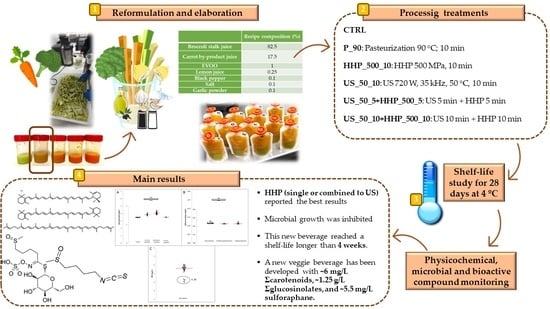
Effect of Ultrasound and High Hydrostatic Pressure Processing on Quality and Bioactive Compounds during the Shelf Life of a Broccoli and Carrot By-Products Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beverage Formulation and Preparation
2.2. Processing Treatments and Storage Conditions
- CTRL: Fresh blended beverage without any processing treatment. This treatment was used as a control.
- P_90: Pasteurization (90 °C; 10 min) in an agitated water bath to ensure the temperature distribution (J.P. Selecta, Barcelona, Spain). This temperature and time were chosen according to our previous experiments and previous works on similar beverages, since this kind of product with a pH >4.6 needs stronger thermal treatments to avoid microbial spoilage [26,27,28,29].
- US_50_5+HHP_500_5: US (720 W, 35 kHz, 50 °C, 5 min) combined with HHP (500 MPa, 5 min).
- US_50_10+HHP_500_10: US (720 W, 35 kHz, 50 °C, 10 min) combined with HHP (500 MPa, 10 min).
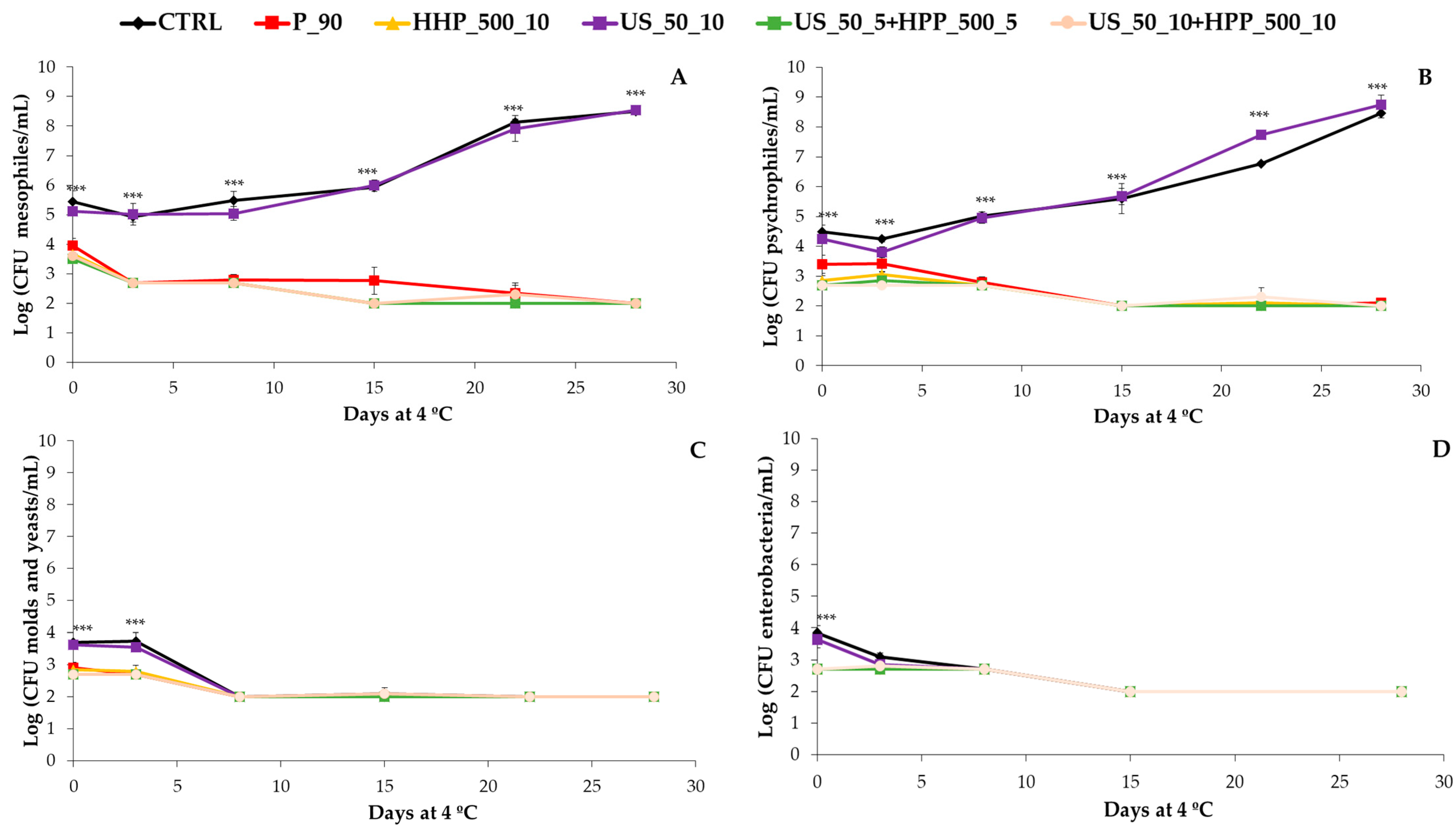
2.3. Microbial Analyses
2.4. Bioactive Compounds
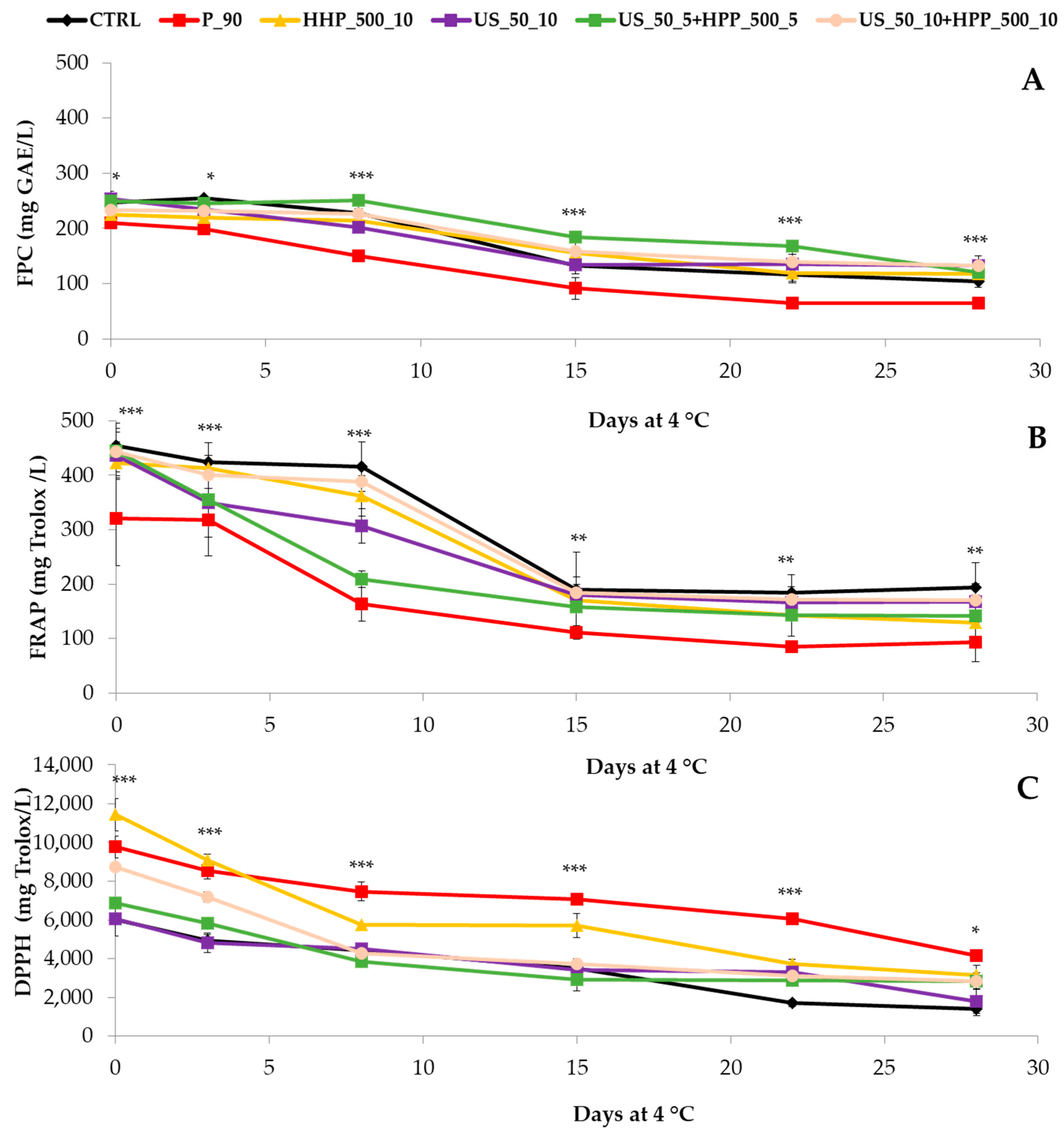
2.4.1. Free Polyphenol Content (FPC) and Antioxidant Capacity (AC)
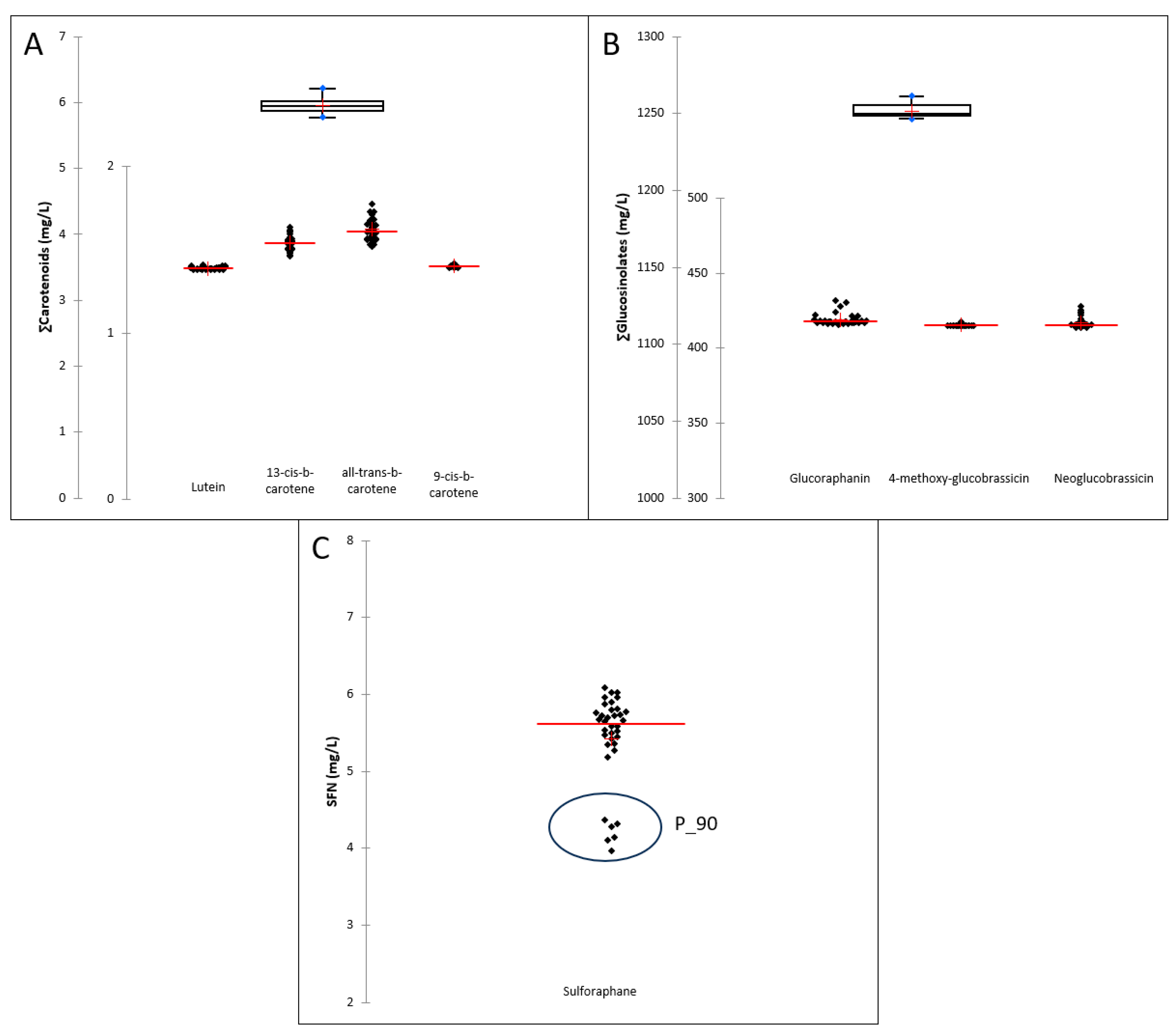
2.4.2. Carotenoid Content
2.4.3. Glucosinolate and Isothiocyanate Content
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
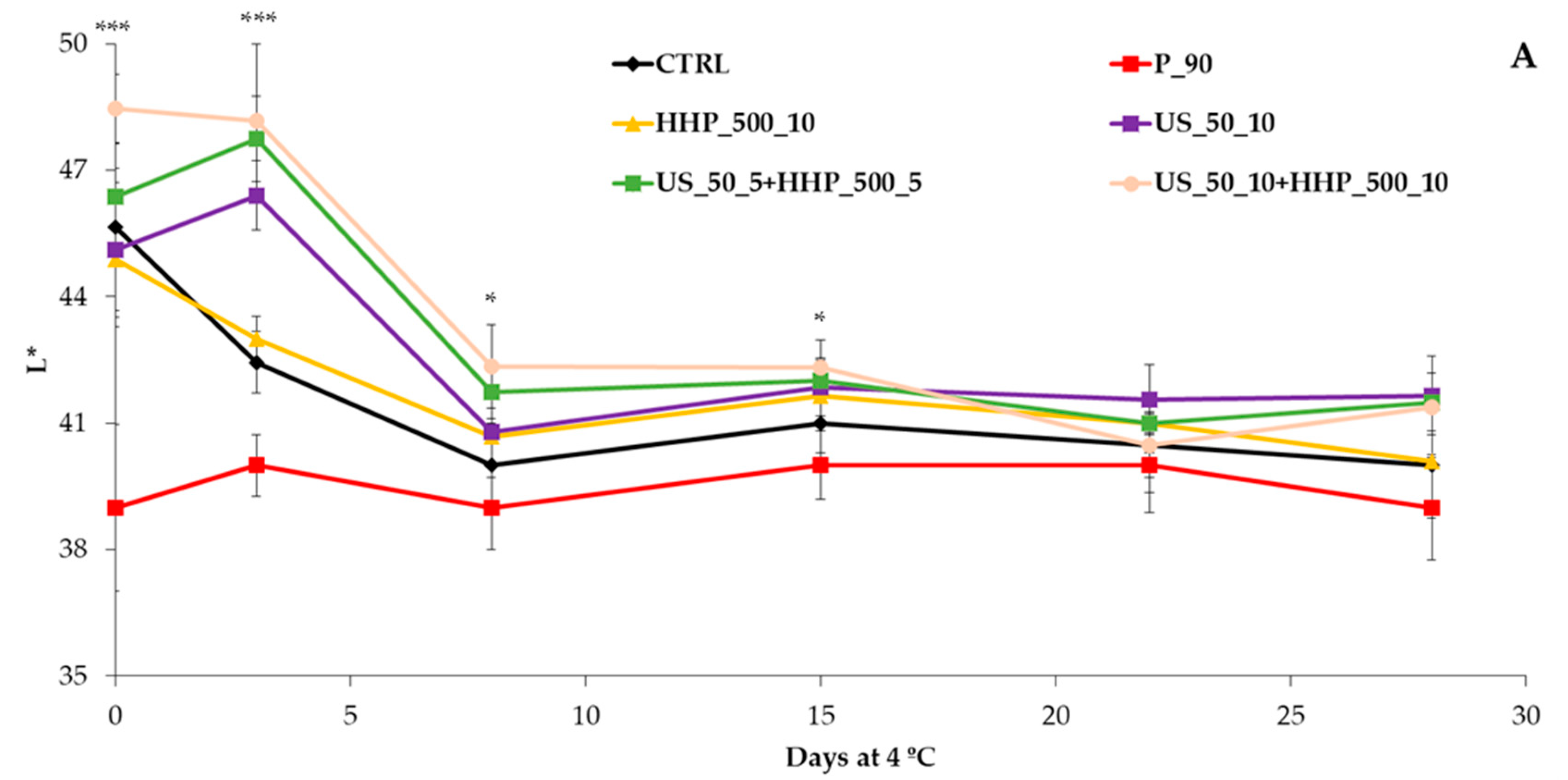
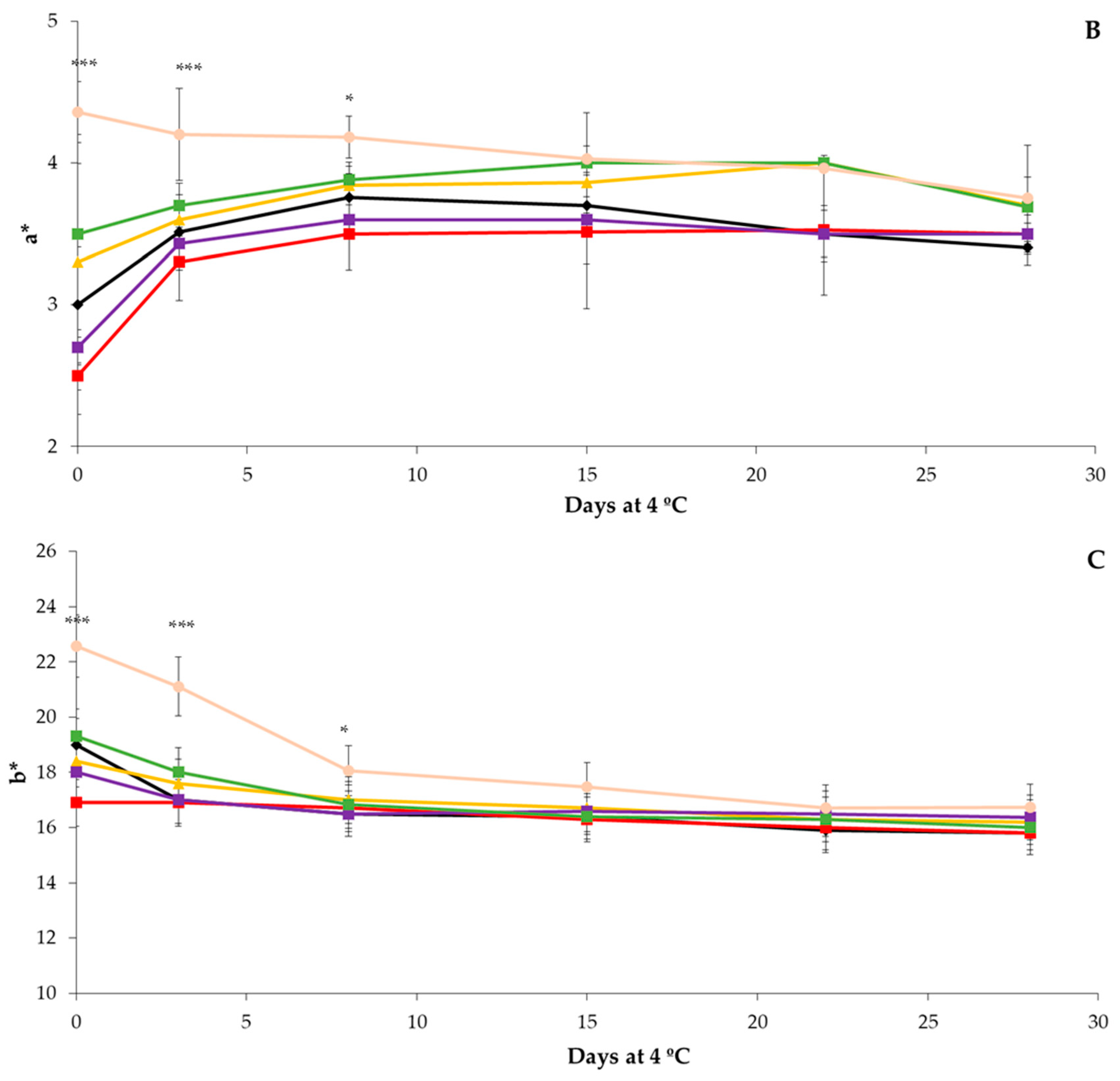
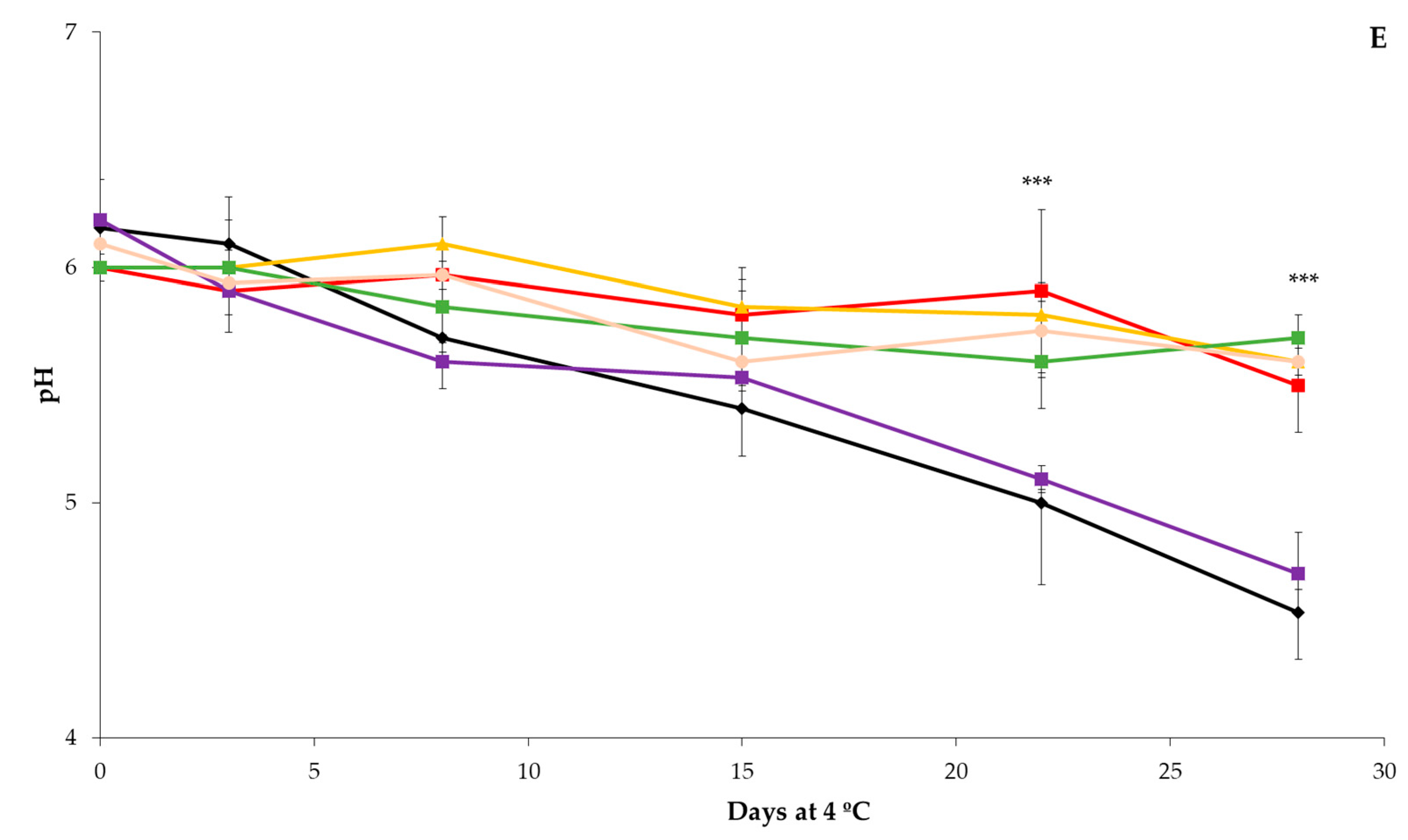
3.1. Physicochemical Analysis
3.2. Microbial Load
3.3. Free Polyphenolic Content (FPC) and Antioxidant Capacity (AC)
3.4. Carotenoids and Sulfur Compounds Content
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.A.; Aguayo, E. Continuous Microwave Pasteurization of a Vegetable Smoothie Improves Its Physical Quality and Hinders Detrimental Enzyme Activity. Food Sci. Technol. Int. 2016, 23, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Klug, T.V.; Martínez-Sánchez, A.; Gómez, P.A.; Collado, E.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Improving Quality of an Innovative Pea Puree by High Hydrostatic Pressure. J. Sci. Food Agric. 2017, 97, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Hamaker, B.R.; Lovejoy, J.C.; Eichelsdoerfer, P.E. Pulse Consumption, Satiety, and Weight Management. Adv. Nutr. 2010, 1, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, N.; Martínez-Hernández, G.B.; Artés-Hernández, F. Revalorized Broccoli By-Products and Mustard Improved Quality during Shelf Life of a Kale Pesto Sauce. Food Sci. Technol. Int. 2021, 27, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Artés-Hernández, F.; Martínez-Zamora, L.; Cano-Lamadrid, M.; Hashemi, S.; Castillejo, N. Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review. Foods 2023, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Campas-Baypoli, O.N.; Sánchez-Machado, D.I.; Bueno-Solano, C.; Núñez-Gastélum, J.A.; Reyes-Moreno, C.; López-Cervantes, J. Biochemical Composition and Physicochemical Properties of Broccoli Flours. Int. J. Food Sci. Nutr. 2009, 60, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli Leaf Powder as an Attractive By-Product Ingredient: Effect on Batter Behaviour, Technological Properties and Sensory Quality of Gluten-Free Mini Sponge Cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived By-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary Sulforaphane, a Histone Deacetylase Inhibitor for Cancer Prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-Targeted Prevention of Cancer by Sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of Many Colors Provide Basic Nutrition and Bioavailable Phytochemicals Acting as a Functional Food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef] [PubMed]
- Nieva, S.G.; Jagus, R.J.; Agüero, M.V.; Fernandez, M. V Fruit and Vegetable Smoothies Preservation with Natural Antimicrobials for the Assurance of Safety and Quality. LWT 2022, 154, 112663. [Google Scholar] [CrossRef]
- Salar, F.J.; Periago, P.M.; Agulló, V.; García-Viguera, C.; Fernández, P.S. High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage. Foods 2021, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.R.; Popović, V.; Bissonnette, S.; Ros, I.; Mats, L.; Duizer, L.; Warriner, K.; Koutchma, T. Quality Changes in Cold Pressed Juices after Processing by High Hydrostatic Pressure, Ultraviolet-c Light and Thermal Treatment at Commercial Regimes. Innov. Food Sci. Emerg. Technol. 2020, 64, 102398. [Google Scholar] [CrossRef]
- Gupta, R.; Balasubramaniam, V.M. Chapter 5—High-Pressure Processing of Fluid Foods. In Novel Thermal and Non-Thermal Technologies for Fluid Foods; Cullen, P.J., Tiwari, B.K., Valdramidis, V.P., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 109–133. ISBN 978-0-12-381470-8. [Google Scholar]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating Decreases Epithiospecifier Protein Activity and Increases Sulforaphane Formation in Broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Song, Y.; Bi, X.; Zhou, M.; Zhou, Z.; Chen, L.; Wang, X.; Ma, Y. Effect of Combined Treatments of Ultrasound and High Hydrostatic Pressure Processing on the Physicochemical Properties, Microbial Quality and Shelf-Life of Cold Brew Tea. Int. J. Food Sci. Technol. 2021, 56, 5977–5988. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Kehoe, K.; Reilly, K.; Rai, D.K.; Barry-Ryan, C. Development of a Novel Functional Soup Rich in Bioactive Sulforaphane Using Broccoli (Brassica Oleracea L. Ssp. Italica) Florets and Byproducts. Food Bioprocess Technol. 2014, 7, 1310–1321. [Google Scholar] [CrossRef]
- Klug, T.V.; Martínez-Hernández, G.B.; Collado, E.; Artés, F.; Artés-Hernández, F. Effect of Microwave and High-Pressure Processing on Quality of an Innovative Broccoli Hummus. Food Bioprocess Technol. 2018, 11, 1464–1477. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. Red Fresh Vegetables Smoothies with Extended Shelf Life as an Innovative Source of Health-Promoting Compounds. J. Food Sci. Technol. 2016, 53, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of Temperature-Controlled Ultrasound Treatment on Sensory Properties, Physical Characteristics and Antioxidant Activity of Cloudy Apple Juice. LWT 2021, 142, 111030. [Google Scholar] [CrossRef]
- Dereli, U.; Türkyilmaz, M.; Yemiş, O.; Özkan, M. Effects of Clarification and Pasteurization on the Phenolics, Antioxidant Capacity, Color Density and Polymeric Color of Black Carrot (Daucus carota L.) Juice. J. Food Biochem. 2015, 39, 528–537. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Sattar, S.; Imran, M.; Mushtaq, Z.; Ahmad, M.H.; Arshad, M.S.; Holmes, M.; Maycock, J.; Nisar, M.F.; Khan, M.K. Retention and Stability of Bioactive Compounds in Functional Peach Beverage Using Pasteurization, Microwave and Ultrasound Technologies. Food Sci. Biotechnol. 2020, 29, 1381–1388. [Google Scholar] [CrossRef]
- Mandelová, L.; Totušek, J. Broccoli Juice Treated by High Pressure: Chemoprotective Effects of Sulforaphane and Indole-3-Carbinol. High. Press. Res. 2007, 27, 151–156. [Google Scholar] [CrossRef]
- Gomes, W.F.; Tiwari, B.K.; Rodriguez, Ó.; de Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Effect of Ultrasound Followed by High Pressure Processing on Prebiotic Cranberry Juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef]
- Ferrario, M.; Alzamora, S.M.; Guerrero, S. Study of the Inactivation of Spoilage Microorganisms in Apple Juice by Pulsed Light and Ultrasound. Food Microbiol. 2015, 46, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Oxidants and Antioxidants Part A; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A Rapid and Sensitive Method for Determination of Carotenoids in Plant Tissues by High Performance Liquid Chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts. Antioxidants 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kiddle, G.; Bennett, R.N.; Botting, N.P.; Davidson, N.E.; Robertson, A.A.B.; Wallsgrove, R.M. High-Performance Liquid Chromatographic Separation of Natural and Synthetic Desulphoglucosinolates and Their Chemical Vadation by UV, NMR and Chemical Ionisation-MS Methods. Phytochem. Anal. 2001, 12, 226–242. [Google Scholar] [CrossRef] [PubMed]
- ISO-8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standarization: Geneva, Switzerland, 2012.
- Cano-Lamadrid, M.; Tkacz, K.; Turkiewicz, I.P.; Clemente-Villalba, J.; Sánchez-Rodríguez, L.; Lipan, L.; García-García, E.; Carbonell-Barrachina, Á.A.; Wojdyło, A. How a Spanish Group of Millennial Generation Perceives the Commercial Novel Smoothies? Foods 2020, 9, 1213. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Cattaneo, C.; Pagliarini, E.; Artés-Hernández, F. How Does the Phytochemical Composition of Sprouts and Microgreens from Brassica Vegetables Affect the Sensory Profile and Consumer Acceptability? Postharvest Biol. Technol. 2023, 203, 112411. [Google Scholar] [CrossRef]
- Issa-Issa, H.; Cano-Lamadrid, M.; Calín-Sánchez, Á.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Volatile Composition and Sensory Attributes of Smoothies Based on Pomegranate Juice and Mediterranean Fruit Purées (Fig, Jujube and Quince). Foods 2020, 9, 926. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour Difference ΔE—A Survey. MGV 2011, 20, 383–411. [Google Scholar]
- Izadifar, Z.; Babyn, P.; Chapman, D. Ultrasound Cavitation/Microbubble Detection and Medical Applications. J. Med. Biol. Eng. 2019, 39, 259–276. [Google Scholar] [CrossRef]
- Ahmad, K.; Imran, M.; Ahmad, T.; Ahmad, M.H.; Khan, M.K. Impact of Ultrasound Processing on Physicochemical and Bioactive Attributes of Grape Based Optimized Fruit Beverage. Pak. J. Agric. Sci. 2020, 57, 1117–1124. [Google Scholar]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological Spoilage of Fruits and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 135–183. [Google Scholar]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic Acid Is the Only Bioactive That Is Better Preserved by High Hydrostatic Pressure than by Thermal Treatment of a Vegetable Beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Aggarwal, P. Effect of Processing and Storage on Bioactive Compounds and Antioxidant Activity of Carrot Juice. J. Appl. Hortic. 2014, 16, 80–84. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Abou-Arab, A.A.; Abu Salem, F.M. Antioxidant and Antimicrobial Effects of Some Natural Plant Extracts Added to Lamb Patties during Storage. Grasas Y Aceites 2011, 62, 139–148. [Google Scholar] [CrossRef]
- de Albuquerque, J.G.; Escalona-Buendía, H.B.; de Magalhães Cordeiro, A.M.T.; dos Santos Lima, M.; de Souza Aquino, J.; da Silva Vasconcelos, M.A. Ultrasound Treatment for Improving the Bioactive Compounds and Quality Properties of a Brazilian Nopal (Opuntia Ficus-Indica) Beverage during Shelf-Life. LWT 2021, 149, 111814. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (ATEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, Y.J.; Duan, S.; Park, T.J.; Eom, S.H. Accumulation of Antioxidative Phenolics and Carotenoids Using Thermal Processing in Different Stages of Momordica Charantia Fruit. Molecules 2023, 28, 1500. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O.; Cano, M.P. Impact of High Pressure and Pulsed Electric Fields on Bioactive Compounds and Antioxidant Activity of Orange Juice in Comparison with Traditional Thermal Processing. J. Agric. Food Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Nutritional Characterisation of Commercial Traditional Pasteurised Tomato Juices: Carotenoids, Vitamin C and Radical-Scavenging Capacity. Food Chem. 2006, 98, 749–756. [Google Scholar] [CrossRef]
- Dallagi, W.; Rguez, S.; Hammami, M.; Bettaieb Rebey, I.; Bourgou, S.; Hamrouni Sellami, I. Optimization of Processing Conditions to Enhance Antioxidant and Carotenoid Contents of Carrot Juice. J. Food Meas. Charact. 2023, 17, 4384–4393. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to: Flavonoids and Ascorbic Acid in Fruit Juices, Including Berry Juices; Flavonoids from Citrus; Flavonoids from Citrus Paradisi Macfad.; Flavonoids; Flavonoids in Cranerry Juice; Carotenoids; Polyphenols; among Others Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2082. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of Thermal and Non-Thermal Processing of Cruciferous Vegetables on Glucosinolates and Its Derived Forms. J. Food Sci. Technol. 2018, 55, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.N.; Wolf, W.; Tevini, D.; Bognár, A. Microwave Blanching of Vegetables. J. Food Sci. 2002, 67, 390–398. [Google Scholar] [CrossRef]
- Parchem, K.; Piekarska, A.; Bartoszek, A. Enzymatic Activities behind Degradation of Glucosinolates. Glucosinolates Prop. Recovery Appl. 2020, 2020, 79–106. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Various Foods/Food Constituents and “Immune Function/Immune System”, “Contribution to Body Defences against External Agents”, Stimulation of Immunological Responses, Reduction of Inflammation, among Others Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2061. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Various Food(s)/Food Constituent(s) and Protection of Cells from Premature Aging, Antioxidant Activity, Antioxidant Content and Antioxidant Properties, and Protection of DNA, Proteins and Lipids from Oxidative Damage Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancing the Recovery of Cabbage Glucoraphanin through the Monitoring of Sulforaphane Content and Myrosinase Activity during Extraction by Different Methods. Sep. Purif. Technol. 2017, 174, 338–344. [Google Scholar] [CrossRef]
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced Production of Sulforaphane by Exogenous Glucoraphanin Hydrolysis Catalyzed by Myrosinase Extracted from Chinese Flowering Cabbage (Brassica Rapa Var. Parachinensis). Sci. Rep. 2019, 9, 9882. [Google Scholar] [CrossRef]
- Mahn, A.; Quintero, J.; Castillo, N.; Comett, R. Effect of Ultrasound-Assisted Blanching on Myrosinase Activity and Sulforaphane Content in Broccoli Florets. Catalysts 2020, 10, 616. [Google Scholar] [CrossRef]

| Recipe Composition (%) | |
|---|---|
| Broccoli stalk juice | 82.5 |
| Carrot by-product juice | 17.5 |
| EVOO | 1 |
| Lemon juice | 0.25 |
| Black pepper | 0.1 |
| Salt | 0.1 |
| Garlic powder | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, P.; Hashemi, S.; Cano-Lamadrid, M.; Martínez-Zamora, L.; Gómez, P.A.; Artés-Hernández, F. Effect of Ultrasound and High Hydrostatic Pressure Processing on Quality and Bioactive Compounds during the Shelf Life of a Broccoli and Carrot By-Products Beverage. Foods 2023, 12, 3808. https://doi.org/10.3390/foods12203808
Pérez P, Hashemi S, Cano-Lamadrid M, Martínez-Zamora L, Gómez PA, Artés-Hernández F. Effect of Ultrasound and High Hydrostatic Pressure Processing on Quality and Bioactive Compounds during the Shelf Life of a Broccoli and Carrot By-Products Beverage. Foods. 2023; 12(20):3808. https://doi.org/10.3390/foods12203808
Chicago/Turabian StylePérez, Pablo, Seyedehzeinab Hashemi, Marina Cano-Lamadrid, Lorena Martínez-Zamora, Perla A. Gómez, and Francisco Artés-Hernández. 2023. "Effect of Ultrasound and High Hydrostatic Pressure Processing on Quality and Bioactive Compounds during the Shelf Life of a Broccoli and Carrot By-Products Beverage" Foods 12, no. 20: 3808. https://doi.org/10.3390/foods12203808