Impact of Modified Atmosphere Packaging Conditions on Quality of Dates: Experimental Study and Predictive Analysis Using Artificial Neural Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Samples Preparation
2.2. Dates Quality Measurements
2.2.1. Moisture Content
2.2.2. pH
2.2.3. Water Activity
2.2.4. Total Soluble Solids
2.2.5. Color Parameters
2.2.6. Microbiological Load
2.3. Artificial Neural Networks
- Dataset preparation: The dataset preparation involved two separate stages. In the initial stage of the experiment, experimental data collection was carried out to train, test, and evaluate the ANN models. During this stage, the dataset was divided to 60% for model training, 20% for testing the model’s performance, and an additional 20% for evaluation. In the second stage, new data, entirely separate from the dataset employed in model development, was utilized for the purpose of model validation. This separation was performed to ensure that the ANN models were validated on unseen data, preventing any potential bias or overfitting that might occur if the same data were used for both training and validation.
- Training the ANN model: in this phase, 60% of the dataset was used to train the ANN model. This involved providing the ANN with input data, which consisted of various storage parameters of storage time, storage temperature, packing material, N, O2, and CO2. The model learned to map these input variables to the target output variables of stored date fruit quality attributes, i.e., moisture content, water activity, total soluble solids, pH, color parameters, and microbial counts. The ANN adjusted its internal weights and biases during this phase to minimize the sum of square errors between the predicted values and the actual values in the training dataset.
- Testing the ANN: In this phase, 20% of the dataset that was not used during training was set aside for testing the model’s performance. The ANN was used to predict the quality attributes for this testing dataset based on the same conditions. The error between the predicted values and the actual values for this dataset was calculated to assess how well the model generalized to unseen data.
- Evaluation of the ANN models: In this phase, the remaining 20% of the dataset was employed for the evaluation phase, which served as an independent validation dataset. Again, the ANN was used to predict the quality attributes for this dataset. The error metrics of RMSE and MAPE were calculated for the evaluation of the unseen dataset. This phase was conducted to evaluate the performance of the trained ANN model to assess how well the developed model predicts future outcomes.
- Validation: The validation of the implemented ANN prediction model was conducted after evaluating the developed models on the separate data. By providing the new input data to the trained networks, the model generated predictions based on the patterns it learned during the training phase. The linear regression analysis was performed to compare the predicted values with the observed values for the separated data of various quality parameters to validate the models.
2.4. Statistical Analysis and ANN Evaluation
3. Results and Discussion
3.1. Changes in Gas Concentrations
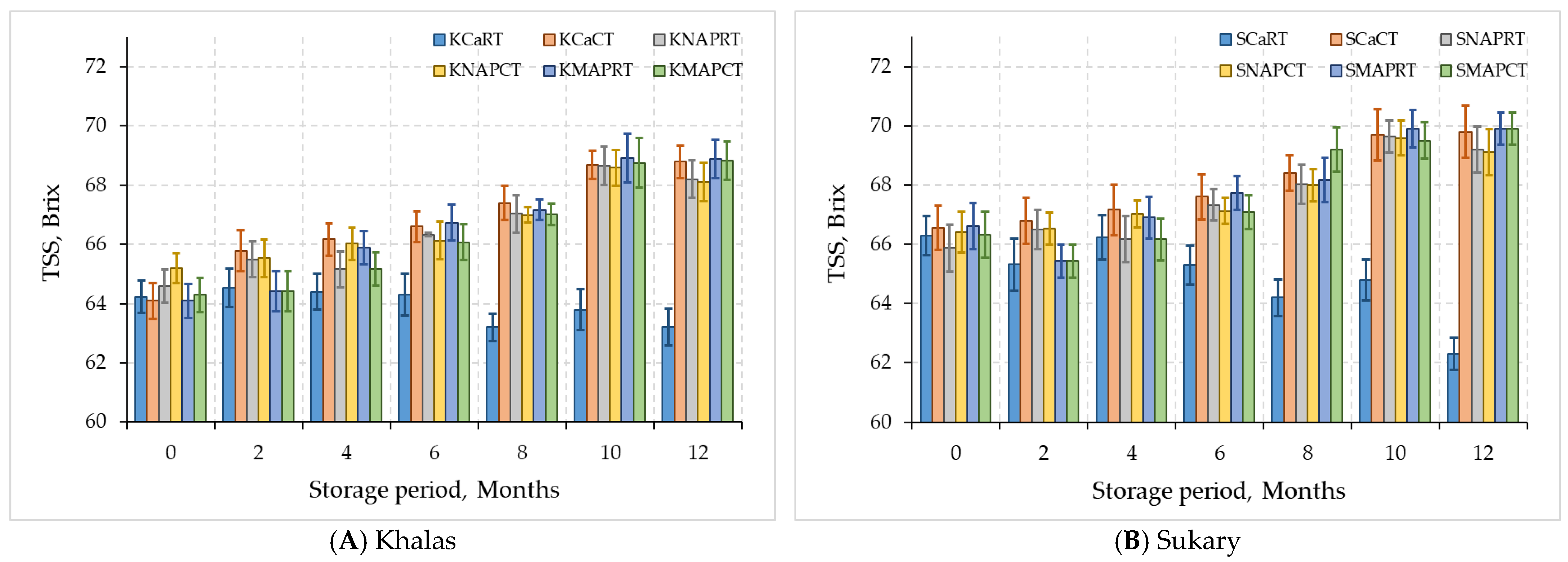
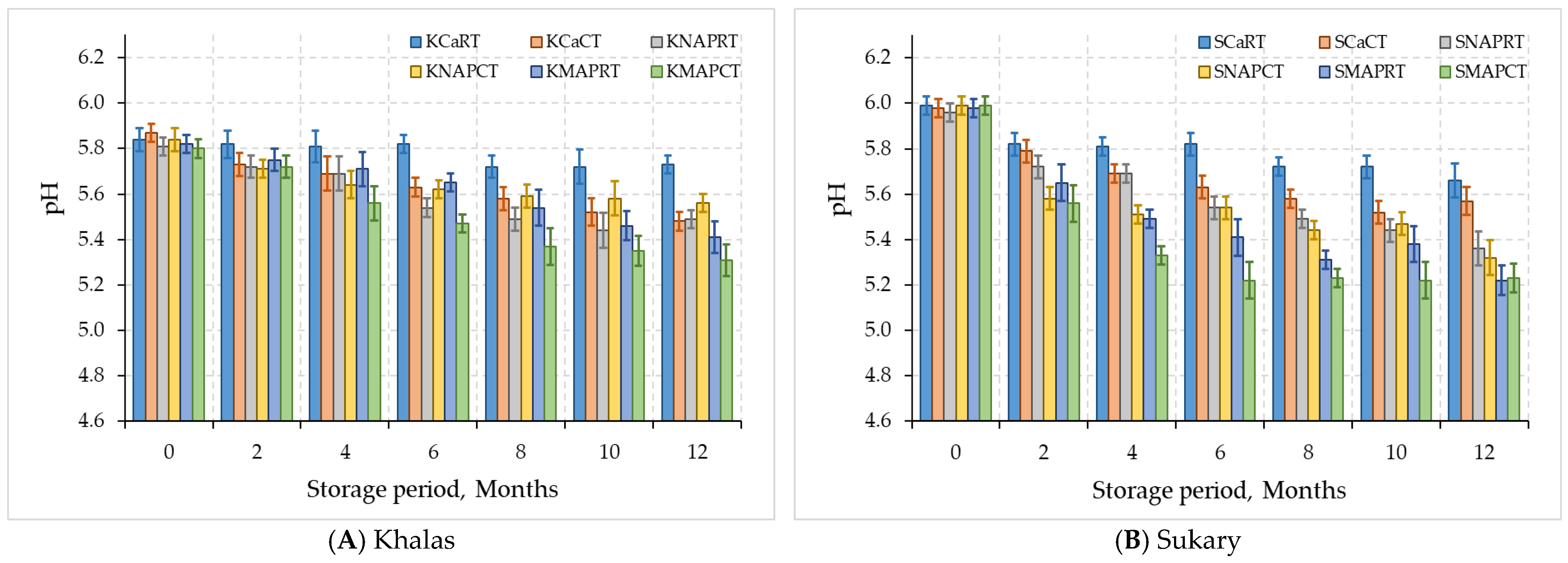
3.2. Quality of Date Fruits Data
3.2.1. Chemical Properties
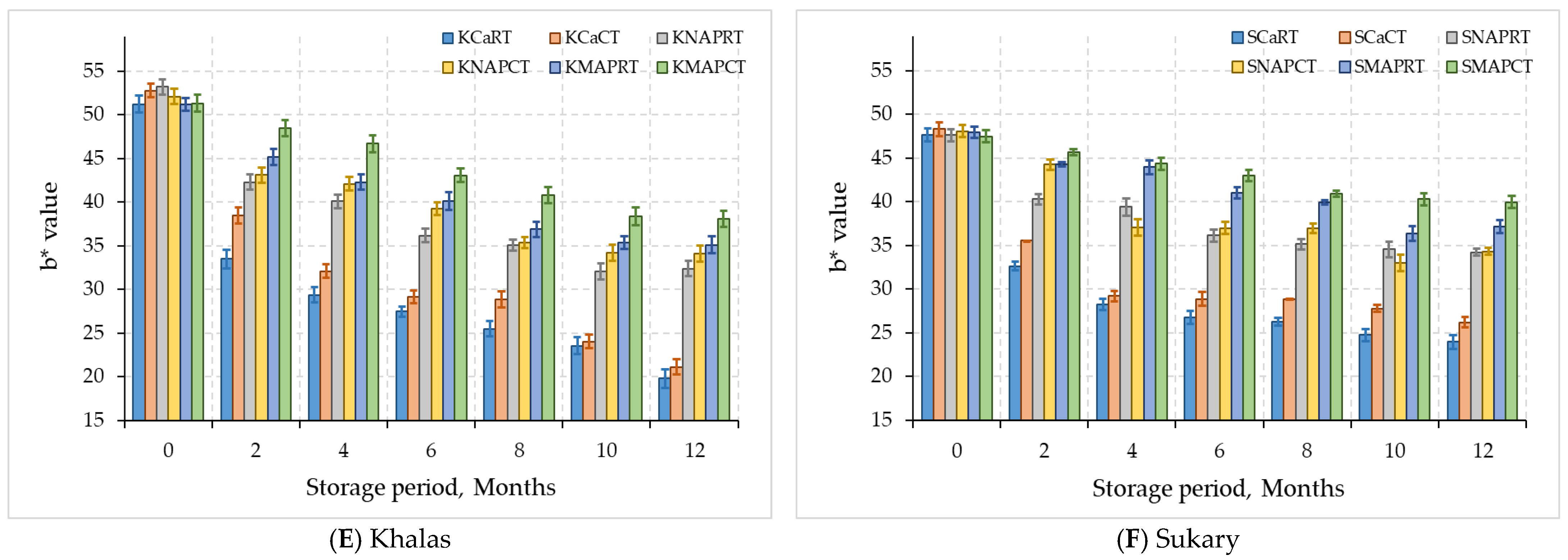
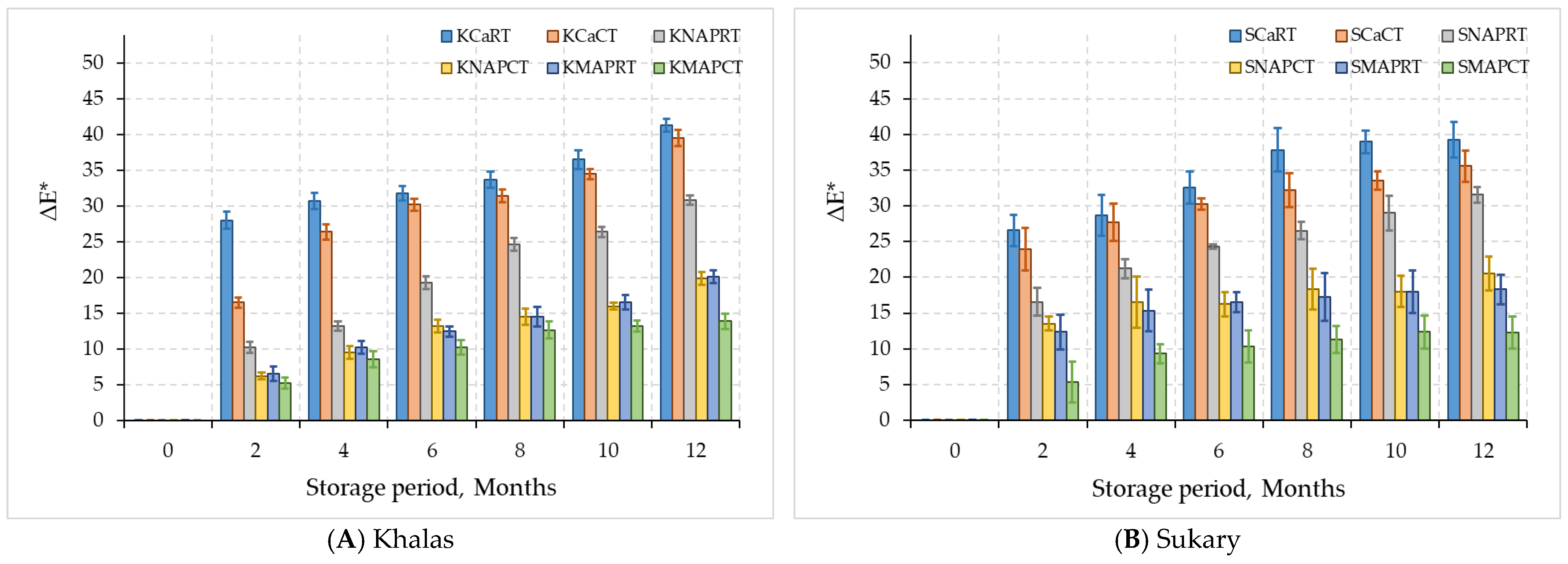
3.2.2. Color Parameters
3.2.3. Microbial Load
3.3. Correlation between the Characteristics and Storage Parameters
3.4. Predictive Analysis Using ANN
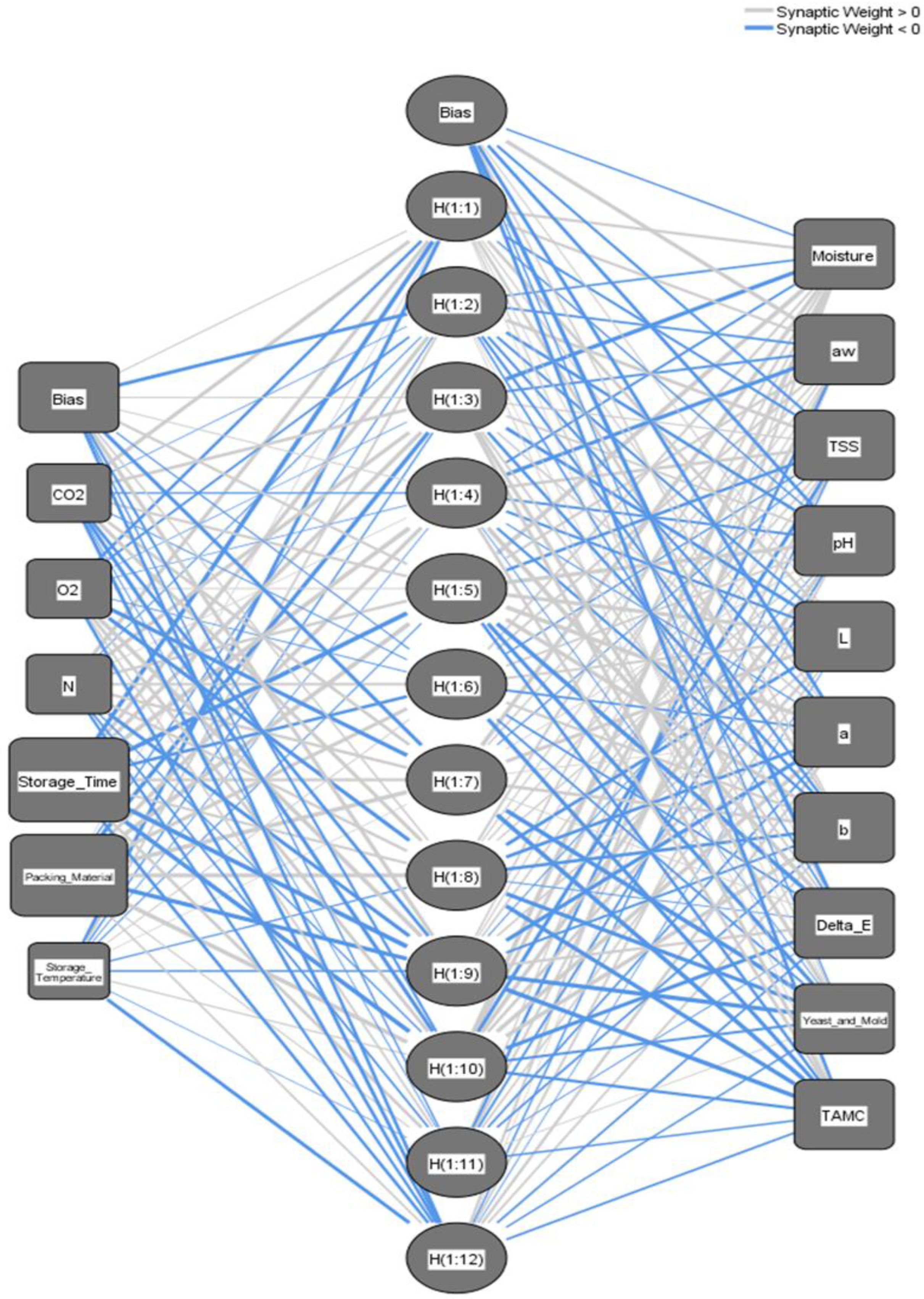
3.4.1. Architecture of the ANN Prediction Models
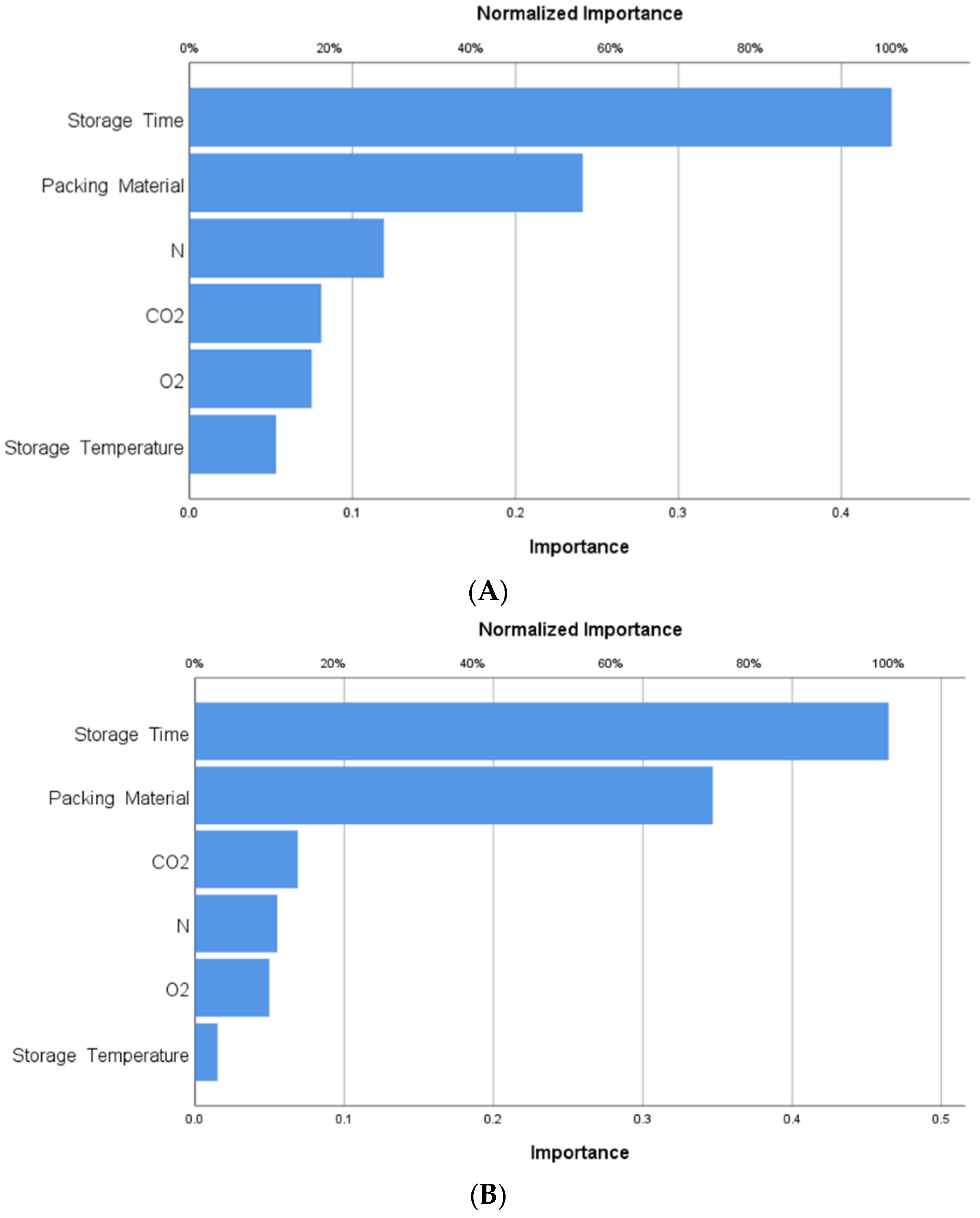
3.4.2. Importance of the Independent Variable
3.4.3. Evaluation of the ANN Prediction Models
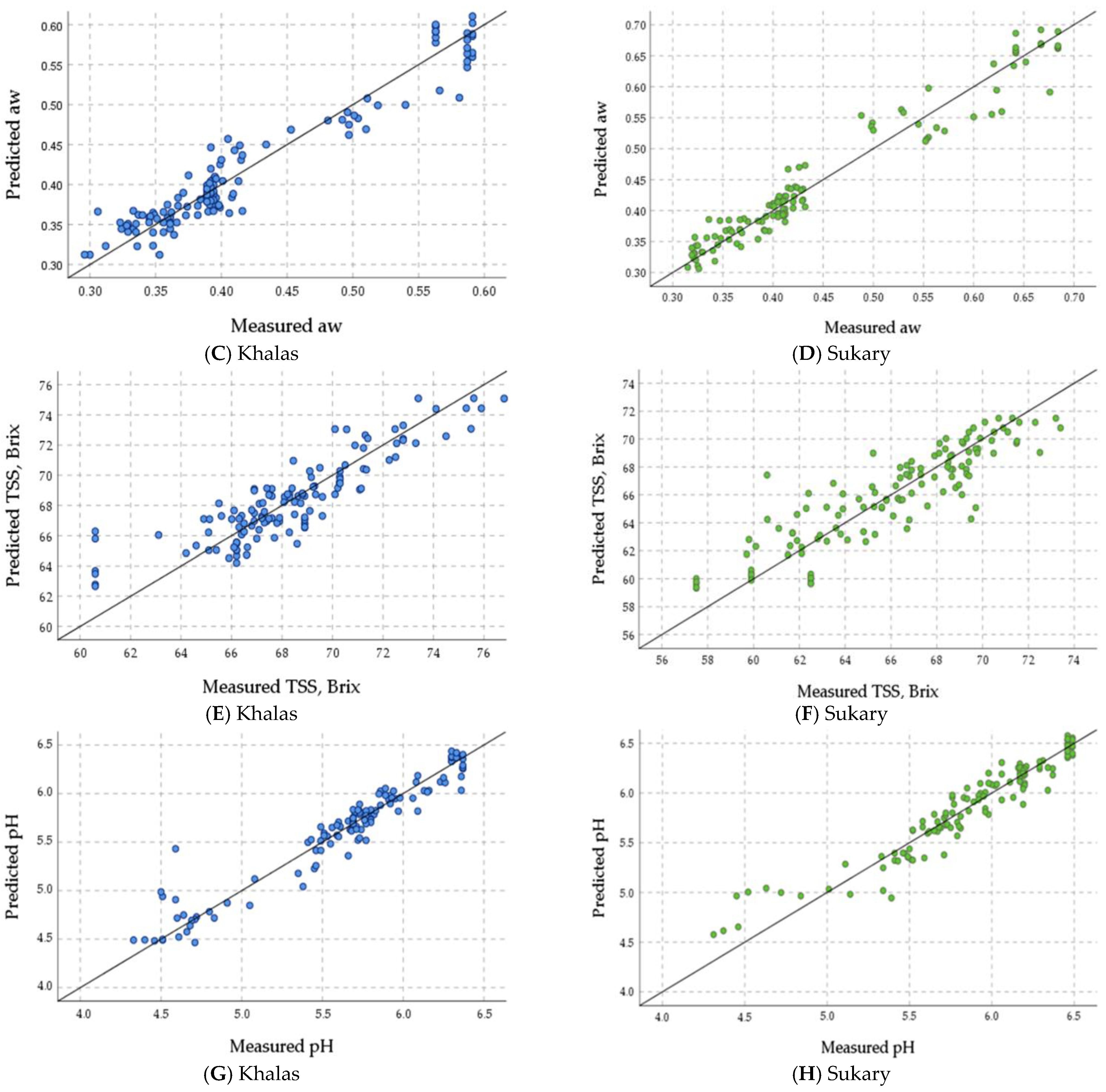
3.4.4. Validation of the ANN Prediction Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Faostat-FAO Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 September 2023).
- Almutawa, A.A. Date Production in the Al-Hassa Region, Saudi Arabia in the Face of Climate Change. J. Water Clim. Chang. 2022, 13, 2627–2647. [Google Scholar] [CrossRef]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M.J. Date Fruit Processing Waste and Approaches to Its Valorization: A Review. Bioresour. Technol. 2021, 340, 125625. [Google Scholar] [CrossRef]
- Eid, N.M.S.; Al-Awadi, B.; Vauzour, D.; Oruna-Concha, M.J.; Spencer, J.P.E. Effect of Cultivar Type and Ripening on the Polyphenol Content of Date Palm Fruit. J. Agric. Food Chem. 2013, 61, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Bouhlali, E.T.; Ramchoun, M.; Alem, C.; Ghafoor, K.; Ennassir, J.; Zegzouti, Y.F. Functional Composition and Antioxidant Activities of Eight Moroccan Date Fruit Varieties (Phoenix dactylifera L.). J. Saudi Soc. Agric. Sci. 2017, 16, 257–264. [Google Scholar] [CrossRef]
- Al-Mssallem, M.Q.; Alqurashi, R.M.; Al-Khayri, J.M. Bioactive Compounds of Date Palm (Phoenix dactylifera L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Reference Series in Phytochemistry; Murthy, H.N., Bapat, V.A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 91–105. [Google Scholar]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The Impact of Date Palm Fruits and Their Component Polyphenols, on Gut Microbial Ecology, Bacterial Metabolites and Colon Cancer Cell Proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef]
- Alsarayrah, N.A.; Omar, E.A.; Alsanad, S.M.; Arsad, H.; Abudahash, M.M.; Alenazi, F.K.; Alenzi, N.D. The Health Values of Phoenix dactylifera (Dates): A Review. Emirates J. Food Agric. 2023, 35, 1–16. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Farag, M.A.; Gong, J.; Su, Q.; Cao, H.; Zhang, W.; Zhao, Y.; Wang, H. Dietary Flavonoids and Gut Microbiota Interaction: A Focus on Animal and Human Studies to Maximize Their Health Benefits. Food Front. 2023, 1–16. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Liu, Z.; Danzengquzhen; Cisangzhuoma; Ma, J.; Li, X.; Huang, X.; Li, B. The Neuromodulatory Effects of Flavonoids and Gut Microbiota through the Gut-Brain Axis. Front. Cell. Infect. Microbiol. 2023, 13, 1197646. [Google Scholar] [CrossRef]
- Aleid, S.M.; Al Saikhan, M.S. Quality, Texture and Colour Properties of Fresh “Ghur” and “Khenaizy” Dates Cold Stored with Modified Atmosphere Packaging. Fresenius Environ. Bull. 2019, 28, 8882–8888. [Google Scholar]
- Lotfy, K.; Al-Qahtani, S.; Al-Harbi, N.; El-Absy, K.; Shulaybi, F.B.; Alali, S.; Mashtoly, T. Decontamination Potential of Date Palm Fruit via Non-Thermal Plasma Technique. Sci. Rep. 2022, 12, 17323. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.W.; Nightingale, K.K. Use of Omics Methods for the Advancement of Food Quality and Food Safety. Anim. Front. 2018, 8, 33–41. [Google Scholar] [CrossRef]
- Rešetar, D.; Pavelić, S.K.; Josić, D. Foodomics for Investigations of Food Toxins. Curr. Opin. Food Sci. 2015, 4, 86–91. [Google Scholar] [CrossRef]
- Sugrue, I.C.; Tobin, R.P.; Ross, C.; Stanton, C.; Hill, C. Raw Milk; Academic Press, Elsevier: Cambridge, MA, USA, 2019; ISBN 9780128105306. [Google Scholar]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite Changes during Postharvest Storage: Effects on Fruit Quality Traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous Modified Atmosphere Packaging to Extend Shelf Life of Fresh Foods: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, R.; Wang, B.; Chen, K. Development and Characterization of Bilayer Films Based on Pea Starch/Polylactic Acid and Use in the Cherry Tomatoes Packaging. Carbohydr. Polym. 2019, 222, 114912. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Sattar Khan, A.; Ullah Malik, A.; Anjum, M.A.; Nawaz, A.; Shoaib Shah, H.M. Modified Atmosphere Packaging Delays Enzymatic Browning and Maintains Quality of Harvested Litchi Fruit during Low Temperature Storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, Plant- and Animal-Derived Compounds as Alternatives to Conventional Fungicides for the Control of Postharvest Diseases of Fresh Horticultural Produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Keshtiban, A.K.; Amini, A.; Eslami, A. Date Canning: A New Approach for the Long Time Preservation of Date. J. Food Sci. Technol. 2015, 52, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of Modified Atmosphere Packaging as a Safety Approach to Fresh-Cut Fruits and Vegetables—A Review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of Biocontrol Agents with Physical Methods against Postharvest Diseases of Fruits and Vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Huang, Y.; Kangas, L.J.; Rasco, B.A. Applications of Artificial Neural Networks (ANNs) in Food Science. Crit. Rev. Food Sci. Nutr. 2007, 47, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Hamdoun, H.; Sagheer, A. Toward Sustainable Farming: Implementing Artificial Intelligence to Predict Optimum Water and Energy Requirements for Sensor-Based Micro Irrigation Systems Powered by Solar PV. Agronomy 2023, 13, 1081. [Google Scholar] [CrossRef]
- Basheer, I.A.; Hajmeer, M. Artificial Neural Networks: Fundamentals, Computing, Design, and Application. J. Microbiol. Methods 2000, 43, 3–31. [Google Scholar] [CrossRef]
- Mohammed, M.; Munir, M.; Aljabr, A. Prediction of Date Fruit Quality Attributes during Cold Storage Based on Their Electrical Properties Using Artificial Neural Networks Models. Foods 2022, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gomariz, M.; López-Gómez, A.; Cerdán-Cartagena, F. Artificial Neural Networks as Artificial Intelligence Technique for Energy Saving in Refrigeration Systems—A Review. Clean Technol. 2023, 5, 116–136. [Google Scholar] [CrossRef]
- Kujawa, S.; Niedbała, G. Artificial Neural Networks in Agriculture. Agriculture 2021, 11, 497. [Google Scholar] [CrossRef]
- Bhotmange, M.; Shastri, P. Application of Artificial Neural Networks to Food and Fermentation Technology. In Artificial Neural Networks—Industrial and Control Engineering Applications; Suzuki, K., Ed.; IntechOpen: London, UK, 2012; pp. 201–222. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Hunter, R.S.; Harold, R.W. The Measurement of Appearance; John Wiley and Sons: Hoboken, NJ, USA, 1987; Volume 29. [Google Scholar]
- Al-Hooti, S.; Sidhu, J.S.; Qabazard, H. Objective Color Measurement of Fresh Date Fruits and Processed Date Products. J. Food Qual. 1997, 20, 257–266. [Google Scholar] [CrossRef]
- Atlas, R.M.; Atlas, R.M. Handbook of Microbiological Media, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780429129032. [Google Scholar]
- González, J.; Ferrer, A.; Oria, R.; Salvador, M.L. Determination of O2 and CO2 Transmission Rates through Microperforated Films for Modified Atmosphere Packaging of Fresh Fruits and Vegetables. J. Food Eng. 2008, 86, 194–201. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Iqbal, Y.; Khattak, T.N. Effect of Various Irradiation Doses on Some Nutrients of Pakistani Date. J. Radioanal. Nucl. Chem. 2005, 266, 361–366. [Google Scholar] [CrossRef]
- Khan, R.U.; Rashid, A.; Khan, M.S. Evaluation of Date Palm Cultivars with Their Monetary Returns Under Ecological Zone of Dera Ismail Khan. J. Agric. Res. 2008, 46, 93–98. [Google Scholar]
- Zare, Z.; Sohrabpour, M.; Fazeli, T.Z.; Kohan, K.G. Evaluation of Invertase (B-fructo furanosidase) Activity in Irradiated Mazafaty Dates during Storage. Radiat. Phys. Chem. 2002, 65, 289–291. [Google Scholar] [CrossRef]
- Baloch, M.K.; Saleem, S.A.; Baloch, A.K.; Baloch, W.A. Impact of Controlled Atmosphere on the Stability of Dhakki Dates. LWT 2006, 39, 671–676. [Google Scholar] [CrossRef]
- Mohammed, M.; Alqahtani, N.; El-Shafie, H. Development and Evaluation of an Ultrasonic Humidifier to Control Humidity in a Cold Storage Room for Postharvest Quality Management of Dates. Foods 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Riad, K.; Alqahtani, N. Design of a Smart IoT-Based Control System for Remotely Managing Cold Storage Facilities. Sensors 2022, 22, 4680. [Google Scholar] [CrossRef] [PubMed]
- Al-Eid, S.M.; Barber, A.R.; Rettke, M.; Leo, A.; Alsenaien, W.A.; Sallam, A.A.; Aleid, S.M.; Barber, A.R.; Rettke, M.; Leo, A.; et al. Utilisation of Modified Atmosphere Packaging to Extend the Shelf Life of Khalas Fresh Dates. Int. J. Food Sci. Technol. 2012, 47, 1518–1525. [Google Scholar] [CrossRef]
- Al-Redhaiman, K.N. Modified Atmosphere Improves Storage Ability, Controls Decay, and Maintains Quality and Antioxidant Contents of Barhi Date Fruits. Food Agric. Environ. 2004, 2, 25–32. [Google Scholar]
- Rastegar, S.; Rahemi, M.; Baghizadeh, A.; Gholami, M. Enzyme Activity and Biochemical Changes of Three Date Palm Cultivars with Different Softening Pattern during Ripening. Food Chem. 2012, 134, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.K.; Srinivasan, P.; Choi, J.; Kim, J.H.; Han, S.B.; Kim, D.J.; Byun, M.W. Effect of Gamma Irradiation on Microbial Analysis, Antioxidant Activity, Sugar Content and Color of Ready-to-Use Tamarind Juice during Storage. LWT 2009, 42, 101–105. [Google Scholar] [CrossRef]
- Azelmat, K.; Sayah, F.; Mouhib, M.; Ghailani, N.; ElGarrouj, D. Effects of Gamma Irradiation on Fourth-Instar Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2005, 41, 423–431. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; El-Masry, A.M.; Fikry, M.; El-Kholy, M.F.; Shaban, A.E.; Sami, R.; Algarni, E.; Alshehry, G.; Aljumayi, H.; Benajiba, N.; et al. Utilization of Active Edible Films (Chitosan, Chitosan Nanoparticle, and CaCl2) for Enhancing the Quality Properties and the Shelf Life of Date Palm Fruits (Barhi Cultivar) during Cold Storage. Coatings 2022, 12, 255. [Google Scholar] [CrossRef]
- Akhavan, H.R.; Hosseini, F.S.; Amiri, S.; Radi, M. Cinnamaldehyde-Loaded Nanostructured Lipid Carriers Extend the Shelf Life of Date Palm Fruit. Food Bioprocess Technol. 2021, 14, 1478–1489. [Google Scholar] [CrossRef]
- Kader, A.A.; Hussein, A.M.; Adel, A.K.; Awad, M.H. Harvesting and Postharvest Handling of Dates; ICARDA: Aleppo, Syria, 2009; Volume 4, p. 15. [Google Scholar]
- Kader, A.A. Modified Atmospheres during Transport and Storage. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Oakland, CA, USA, 2002; pp. 135–148. [Google Scholar]
- Hazbavi, E.; Khoshtaghaza, M.H.; Mostaan, A.; Banakar, A. Effect of Storage Duration on Some Physical Properties of Date Palm (Cv. Stamaran). J. Saudi Soc. Agric. Sci. 2015, 14, 140–146. [Google Scholar] [CrossRef]
- Radi, M.; Firouzi, E.; Akhavan, H.; Amiri, S. Effect of Gelatin-Based Edible Coatings Incorporated with Aloe Vera and Black and Green Tea Extracts on the Shelf Life of Fresh-Cut Oranges. J. Food Qual. 2017, 2017, 9764650. [Google Scholar] [CrossRef]
- Barreveld, W.H. Date Palm Products. Food Agric. Organ. Agric. Serv. Bull. 1993, 101, 1010–1365. [Google Scholar]
- Sivertsvic, M.; Rosnes, J.T.; Bergslien, H. Modified Atmosphere Packaging. In Minimal Processing Technologies in Food Industry; Woodhead Publishing Limited: Cambridge, UK, 2002; pp. 61–87. [Google Scholar]
- Ramalingam, S.; Bahuguna, A.; Lim, S.; Joe, A.-R.; Lee, J.-S.; Kim, S.-Y.; Kim, M. Physicochemical, Microbial, and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries. Foods 2022, 11, 375. [Google Scholar] [CrossRef]
- Mohammed, M.; Alqahtani, N.K. Design and Validation of Automated Sensor-Based Artificial Ripening System Combined with Ultrasound Pretreatment for Date Fruits. Agronomy 2022, 12, 2805. [Google Scholar] [CrossRef]
- Mohammed, M.; Sallam, A.; Alqahtani, N.; Munir, M. The Combined Effects of Precision-Controlled Temperature and Relative Humidity on Artificial Ripening and Quality of Date Fruit. Foods 2021, 10, 2636. [Google Scholar] [CrossRef]
- Abu-Shama, H.S.; Abou-Zaid, F.O.F.; El-Sayed, E.Z. Effect of Using Edible Coatings on Fruit Quality of Barhi Date Cultivar. Sci. Hortic. 2020, 265, 109262. [Google Scholar] [CrossRef]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef]
- Diboun, I.; Mathew, S.; Al-Rayyashi, M.; Elrayess, M.; Torres, M.; Halama, A.; Méret, M.; Mohney, R.P.; Karoly, E.D.; Malek, J.; et al. Metabolomics of Dates (Phoenix dactylifera) Reveals a Highly Dynamic Ripening Process Accounting for Major Variation in Fruit Composition. BMC Plant Biol. 2015, 15, 291. [Google Scholar] [CrossRef]
- Serrano, M.; Pretel, M.T.; Botella, M.A.; Amoros, A. Physicochemical Changes during Date Ripening Related to Ethylene Production. Food Sci. Technol. Int. 2001, 7, 31–36. [Google Scholar] [CrossRef]
- Aleid, S.M.; Hassan, B.H.; Almaiman, S.A.; Al-Kahtani, S.H.; Ismail, S.M. Microbial Loads and Physicochemical Characteristics of Fruits from Four Saudi Date Palm Tree Cultivars: Conformity with Applicable Date Standards. Food Nutr. Sci. 2014, 05, 316–327. [Google Scholar] [CrossRef]
- Hasanaoui, A.; Elhoumaizi, M.A.; Hakkou, A.; Wathelet, B.; Sindic, M. Physico-Chemical Characterization, Classification and Quality Evaluation of Date Palm Fruits of Some Moroccan Cultivars. J. Sci. Res. 2010, 3, 139. [Google Scholar] [CrossRef]
- Kader, A.A. Date: Recommendations for Maintaining Postharvest Quality; University of California: Davis, CA, USA, 2007; pp. 1–3. [Google Scholar]
- Vandercook, C.E.; Hasegawa, S.; Maier, V.P. Dates. In Tropical and Subtropical Fruits: Composition, Properties and Uses; Nagy, S., Shaw, P.E., Eds.; AVI Publishing Company: Westport, CT, USA, 1980; pp. 506–541. [Google Scholar]
- Gomez, S.; Kuruvila, B.; Maneesha, P.K.; Joseph, M. Variation in Physico-Chemical, Organoleptic and Microbial Qualities of Intermediate Moisture Pineapple (Ananas comosus (L.) Merr.) Slices during Storage. Food Prod. Process. Nutr. 2022, 4, 5. [Google Scholar] [CrossRef]
- Abekhti, A.; Zarour, K.; Boulal, A.; Benmechernene, Z.; Kihal, M. Evaluation of Microbiological Quality of the Date Fruit Product “Btana” Produced in Adrar South Algeria. J. Microbiol. Res. 2013, 2013, 163–170. [Google Scholar]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Dehydrated Foods: Are They Microbiologically Safe? Crit. Rev. Food Sci. Nutr. 2019, 59, 2734–2745. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of Survival, Responses and Sources of Salmonella in Low-Moisture Environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef]
- Kalia, A.; Gupta, R.P. Fruit Microbiology. In Handbook of Fruits and Fruit Processing; Wiley: Ames, IA, USA, 2006; pp. 1–28. ISBN 0813819814. [Google Scholar]
- Benkeblia, N. Storage Technologies for Date Palm Fruits (Phoenix dactylifera L.): Current and Potential Techniques. Acta Hortic. 2013, 994, 151–154. [Google Scholar] [CrossRef]
- Aziz Elhoumaizi, M.; Jdaini, K.; Alla, F.; Parmar, A. Variations in Physicochemical and Microbiological Characteristics of ‘Mejhoul’ Dates (Phoenix dactylifera L.) from Morocco and New Countries of Its Expansion. J. Saudi Soc. Agric. Sci. 2023, 22, 318–326. [Google Scholar] [CrossRef]
- Jdaini, K.; Alla, F.; M’hamdi, H.; Guerrouj, K.; Parmar, A.; Elhoumaizi, M.A. Effect of Harvesting and Post-Harvest Practices on the Microbiological Quality of Dates Fruits (Phoenix dactylifera L.). J. Saudi Soc. Agric. Sci. 2022, 21, 552–559. [Google Scholar] [CrossRef]
- Mohammed, M.E.A.; Eissa, A.H.A.; Aleid, S.M. Application of Pulsed Electric Field for Microorganisms Inactivation in Date Palm Fruits. J. Food Nutr. Res. 2016, 4, 646–652. [Google Scholar]
- Zamir, R.; Islam, A.B.M.N.; Rahman, A.; Ahmed, S.; Omar Faruque, M. Microbiological Quality Assessment of Popular Fresh Date Samples Available in Local Outlets of Dhaka City, Bangladesh. Int. J. Food Sci. 2018, 2018, 7840296. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.H. The Microbial Quality of Processed Date Fruits Collected from a Factory in Al-Hofuf City, Kingdom of Saudi Arabia. Emirates J. Food Agric. 2012, 24, 105–112. [Google Scholar]
- Aleid, S.M.; Elansari, A.M.; Zhen-Xing, T.; Sallam, A.A. Effect of Cold Storage and Packing Type on Khalas and Sukkary Dates Quality. Adv. J. Food Sci. Technol. 2014, 6, 603–608. [Google Scholar] [CrossRef]
- Srinivasagan, R.; Mohammed, M.; Alzahrani, A. TinyML-Sensor for Shelf Life Estimation of Fresh Date Fruits. Sensors 2023, 23, 7081. [Google Scholar] [CrossRef]
- Mohammed, M.; Srinivasagan, R.; Alzahrani, A.; Alqahtani, N.K. Machine-Learning-Based Spectroscopic Technique for Non-Destructive Estimation of Shelf Life and Quality of Fresh Fruits Packaged under Modified Atmospheres. Sustainability 2023, 15, 12871. [Google Scholar] [CrossRef]











| Film Type | Material | Width (mm) | Thickness (µm) | WVTR (g/m2/24 h) | O2TR (cm3/m2/24 h) | CO2TR (cm3/m2/24 h) |
|---|---|---|---|---|---|---|
| High Barrier | PHB/PpHB | 320 | 65 | 3.74 | 8.65 | 323.04 |
| Fruit Cultivars | Packaging Material | Gas Concentrations | Storage Temperature | Treatment Codes | ||
|---|---|---|---|---|---|---|
| N (%) | O2 (%) | CO2 (%) | ||||
| Khalas | Cardboard box | 78.08 ± 0.05 | 20.95 ± 0.05 | 0.03 ± 0.01 | 24 ± 1 °C | KCaRT |
| 4 ± 0.5 °C | KCaCT | |||||
| MAP tray | 78.08 ± 0.05 | 20.95 ± 0.05 | 0.03 ± 0.01 | 24 ± 1 °C | KNAPRT | |
| 4 ± 0.5 °C | KNAPCT | |||||
| 80 ± 0.05 | 0 | 20 ± 0.05 | 24 ± 1 °C | KMAPRT | ||
| 4 ± 0.5 °C | KMAPCT | |||||
| Sukary | Cardboard box | 78.08 ± 0.05 | 20.95 ± 0.05 | 0.03 ± 0.01 | 24 ± 1 °C | SCaRT |
| 4 ± 0.5 °C | SCaCT | |||||
| MPA tray | 78.08 ± 0.05 | 20.95 ± 0.05 | 0.03 ± 0.01 | 24 ± 1 °C | SNAPRT | |
| 4 ± 0.5 °C | SNAPCT | |||||
| 80 ± 0.05 | 0 | 20 ± 0.05 | 24 ± 1 °C | SMAPRT | ||
| 4 ± 0.5 °C | SMAPCT | |||||
| Fruit Cultivars | Storage Conditions | Chemical Properties | |||
|---|---|---|---|---|---|
| MC | aw | TSS | pH | ||
| Khalas | KCaRT | 14.14 ± 1.96 B | 0.41 ± 0.08 B | 62.95 ± 0.72 B | 5.78 ± 0.06 A |
| KCaCT | 18.48 ± 0.42 A | 0.58 ± 0.03 A | 65.79 ± 1.67 A | 5.64 ± 0.13 B | |
| KNAPRT | 18.16 ± 0.39 A | 0.57 ± 0.03 A | 65.52 ± 1.55 A | 5.61 ± 0.14 B | |
| KNAPCT | 18.12 ± 0.34 A | 0.58 ± 0.04 A | 65.66 ± 1.33 A | 5.65 ± 0.12 B | |
| KMAPRT | 18.11 ± 0.33 A | 0.58 ± 0.03 A | 65.59 ± 1.92 A | 5.62 ± 0.15 B | |
| KMAPCT | 18.18 ± 0.31 A | 0.59 ± 0.04 A | 65.37 ± 1.88 A | 5.51 ± 0.18 C | |
| Sukary | SCaRT | 9.64 ± 2.23 C | 0.42 ± 0.11 B | 64.92 ± 1.41 B | 5.79 ± 0.11 A |
| SCaCT | 15.16 ± 0.45 A | 0.64 ± 0.03 A | 68.22 ± 1.39 A | 5.68 ± 0.16 AB | |
| SNAPRT | 14.57 ± 0.32 AB | 0.63 ± 0.03 A | 67.54 ± 1.52 A | 5.63 ± 0.22 BC | |
| SNAPCT | 14.73 ± 0.38 AB | 0.63 ± 0.04 A | 67.69 ± 1.32 A | 5.55 ± 0.23 BC | |
| SMAPRT | 14.49 ± 0.38 B | 0.62 ± 0.03 A | 67.81 ± 1.69 A | 5.49 ± 0.24 CD | |
| SMAPCT | 14.57 ± 0.37 AB | 0.63 ± 0.04 A | 67.66 ± 1.83 A | 5.42 ± 0.28 D | |
| Fruit Cultivars | Storage Conditions | Color Parameters | |||
|---|---|---|---|---|---|
| L* | a* | b* | ∆E* | ||
| Khalas | KCaRT | 31.58 ± 7.2 B | 12.4 ± 3.39 B | 30.07 ± 9.79 C | 28.87 ± 12.75 A |
| KCaCT | 33.94 ± 6.12 B | 13.44 ± 3.08 B | 32.38 ± 10.07 C | 25.51 ± 12.65 A | |
| KNAPRT | 40.76 ± 3.57 A | 16.34 ± 1.28 A | 38.77 ± 7.04 B | 17.8 ± 10.17 B | |
| KNAPCT | 42.18 ± 2.97 A | 16.31 ± 1.26 A | 40.05 ± 6.16 AB | 11.33 ± 6.32 C | |
| KMAPRT | 40.87 ± 3.7 A | 16.55 ± 1.2 A | 40.89 ± 5.61 AB | 11.49 ± 6.35 C | |
| KMAPCT | 42.43 ± 2.86 A | 16.82 ± 1.12 A | 43.84 ± 4.9 A | 9.11 ± 4.78 C | |
| Sukary | SCaRT | 32.22 ± 6.75 D | 12.52 ± 2.62 B | 30.05 ± 7.87 C | 29.11 ± 13.08 A |
| SCaCT | 34.13 ± 5.85 CD | 13.24 ± 2.02 B | 32.13 ± 7.36 C | 26.16 ± 11.54 AB | |
| SNAPRT | 35.75 ± 4.62 BC | 14.84 ± 1.49 A | 38.22 ± 4.58 B | 21.31 ± 10.08 B | |
| SNAPCT | 38.72 ± 4.26 B | 15.27 ± 1.23 A | 38.68 ± 5.22 B | 14.74 ± 6.51 C | |
| SMAPRT | 38.21 ± 3.63 B | 15.53 ± 1.17 A | 41.55 ± 3.98 AB | 13.96 ± 6.15 CD | |
| SMAPCT | 43.06 ± 2.55 A | 15.87 ± 0.97 A | 43.11 ± 2.79 A | 8.74 ± 4.31 D | |
| Fruit Cultivars | Storage Conditions | Microbial Load | |
|---|---|---|---|
| Yeast and Molds (Log cfu/g) | Total Bacteria (Log cfu/g) | ||
| Khalas | KCaRT | 1.66 ± 0.28 E | 1.24 ± 0.43 C |
| KCaCT | 3.27 ± 0.72 A | 2.96 ± 0.85 A | |
| KNAPRT | 2.82 ± 0.52 B | 2.14 ± 0.58 B | |
| KNAPCT | 2.62 ± 0.43 BC | 2.01 ± 0.55 B | |
| KMAPRT | 2.46 ± 0.47 C | 2.01 ± 0.55 B | |
| KMAPCT | 2.16 ± 0.24 D | 2.03 ± 0.46 B | |
| Sukary | SCaRT | 1.96 ± 0.33 C | 1.61 ± 0.44 C |
| SCaCT | 2.84 ± 0.73 A | 2.19 ± 0.64 A | |
| SNAPRT | 2.51 ± 0.59 AB | 1.97 ± 0.56 AB | |
| SNAPCT | 2.37 ± 0.58 B | 1.83 ± 0.49 BC | |
| SMAPRT | 2.32 ± 0.49 B | 1.73 ± 0.46 BC | |
| SMAPCT | 2.19 ± 0.41 BC | 2.07 ± 0.63 AB | |
| Fruit cv. | Fruit Characteristics | Storage Parameters | ||||
|---|---|---|---|---|---|---|
| Storage Time | Storage Temperature | N | O2 | CO2 | ||
| Khalas | MC | −0.241 ** | −0.207 * | 0.350 ** | −0.350 ** | 0.350 ** |
| aw | −0.315 ** | −0.051 | 0.111 | −0.111 | 0.111 | |
| TSS | 0.324 ** | 0.022 | −0.170 | 0.170 | −0.170 | |
| pH | −0.301 ** | −0.127 | 0.285 ** | −0.285 ** | 0.285 ** | |
| L* | −0.321 ** | −0.162 | 0.298 ** | −0.298 ** | 0.298 ** | |
| A* | −0.415 ** | −0.054 | 0.172 | −0.172 | 0.172 | |
| B* | −0.312 ** | −0.159 | 0.296 ** | −0.296 ** | 0.296 ** | |
| ∆E | 0.354 ** | 0.059 | −0.125 | 0.125 | −0.125 | |
| Y&MC | 0.105 | 0.102 | −0.249 ** | 0.249 ** | −0.249 ** | |
| TAMC | 0.134 | 0.098 | −0.271 ** | 0.271 ** | −0.271 ** | |
| Sukary | MC | −0.497 ** | −0.184 * | 0.437 ** | −0.437 ** | 0.437 ** |
| aw | −0.347 ** | −0.095 | 0.191 * | −0.191 * | 0.191 * | |
| TSS | 0.265 ** | 0.067 | −0.148 | 0.148 | −0.148 | |
| pH | −0.324 ** | −0.155 | 0.259 ** | −0.259 ** | 0.259 ** | |
| L* | −0.314 ** | −0.156 | 0.469 ** | −0.469 ** | 0.469 ** | |
| A* | −0.254 ** | −0.151 | 0.302 ** | −0.302 ** | 0.302 ** | |
| B* | −0.321 ** | −0.103 | 0.375 ** | −0.375 ** | 0.375 ** | |
| ∆E | 0.425 ** | −0.007 | −0.143 | 0.143 | −0.143 | |
| Y&MC | 0.110 | 0.123 | −0.226 * | 0.226 * | −0.226 * | |
| TAMC | 0.173 | 0.145 | −0.326 ** | 0.326 ** | −0.326 ** | |
| Networks Information | |||
|---|---|---|---|
| Input Layer | Covariates | 1 | CO2 |
| 2 | O2 | ||
| 3 | N | ||
| 4 | Storage Time | ||
| 5 | Packing Material | ||
| 6 | Storage Temperature | ||
| Number of Units (Excluding the bias unit) | 6 | ||
| Rescaling Method for Covariates | Normalized | ||
| Hidden Layer(s) | Number of Hidden Layers | 1 | |
| Number of Units in Hidden Layer (Excluding the bias) unit | 13 for Khalas 12 for Sukary | ||
| Activation Function | Hyperbolic tangent | ||
| Output Layer | Dependent Variables | 1 | Moisture |
| 2 | aw | ||
| 3 | TSS | ||
| 4 | pH | ||
| 5 | L | ||
| 6 | a | ||
| 7 | b | ||
| 8 | ∆E | ||
| 9 | Yeast and Mold | ||
| 10 | TAMC | ||
| Number of Units | 10 | ||
| Rescaling Method for Scale Dependents | Standardized | ||
| Activation Function | Identity | ||
| Error Function | Sum of Squares | ||
| Date Fruit Cultivars | |||||||
|---|---|---|---|---|---|---|---|
| Khalas | Sukary | ||||||
| Phases | Training | Testing | Evaluating | Training | Testing | Evaluating | |
| Sum of squares error | 140.172 | 40.2319 | 134.158 | 41.473 | |||
| Average overall relative error | 0.374 | 0.2914 | 0.4323 | 0.363 | 0.419 | 0.398 | |
| Relative error | MC | 0.313 | 0.2455 | 0.3860 | 0.509 | 0.546 | 0.485 |
| aw | 0.120 | 0.0636 | 0.2189 | 0.089 | 0.124 | 0.078 | |
| TSS | 0.401 | 0.4598 | 0.3655 | 0.241 | 0.309 | 0.277 | |
| pH | 0.234 | 0.1708 | 0.2610 | 0.197 | 0.235 | 0.226 | |
| L* | 0.217 | 0.1727 | 0.4759 | 0.225 | 0.202 | 0.423 | |
| a* | 0.516 | 0.2601 | 0.4362 | 0.371 | 0.556 | 0.324 | |
| b* | 0.268 | 0.1224 | 0.3361 | 0.294 | 0.567 | 0.331 | |
| ∆E | 0.199 | 0.0716 | 0.3795 | 0.250 | 0.472 | 0.253 | |
| Y&MC | 0.753 | 0.6675 | 1.3610 | 0.837 | 3.862 | 0.895 | |
| TAMC | 0.716 | 0.6636 | 1.5660 | 0.613 | 0.749 | 0.745 | |
| Quality Parameters | Khalas | Sukary | ||
|---|---|---|---|---|
| RMSE | MAPE, % | RMSE | MAPE, % | |
| MC | 1.251 | 6.321 | 1.142 | 7.913 |
| aw | 0.023 | 4.332 | 0.035 | 4.824 |
| TSS | 1.696 | 2.151 | 1.787 | 1.911 |
| pH | 0.243 | 2.721 | 0.219 | 2.204 |
| L* | 2.707 | 6.712 | 4.555 | 9.214 |
| a* | 1.418 | 7.121 | 1.465 | 8.523 |
| b* | 2.263 | 6.132 | 4.947 | 10.202 |
| ∆E | 2.996 | 8.374 | 4.141 | 9.413 |
| YM | 0.316 | 8.152 | 0.311 | 7.532 |
| TB | 0.271 | 8.243 | 0.287 | 10.309 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.R.; Aleid, S.M.; Mohammed, M. Impact of Modified Atmosphere Packaging Conditions on Quality of Dates: Experimental Study and Predictive Analysis Using Artificial Neural Networks. Foods 2023, 12, 3811. https://doi.org/10.3390/foods12203811
Ahmed AR, Aleid SM, Mohammed M. Impact of Modified Atmosphere Packaging Conditions on Quality of Dates: Experimental Study and Predictive Analysis Using Artificial Neural Networks. Foods. 2023; 12(20):3811. https://doi.org/10.3390/foods12203811
Chicago/Turabian StyleAhmed, Abdelrahman R., Salah M. Aleid, and Maged Mohammed. 2023. "Impact of Modified Atmosphere Packaging Conditions on Quality of Dates: Experimental Study and Predictive Analysis Using Artificial Neural Networks" Foods 12, no. 20: 3811. https://doi.org/10.3390/foods12203811







