.; Pérez-Martínez, J.D.; del Pilar Escalante-Minakata, M.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Wang, L.; Fu, H.; Wang, W.; Wang, Y.; Zheng, F.; Ni, H.; Chen, F. Analysis of reducing sugars, organic acids and minerals in 15 cultivars of jujube (Ziziphus jujuba Mill.) fruits in China. J. Food Compos. Anal. 2018, 73, 10–16. [Google Scholar] [CrossRef]
- Lu, D.; Wu, Y.; Pan, Q.; Zhang, Y.; Qi, Y.; Bao, W. Identification of key genes controlling L-ascorbic acid during Jujube (Ziziphus jujuba Mill.) fruit development by integrating transcriptome and metabolome analysis. Front. Plant Sci. 2022, 13, 950103. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.; Li, X. Transcriptomic analysis reveals the metabolic mechanism of L-ascorbic acid in Ziziphus jujuba Mill. Front. Plant Sci. 2016, 7, 122. [Google Scholar] [CrossRef]
- Wang, W.; Pu, Y.; Wen, H.; Lu, D.; Yan, M.; Liu, M.; Wu, M.; Bai, H.; Shen, L.; Wu, C. Transcriptome and weighted gene co-expression network analysis of jujube (Ziziphus jujuba Mill.) fruit reveal putative genes involved in proanthocyanin biosynthesis and regulation. Food Sci. Hum. Wellness 2023, 12, 1557–1570. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Miskinis, R.d.A.S.; do Nascimento, L.Á.; Colussi, R. Bioactive compounds from acerola pomace: A review. Food Chem. 2023, 404, 134613. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef]
- Wei, X.; Jia, W.; Ma, J.; Wang, Y.; Li, J.; Wu, L. Review on the Effects of Plant Growth Regulators on Plant Growth and Development. North. Hortic. 2022, 4, 118–125. [Google Scholar]
- Zhang, Y.; Liu, W.; Shi, X.; Zhang, Y.; Du, G. The characteristic of Yatu morphogenesis and the efficacy of exogenous hormones on the development of Yatu during fruit development in ‘Yali’ pear (Pyrus bretschneideri Rehd.). Plant Signal. Behav. 2022, 17, 2106075. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, Z.; Jiang, M.; Zhang, B.; Chen, Q.; Zhang, C.; Zheng, Z.; Qiao, X. Visualizing the spatial distribution of Arctium lappa L. root components by MALDI-TOF mass spectrometry imaging. Foods 2022, 11, 3957. [Google Scholar] [CrossRef]


| Group | Compounds | Formula | Precursor (Da) m/z | Exact Precursor (Da) m/z | Adduct | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| Soluble sugars | Glucose | C6H12O6 | 203.0529 | 203.052609 | M+Na | 1.43 |
| Fructose | C6H12O6 | 203.0529 | 203.052609 | M+Na | 1.43 | |
| Sucrose | C12H22O11 | 365.1053 | 365.105432 | M+Na | −3.62 | |
| Maltose | C12H22O11 | 365.1053 | 365.105432 | M+Na | −3.62 | |
| Organic acids | Succinic acid | C4H6O4 | 136.0622 | 136.060434 | M+NH4 | 1.30 |
| Citric acid | C6H8O7 | 215.0169 | 215.016223 | M+Na | 3.15 | |
| Isocitric acid | C6H8O7 | 215.0169 | 215.016223 | M+Na | 3.15 | |
| Quinic acid | C7H12O6 | 210.0890 | 210.097214 | M+NH4 | −3.91 | |
| Ascorbic acid | C6H8O6 | 199.0212 | 199.021309 | M+Na | −0.55 | |
| Procyanidins and flavonoid | Procyanidin A1 | C30H24O12 | 577.1345 | 577.134053 | M+H | 7.75 |
| Procyanidin A2 | C30H24O12 | 577.1345 | 577.134053 | M+H | 7.75 | |
| Procyanidin B1 | C30H26O12 | 601.1325 | 601.131647 | M+Na | 1.42 | |
| Procyanidin B2 | C30H26O12 | 601.1325 | 601.131647 | M+Na | 1.42 | |
| Procyanidin B3 | C30H26O12 | 601.1325 | 601.131647 | M+Na | 1.42 | |
| Procyanidin C1 | C45H38O18 | 889.1980 | 889.195035 | M+Na | 3.33 | |
| Rutin | C27H30O16 | 633.1424 | 633.142606 | M+Na | −3.25 | |
| Plant hormones | Indole-3-acetic acid | C10H9NO2 | 198.0534 | 198.052549 | M+Na | 4.30 |
| 6-Benzylaminopurine | C12H11N5 | 226.1054 | 226.108722 | M+H | −1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Wu, Y.; Zhang, J.; Qi, Y.; Zhang, Y.; Pan, Q. Visualizing the Distribution of Jujube Metabolites at Different Maturity Stages Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Foods 2023, 12, 3795. https://doi.org/10.3390/foods12203795
Lu D, Wu Y, Zhang J, Qi Y, Zhang Y, Pan Q. Visualizing the Distribution of Jujube Metabolites at Different Maturity Stages Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Foods. 2023; 12(20):3795. https://doi.org/10.3390/foods12203795
Chicago/Turabian StyleLu, Dongye, Yang Wu, Junmin Zhang, Yuanyong Qi, Yuping Zhang, and Qinghua Pan. 2023. "Visualizing the Distribution of Jujube Metabolites at Different Maturity Stages Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging" Foods 12, no. 20: 3795. https://doi.org/10.3390/foods12203795
APA StyleLu, D., Wu, Y., Zhang, J., Qi, Y., Zhang, Y., & Pan, Q. (2023). Visualizing the Distribution of Jujube Metabolites at Different Maturity Stages Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Foods, 12(20), 3795. https://doi.org/10.3390/foods12203795






