Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Standard
2.2. Cold Brew Coffee Extract
2.3. Analysis of pH and Titratable Acidity
2.4. Analysis of Antioxidant Activity
2.5. LC-MS Analysis of Polyphenols
2.6. Microbial Analysis
2.7. Color Analysis
2.8. Statistics
3. Results
3.1. Titratable Acidity and pH
3.2. Antioxidant Capacity
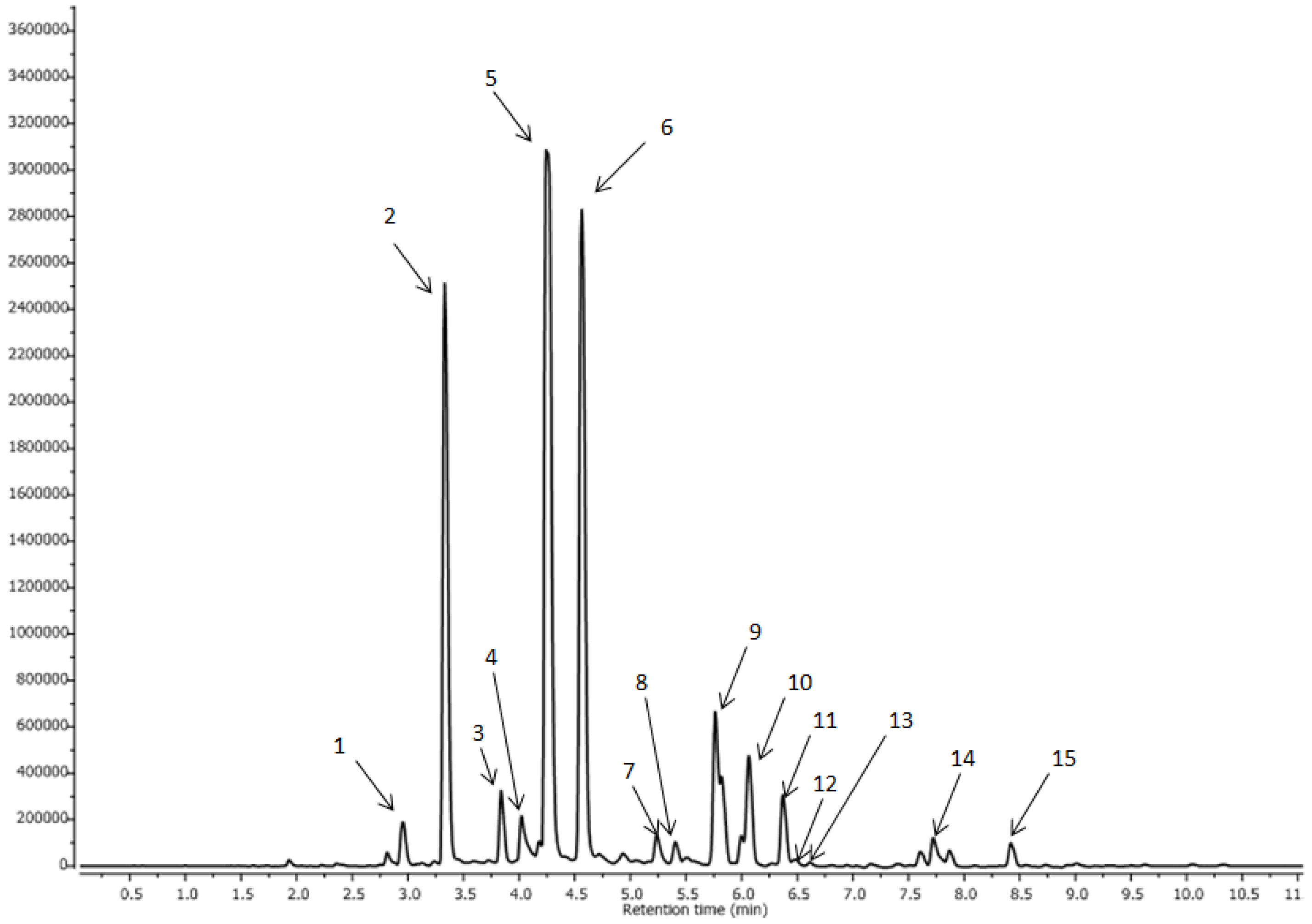
3.3. Characterization of Phenolic Compounds by LC–MS
3.4. Microbial Analysis
3.5. Color Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2017, 128, 110–117. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/industry-analysis/hot-drinks-packaging-market (accessed on 2 September 2023).
- Yu, D.; Wang, X.; Lim, L. Investigation of the factors affecting foamability and foam stability of cold brew coffee. J. Sci. Food Agric. 2022, 102, 5875–5882. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2018, 99, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Larkin, J.; Cole, M.; Skinner, G.; Whiting, R.; Gorris, L.; Rodriguez, A.; Buchanan, R.; Stewart, C.; Hanlin, J.; et al. Food Safety Objective Approach for Controlling Clostridium botulinum Growth and Toxin Production in Commercially Sterile Foods. J. Food Prot. 2011, 74, 1956–1989. [Google Scholar] [CrossRef]
- Bellumori, M.; Angeloni, G.; Guerrini, L.; Masella, P.; Calamai, L.; Mulinacci, N.; Parenti, A.; Innocenti, M. Effects of different stabilization techniques on the shelf life of cold brew coffee: Chemical composition, flavor profile and microbiological analysis. LWT 2021, 142, 111043. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Fei, Y.; Zhou, Y.; Jin, L.; Xing, Z. Effect of sterilization methods on the flavor of cold brew coffee. Beverage Plant Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- De Sousa, P.H.M.; Maia, G.A.; De Azeredo, H.M.C.; Ramos, A.M.; De Figueiredo, R.W. Storage stability of a tropical fruit (cashew apple, acerola, papaya, guava and passion fruit) mixed nectar added caffeine. Int. J. Food Sci. Technol. 2010, 45, 2162–2166. [Google Scholar] [CrossRef]
- PN-90/A-75101/04; Fruit and Vegetable Preserves. Preparation of Samples and Methods of Physicochemical Research. Determination of Total Acidity. Available online: http://www.ydylstandards.org.cn/static/down/pdf/PN%20A75101-04-1990_5000.pdf (accessed on 30 September 2023).
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing Ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- PN-EN ISO 6579-12017-04/A1:2020; Food Chain Microbiology—Horizontal Method for Detection, Enumeration and Serotyping of Salmonella. ISO: Geneva, Switzerland, 2020.
- Aguiar, J.; Estevinho, B.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kang, D.-E.; Lee, H.-U.; Davaatseren, M.; Chung, M.-S. Comparison of acrylamide and furan concentrations, antioxidant activities, and volatile profiles in cold or hot brew coffees. Food Sci. Biotechnol. 2020, 29, 141–148. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef]
- Pokorná, J.; Venskutonis, P.R.; Kraujalyte, V.; Kraujalis, P.; Dvořák, P.; Tremlová, B.; Kopřiva, V.; Ošťádalová, M. Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Aliment. 2015, 44, 454–460. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of Roasting on the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Schwarzmann, E.T.; Washington, M.P.; Rao, N.Z. Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee. Processes 2022, 10, 1989. [Google Scholar] [CrossRef]
- Wallace, J.T. An Analysis of the Acid Profile of Coffee Brews: Caffeine and Chlorogenic Acid Concentrations in Different Forms of Coffee Brew. Undergraduate Thesis, University of Mississippi, Oxford, MS, USA, 2017. [Google Scholar]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and Characterization of the Chlorogenic Acids in Green Robusta Coffee Beans by LC-MSn: Identification of Seven New Classes of Compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC–MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MSn of Four New Classes of Chlorogenic Acids in Green Coffee Beans: Dimethoxycinnamoylquinic Acids, Diferuloylquinic Acids, Caffeoyl-dimethoxycinnamoylquinic Acids, and Feruloyl-dimethoxycinnamoylquinic Acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of polyphenol oxidase in coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and Antioxidant Activity of Cold Brew Coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Feng, Z.; Song, L.; Wu, J.; Hu, X. Influence of Thermal and Dense-Phase Carbon Dioxide Pasteurization on Physicochemical Properties and Flavor Compounds in Hami Melon Juice. J. Agric. Food Chem. 2009, 57, 5805–5808. [Google Scholar] [CrossRef]
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Jambrak, A.R.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Kovačević, D.B. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Lee Wee Ting, K.; Schwarz, S.; Claassen, L.; Lachenmeier, D.W. Current Challenges of Cold Brew Coffee—Roasting, Extraction, Flavor Profile, Contamination, and Food Safety. Challenges 2020, 11, 26. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Manickavasagan, A.; Lim, L.-T. Extraction and physicochemical characteristics of high pressure-assisted cold brew coffee. Futur. Foods 2022, 5, 100113. [Google Scholar] [CrossRef]

| Variant | HTST Pasteurization | Storage Time at Room Temperature | Frozen Time |
|---|---|---|---|
| CB1 | not pasteurized | not stored | 270 days |
| CB2 | pasteurized | not stored | 270 days |
| CB3 | pasteurized | stored 90 days | 180 days |
| CB4 | pasteurized | stored 180 days | 90 days |
| CB5 | pasteurized | stored 270 days | not frozen |
| Variant | pH | Titratable Acidity (TA) * |
|---|---|---|
| CB1 | 4.67 ± 0.01 a | 0.10 ± 0.00 d |
| CB2 | 4.33 ± 0.00 b | 0.12 ± 0.0 c |
| CB3 | 4.25 ± 0.00 c | 0.16 ± 0.01 a |
| CB4 | 4.28 ± 0.01 c | 0.14 ± 0.01 b |
| CB5 | 4.32 ± 0.00 b | 0.11 ± 0.00 d |
| Variant | ABTS [µMol/100 mL] | FRAP [µMol/100 mL] |
|---|---|---|
| CB1 | 163.33 ± 12.67 b | 197.89 ± 16.32 b |
| CB2 | 195.00 ± 17.98 a | 212.04 ± 19.47 a |
| CB3 | 151.67 ± 12.34 c | 176.72 ± 12.65 c |
| CB4 | 154.33 ± 12.17 c | 178.38 ± 14.39 c |
| CB5 | 123.67 ± 11.56 d | 158.16 ± 15.22 d |
| No. | Compounds | Rt (min) | [M-H]- | MS-MS |
|---|---|---|---|---|
| 1 | 3,4-di-O-caffeoylquinic acid | 2.95 | 515.143 | 353.096; 191.068; |
| 2 | 3,5-diO-caffeoylquinic acid | 3.30 | 515.145 | 353.096; 191.066 |
| 3 | 1-O-caffeoylquinic acid | 3.83 | 353.095 | 191.096; 179.042 |
| 4 | 3,4,5-tri-O-caffeoylquinic acid | 4.02 | 677.1816 | 353.0576, 191.0683 |
| 5 | 3-O-caffeoylquinic acid | 4.25 | 353.096 | 191.069; 179.048; 135.059 |
| 6 | 5-O-caffeoylquinic acid | 4.56 | 353.096 | 191.069 |
| 7 | 3-O-feruloylquinic acid | 5.24 | 367.1126 | 191.0689 |
| 8 | 3-O-p-coumaroylquinic acid | 5.40 | 337.0996 | 191.0685 |
| 9 | cis-3,5-di-O-caffeoylquinic acid | 5.76 | 515.121 | 353.096; 193.062; |
| 10 | 5-O-feruloylquinic acid | 6.06 | 367.112 | 193.064; 173.059 |
| 11 | 4-O-p-coumaroylquinic acid | 6.37 | 337.087 | 161.038; 135.059 |
| 12 | 5-O-p-coumaroylquinic acid | 6.47 | 337.0968 | 191.0697 |
| 13 | cis-3-O-feruloylquinic acid | 6.59 | 367.1129 | 191.0689 |
| 14 | 4,5-di-O-caffeoylquinic acid | 7.71 | 515.12 | 353.094; 191.069 |
| 15 | cis-4,5-di-O-caffeoylquinic acid | 8.41 | 515.114 | 353.098; 191.069 |
| No. | CB1 | CB2 | CB3 | CB4 | CB5 |
|---|---|---|---|---|---|
| 1 | 55.09 ± 2.19 | 88.14 ± 4.36 | 153.72 ± 9.89 | 92.90 ± 3.45 | 75.50 ± 5.32 |
| 2 | 599.61 ± 23.76 | 692.44 ± 46.72 | 843.24 ± 67.33 | 663.03 ± 23.78 | 597.92 ± 21.89 |
| 3 | 70.18 ± 4.91 | 77.82 ± 4.66 | 130.12 ± 11.72 | 79.65 ± 5.61 | 86.10 ± 4.46 |
| 4 | 34.03 ± 1.77 | 40.59 ± 1.98 | 52.56 ± 2.55 | 39.80 ± 2.44 | 33.69 ± 1.21 |
| 5 | 1223.18 ± 99.45 | 1235.39 ± 11.34 | 28.28 ± 14.77 | 27.23 ± 1.46 | 18.55 ± 1.17 |
| 6 | 986.27 ± 51.28 | 1027.53 ± 10.02 | 1199.62 ± 9.54 | 987.94 ± 7.89 | 821.82 ± 62.44 |
| 7 | 13.82 ± 12.44 | 12.71 ± 11.12 | 19.99 ± 17.36 | 13.03 ± 11.14 | 10.57 ± 9.52 |
| 8 | 14.36 ± 1.23 | 14.22 ± 1.22 | 25.87 ± 1.33 | 15.75 ± 1.12 | 18.98 ± 1.61 |
| 9 | 151.86 ± 14.22 | 148.01 ± 14.63 | 216.76 ± 19.44 | 143.64 ± 11.38 | 103.50 ± 9.98 |
| 10 | 69.76 ± 5.54 | 14.45 ± 1.44 | 0.00 | 0.00 | 0.00 |
| 11 | 24.41 ± 1.99 | 2.33 ± 0.22 | 0.00 | 0.00 | 0.00 |
| 12 | 0.00 | 0.00 | 1.53 ± 0.12 | 0.63 ± 0.05 | 0.82 ± 0.06 |
| 13 | 0.00 | 0.00 | 0.78 ± 0.05 | 1.54 ± 0.44 | 1.68 ± 0.53 |
| 14 | 13.62 ± 1.33 | 16.22 ± 1.18 | 8.34 ± 0.72 | 15.42 ± 1.13 | 32.05 ± 2.22 |
| 15 | 23.29 ± 2.15 | 22.74 ± 2.12 | 26.13 ± 2.53 | 19.12 ± 1.19 | 3.59 ± 0.26 |
| sum | 3279.50 | 3392.61 | 2706.96 | 2099.67 | 1804.77 |
| No. | Tested Parameter | Unit | Research Methodology PN-ISO/PN-EN ISO | Variant | Result |
|---|---|---|---|---|---|
| 1 | Molds | CFU/mL | PN-ISO 21527-1:2009 | CB2 | <1.0 × 100 |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 2 | Yeasts | CFU/mL | PN-ISO 21527-1:2009 | CB2 | <1.0 × 100 |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 3 | Salomonella spp. | in 25 mL | PN-EN ISO 6579-1:2017-04 PN-EN ISO 6579-1:2017-04/A1:2020-09 | CB2 | Not detected |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 4 | Number of coagulase-positive Staphylococus aureus | CFU/mL | PN-EN ISO 6888-2:2022-03 | CB2 | <1.0 × 100 |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 5 | Presence of coli form bacteria | in 0.1 mL | PN-ISO 4831:2007 | CB2 | Not detected |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 6 | Total microbial count | CFU/mL | PN-EN ISO 4833-1:2013-12 PN-EN ISO 4833-1:2013-12/Ap1:2016-11 | CB2 | <1.0 × 100 |
| CB3 | |||||
| CB4 | |||||
| CB5 | |||||
| 7 | Number of Listeria monocytogenes | CFU/mL | PN-EN ISP11290-2:2017-07 | CB2 | <1.0 × 100 |
| CB3 | |||||
| CB4 | |||||
| CB5 |
| Variant | L | a | b |
|---|---|---|---|
| CB1 | 24.65 ± 1.23 a | 0.67 ± 0.03 c | 1.12 ± 0.15 c |
| CB2 | 23.36 ± 1.45 b | 0.91 ± 0.04 b | 1.38 ± 0.23 b |
| CB3 | 22.33 ± 1.12 d | 0.61 ± 0.01 d | 1.05 ± 0.17 d |
| CB4 | 22.23 ± 1.17 d | 0.60 ± 0.01 d | 1.07 ± 0.11 d |
| CB5 | 21.28 ± 1.06 e | 0.98 ± 0.09 a | 1.47 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimowski, D.; Oziembłowski, M.; Kolniak-Ostek, J.; Stach, M.; Zubaidi, M.A.; Nawirska-Olszańska, A. Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product. Foods 2023, 12, 3840. https://doi.org/10.3390/foods12203840
Maksimowski D, Oziembłowski M, Kolniak-Ostek J, Stach M, Zubaidi MA, Nawirska-Olszańska A. Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product. Foods. 2023; 12(20):3840. https://doi.org/10.3390/foods12203840
Chicago/Turabian StyleMaksimowski, Damian, Maciej Oziembłowski, Joanna Kolniak-Ostek, Marcelina Stach, Muhamad Alfiyan Zubaidi, and Agnieszka Nawirska-Olszańska. 2023. "Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product" Foods 12, no. 20: 3840. https://doi.org/10.3390/foods12203840
APA StyleMaksimowski, D., Oziembłowski, M., Kolniak-Ostek, J., Stach, M., Zubaidi, M. A., & Nawirska-Olszańska, A. (2023). Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product. Foods, 12(20), 3840. https://doi.org/10.3390/foods12203840










