Anti-Influenza Virus Activity of Citrullus lanatus var. citroides as a Functional Food: A Review
Abstract
1. Introduction
2. Cucurbitaceae Family and Wild Watermelon
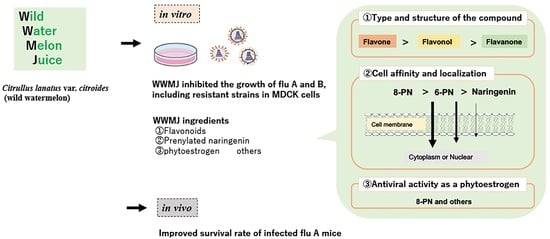
3. Anti-Influenza Virus Activity of Wild Watermelon Juice (WWMJ) and Its Flavonoids
4. Constituents of WWMJ
5. Potential of Prenylated Flavonoids in Antiviral Strategies
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Ishida, H.; Li, J.Y.; Endo, M.; Kobayashi, T.; Kamiki, H.; Matsugo, H.; Takenaka-Uema, A.; Murakami, S.; Horimoto, T. Antigenic heterogeneity among phylogenetic clusters of influenza D viruses. J. Vet. Med. Sci. 2018, 80, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- CDC. Influenza (Flu). 2023. Available online: https://www.cdc.gov/flu/about/viruses/types.htm (accessed on 3 October 2023).
- Bao, P.; Liu, Y.; Zhang, X.; Fan, H.; Zhao, J.; Mu, M.; Li, H.; Wang, Y.; Ge, H.; Li, S.; et al. Human infection with a reassortment avian influenza A H3N8 virus: An epidemiological investigation study. Nat. Commun. 2022, 13, 6817. [Google Scholar] [CrossRef]
- Sullivan, S.J.; Jacobson, R.M.; Dowdle, W.R.; Poland, G.A. 2009 H1N1 influenza. Mayo. Clin. Proc. 2010, 85, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, X.; Liu, L. Influenza and universal vaccine research in China. Viruses 2022, 15, 116. [Google Scholar] [CrossRef]
- Tang, C.Y.; Boftsi, M.; Staudt, L.; McElroy, J.A.; Li, T.; Duong, S.; Ohler, A.; Ritter, D.; Hammer, R.; Hang, J.; et al. SARS-CoV-2 and influenza co-infection: A cross-sectional study in central Missouri during the 2021–2022 influenza season. Virology 2022, 576, 105–110. [Google Scholar] [CrossRef]
- CDC; Fiore, A.E.; Fry, A.; Shay, D.; Gubareva, L.; Bresee, J.S.; Uyeki, T.M. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2011, 60, 1–24. [Google Scholar]
- Mohan, T.; Nguyen, H.T.; Kniss, K.; Mishin, V.P.; Merced-Morales, A.A.; Laplante, J.; St George, K.; Blevins, P.; Chesnokov, A.; De La Cruz, J.A.; et al. Cluster of oseltamivir-resistant and hemagglutinin antigenically drifted influenza A(H1N1)pdm09 viruses, Texas, USA, January 2020. Emerg. Infect. Dis. 2021, 27, 1953–1957. [Google Scholar] [CrossRef]
- Choo, D.; Hossain, M.; Liew, P.; Chowdhury, S.; Tan, J. Side effects of oseltamivir in end-stage renal failure patients. Nephrol. Dial. Transplant. 2011, 26, 2339–2344. [Google Scholar] [CrossRef]
- Fares, R.; Zgheib, A.; Hallit, R.; Hallit, S. Oseltamivir-induced behavioral changes in a female Lebanese adolescent: A case report of a usual drug with unusual side effect. Future Sci. OA 2020, 6, FSO602. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Hayden, F.G.; Kawaguchi, K.; Omoto, S.; Hurt, A.C.; de Jong, M.D.; Hirotsu, N.; Sugaya, N.; Lee, N.; Baba, K.; et al. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: Impact on clinical and virologic outcomes in uncomplicated Influenza. J. Infect. Dis. 2020, 221, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Patel, M.C.; Mishin, V.P.; De La Cruz, J.A.; Chesnokov, A.; Nguyen, H.T.; Wilson, M.M.; Barnes, J.; Kondor, R.J.G.; Wentworth, D.E.; Gubareva, L.V. Detection of baloxavir resistant influenza A viruses using next generation sequencing and pyrosequencing methods. Antivir. Res. 2020, 182, 104906. [Google Scholar] [CrossRef]
- Goldhill, D.H.; Yan, A.; Frise, R.; Zhou, J.; Shelley, J.; Gallego Cortés, A.; Miah, S.; Akinbami, O.; Galiano, M.; Zambon, M.; et al. Favipiravir-resistant influenza A virus shows potential for transmission. PLoS Pathog. 2021, 17, e1008937. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kim, S.; Lee, N.; Kwon, M.; Yang, I.; Kim, M.J.; Cheong, S.G.; Kwon, D.; Lee, J.Y.; Oh, H.B.; et al. Amantadine-resistant influenza A viruses isolated in South Korea from 2003 to 2009. Antivir. Res. 2009, 84, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.R.; Chittaganpitch, M.; Kanai, Y.; Li, Y.G.; Auwanit, W.; Ikuta, K.; Sawanpanyalert, P. Amantadine- and oseltamivir-resistant variants of influenza A viruses in Thailand. Biochem. Biophys. Res. Commun. 2009, 390, 897–901. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, B.W.; Ryu, B.; Cho, H.M.; Kim, S.K.; Yoo, S.S.; Kim, E.; Oh, W.K. Antiviral activity of CAVAC-1901, a combination of 3 standardized medicinal plants, against highly pathogenic influenza A virus in chickens. Poult. Sci. 2023, 102, 102315. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.H.; Shim, A.; Lee, B.R.; Kwon, B.E.; Song, H.H.; Kim, Y.J.; Chang, S.Y.; Jeong, H.G.; Kim, J.G.; et al. Coadministration of hedera helix L. extract enabled mice to overcome insufficient protection against influenza A/PR/8 virus infection under suboptimal treatment with oseltamivir. PLoS ONE. 2015, 10, e0131089. [Google Scholar] [CrossRef][Green Version]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Alzate-Yepes, T.; Pérez-Palacio, L.; Martínez, E.; Osorio, M. Mechanisms of action of fruit and vegetable phytochemicals in colorectal cancer prevention. Molecules 2023, 28, 4322. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxid. Med. Cell Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Xu, Y.M.; Lau, A.T.Y. Anti-cancer and medicinal potentials of moringa isothiocyanate. Molecules 2021, 26, 7512. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Liu, Y. Terpenoids: Natural compounds for non-alcoholic fatty liver disease (NAFLD) therapy. Molecules 2022, 28, 272. [Google Scholar] [CrossRef]
- Kamei, M.; Nishimura, H.; Takahashi, T.; Takahashi, N.; Inokuchi, K.; Mato, T.; Takahashi, K. Anti-influenza virus effects of cocoa. J. Sci. Food Agric. 2016, 96, 1150–1158. [Google Scholar] [CrossRef]
- Lee, J.B.; Miyake, S.; Umetsu, R.; Hayashi, K.; Chijimatsu, T.; Hayashi, T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.). Food Chem. 2012, 134, 2164–2168. [Google Scholar] [CrossRef]
- Sekizawa, H.; Ikuta, K.; Mizuta, K.; Takechi, S.; Suzutani, T. Relationship between polyphenol content and anti-influenza viral effects of berries. J. Sci. Food Agric. 2013, 93, 2239–2241. [Google Scholar] [CrossRef]
- Nakashima, A.; Horio, Y.; Suzuki, K.; Isegawa, Y. Antiviral activity and underlying action mechanism of euglena extract against influenza virus. Nutrients 2021, 13, 3911. [Google Scholar] [CrossRef]
- Nagai, E.; Iwai, M.; Koketsu, R.; Okuno, Y.; Suzuki, Y.; Morimoto, R.; Sumitani, H.; Ohshima, A.; Enomoto, T.; Isegawa, Y. Anti-influenza virus activity of adlay tea components. Plant Foods Hum. Nutr. 2019, 74, 538–543. [Google Scholar] [CrossRef]
- Horio, Y.; Sogabe, R.; Shichiri, M.; Ishida, N.; Morimoto, R.; Ohshima, A.; Isegawa, Y. Induction of a 5-lipoxygenase product by daidzein is involved in the regulation of influenza virus replication. J. Clin. Biochem. Nutr. 2020, 66, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Horio, Y.; Isegawa, Y.; Shichiri, M. Daidzein phosphorylates and activates 5-lipoxygenase via the MEK/ERK pathway: A mechanism for inducing the production of 5-lipoxygenase metabolite that inhibit influenza virus intracellular replication. J. Nutr. Biochem. 2023, 114, 109276. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, R.; Morimoto, R.; Horio, Y.; Sumitani, H.; Isegawa, Y. Inhibition of influenza virus replication by Apiaceae plants, with special reference to Peucedanum japonicum (Sacna) constituents. J. Ethnopharmacol. 2022, 292, 115243. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Yoshioka, K.; Nakayama, M.; Nagai, E.; Okuno, Y.; Nakashima, A.; Ogawa, T.; Suzuki, K.; Enomoto, T.; Isegawa, Y. Juice of Citrullus lanatus var. citroides (wild watermelon) inhibits the entry and propagation of influenza viruses in vitro and in vivo. Food Sci. Nutr. 2021, 9, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Hanada, A.; Morimoto, R.; Horio, Y.; Shichiri, M.; Nakashima, A.; Ogawa, T.; Suzuki, K.; Sumitani, H.; Ogata, T.; Isegawa, Y. Influenza virus entry and replication inhibited by 8-prenylnaringenin from Citrulls lanatus var. citroides (Wild watermelon). Food Sci. Nutr. 2022, 10, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Hanada, A.; Matsubara, C.; Horio, Y.; Sumitani, H.; Ogata, T.; Isegawa, Y. Anti-influenza A virus activity of flavonoids in vitro: A structure-activity relationship. J. Nat. Med. 2023, 77, 219–227. [Google Scholar] [CrossRef]
- Morimoto, R.; Matsubara, C.; Hanada, A.; Omoe, Y.; Ogata, T.; Isegawa, Y. Effect of structural differences in naringenin, prenylated naringenin, and their derivatives on the anti-influenza virus activity and cellular uptake of their flavanones. Pharmaceuticals 2022, 15, 1480. [Google Scholar] [CrossRef]
- Xu, X.; Miao, J.; Shao, Q.; Gao, Y.; Hong, L. Apigenin suppresses influenza A virus-induced RIG-I activation and viral replication. J. Med. Virol. 2020, 92, 3057–3066. [Google Scholar] [CrossRef]
- Yan, H.; Ma, L.; Wang, H.; Wu, S.; Huang, H.; Gu, Z.; Jiang, J.; Li, Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019, 73, 487–496. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent advances in Momordica charantia: Functional components and biological activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Hassan, L.E.A.; Sirat, H.M.; Yagi, S.M.A.; Koko, W.S.; Mohan, S.; Taha, M.M.E.; Ahmad, S.; Chuen, C.S.; Narrima, P.; et al. Anti-inflammatory activities of cucurbitacin E isolated from Citrullus lanatus var. citroides: Role of reactive nitrogen species and cyclooxygenase enzyme inhibition. Fitoterapia 2011, 82, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Freeman, M.; Zhang, X.; Sandhu, A.; Edirisinghe, I. Watermelon and L-citrulline in cardio-metabolic health: Review of the evidence 2000–2020. Curr. Atheroscler. Rep. 2021, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Mamabolo, M.M.; Tabit, F.T. Process optimization for enzymatic clarification of indigenous wild watermelon (Citrullus lanatus) juice. Food Sci. Technol. Int. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Nishimura, N.; Ishida, Y.; Yokota, A. Potent hydroxyl radical-scavenging activity of drought-induced type-2 metallothionein in wild watermelon. Biochem. Biophys. Res. Commun. 2004, 323, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 protein (ArgE homologue) in drought tolerance of wild watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef]
- Fujie, S.; Iemitsu, K.; Inoue, K.; Ogawa, T.; Nakashima, A.; Suzuki, K.; Iemitsu, M. Wild Watermelon-extracted juice ingestion reduces peripheral arterial stiffness with an increase in nitric oxide production: A randomized crossover pilot study. Nutrients 2022, 14, 5199. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Entry of influenza virus. Adv. Exp. Med. Biol. 2013, 790, 72–82. [Google Scholar] [CrossRef]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomed. Rep. 2018, 10, 33–38. [Google Scholar] [CrossRef]
- Zu, M.; Yang, F.; Zhou, W.; Liu, A.; Du, G.; Zheng, L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antivir. Res. 2012, 94, 217–224. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y. Bilobetin, a novel small molecule inhibitor targeting influenza virus polymerase acidic (PA) endonuclease was screened from plant extracts. Nat. Prod. Res. 2021, 35, 5968–5971. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.P.K.; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive oxygen species associated immunoregulation post influenza virus infection. Front. Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khanna, M.; Srivastava, V.; Tyagi, Y.K.; Raj, H.G.; Ravi, K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp. Lung Res. 2005, 31, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Kreiser, T.; Zaguri, D.; Sachdeva, S.; Zamostiano, R.; Mograbi, J.; Segal, D.; Bacharach, E.; Gazit, E. Inhibition of respiratory RNA viruses by a composition of ionophoric polyphenols with metal ions. Pharmaceuticals 2022, 15, 377. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Liu, A.L.; Wang, H.D.; Lee, S.M.; Wang, Y.T.; Du, G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.Y.; Lee, H.W.; Shin, J.S.; Kim, P.; Jung, Y.S.; Jeong, H.S.; Hyun, J.K.; Lee, C.K. Inhibition of influenza virus internalization by (-)-epigallocatechin-3-gallate. Antivir. Res. 2013, 100, 460–472. [Google Scholar] [CrossRef]
- Tronina, T.; Popłoński, J.; Bartmańska, A. flavonoids as phytoestrogenic components of hops and beer. Molecules. 2020, 25, 4201. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzym. Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Shahinozzaman, M.; Taira, N.; Ishii, T.; Halim, M.A.; Hossain, M.A.; Tawata, S. Anti-inflammatory, anti-diabetic, and anti-alzheimer’s effects of prenylated flavonoids from Okinawa propolis: An investigation by experimental and computational studies. Molecules 2018, 23, 2479. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Böcker, A.; Leischner, C.; Riepl, H.; et al. 6- and 8-Prenylnaringenin, novel natural histone deacetylase inhibitors found in hops, exert antitumor activity on melanoma cells. Cell Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from Hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef]
- Hartmayer, E.; Hettler, C.; Sarnecka, A.; Wulle, U.; Ehrhardt, C.; Ludwig, S.; Planz, O. Antiviral activity of Ladania067, an extract from wild black currant leaves against influenza A virus in vitro and in vivo. Front. Microbiol. 2014, 5, 171. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Yuan, X.; Shi, J.; Zhang, C.; Yan, N.; Jing, C. Comparison of phenolic and flavonoid compound profiles and antioxidant and α-glucosidase inhibition properties of cultivated soybean (Glycine max) and wild soybean (Glycine soja). Plants 2021, 10, 813. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; Fridlender, M.; Nallathambi, R.; Selvaraj, G.; Nadarajan, S.; Koltai, H. Review on anti-cancer activity in wild plants of the Middle East. Curr. Med. Chem. 2018, 25, 4656–4670. [Google Scholar] [CrossRef]





| Classification | Compound Name and Type | IC50 Value (μM) | |
|---|---|---|---|
| Flavonoid | Flavone | Acacetin (a) | 33.8 |
Non-flavonoid | Flavanone Flavaprenin Lignan | Naringenin (b) 8-prenylnaringenin (c) Pinoresinol (d) | 290.4 24.0 343.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, R.; Isegawa, Y. Anti-Influenza Virus Activity of Citrullus lanatus var. citroides as a Functional Food: A Review. Foods 2023, 12, 3866. https://doi.org/10.3390/foods12203866
Morimoto R, Isegawa Y. Anti-Influenza Virus Activity of Citrullus lanatus var. citroides as a Functional Food: A Review. Foods. 2023; 12(20):3866. https://doi.org/10.3390/foods12203866
Chicago/Turabian StyleMorimoto, Ryosuke, and Yuji Isegawa. 2023. "Anti-Influenza Virus Activity of Citrullus lanatus var. citroides as a Functional Food: A Review" Foods 12, no. 20: 3866. https://doi.org/10.3390/foods12203866
APA StyleMorimoto, R., & Isegawa, Y. (2023). Anti-Influenza Virus Activity of Citrullus lanatus var. citroides as a Functional Food: A Review. Foods, 12(20), 3866. https://doi.org/10.3390/foods12203866