Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
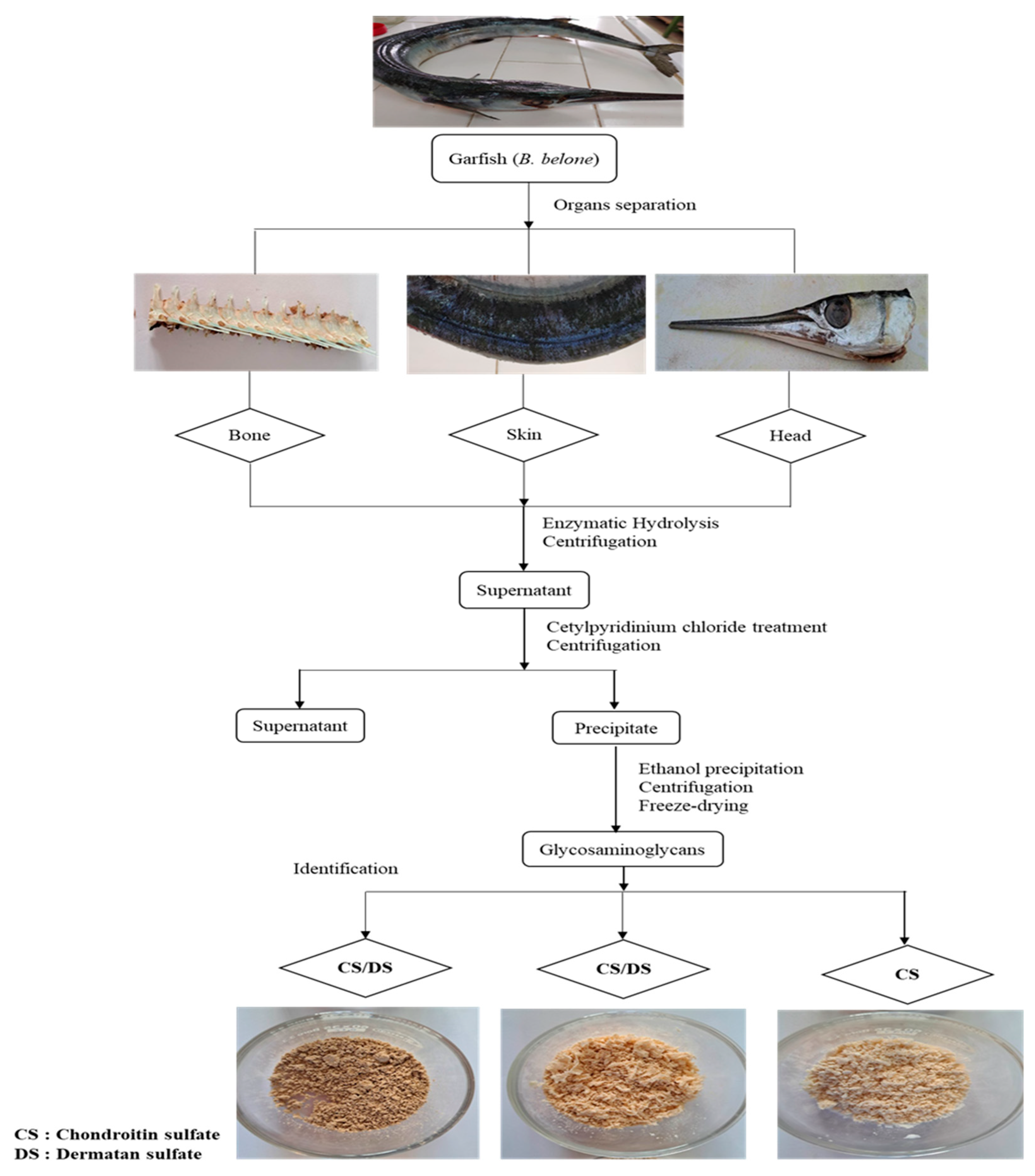
2.2. Material Preparation
2.3. Enzymatic Extraction of GAGs from Garfish By-products
2.4. Purification of GAGs from Garfish by-products
2.5. Glycosaminoglycans Yield Calculation
2.6. Chemical Composition Analysis
2.7. Cellulose Acetate Electrphoresis Analysis
2.8. Visible Ultraviolet Spectrum of GAGs
2.9. Molecular Mass Analysis
2.10. Disaccharide Composition Determination
2.11. In Vitro Anticoagulant Activity
2.11.1. Blood Collection and Preparation of PPP and PRP
2.11.2. Activated Partial Thromboplastin Time Assay
2.11.3. Thrombin Time Assay
2.11.4. Prothrombin Time Assay
2.12. Statistical Analysis
3. Results
3.1. Extraction, Purification, and Chemical Composition of GAGs
3.2. Purity Identifcation and Cellulose Acetate Electrophoresis Analysis
3.3. Molecular Weight Analysis of GAGs
3.4. Disaccharide Composition Analysis
3.5. In Vitro Anticoagulant Activity of Purified GAGs
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cercato, C.; Fonseca, F.A. Cardiovascular Risk and Obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakim, A. General Considerations for Diversifying Heparin Drug Products by Improving the Current Heparin Manufacturing Process and Reintroducing Bovine Sourced Heparin to the US Market. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211052292. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, H.; Zhao, L.; Zhang, Y.; Liu, Z. Oligonucleotide Aptamers: Recent Advances in Their Screening, Molecular Conformation and Therapeutic Applications. Biomed. Pharmacother. 2021, 143, 112232. [Google Scholar] [CrossRef] [PubMed]
- Meher, M.K.; Poluri, K.M. Anticoagulation and Antibacterial Properties of Heparinized Nanosilver with Different Morphologies. Carbohydr. Polym. 2021, 266, 118124. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Rodriguez, R.M.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.C.; Lindahl, U. Glycosaminoglycans and the regulation of blood coagulation. Biochem. J. 1993, 289, 313–330. [Google Scholar] [CrossRef]
- Jang, I.K.; Hursting, M.J. When heparins promote thrombosis. Circulation 2005, 111, 2671–2683. [Google Scholar] [CrossRef]
- Liu, D.; Shriver, Z.; Qi, Y.; Venkataraman, G.; Sasisekharan, R. Dynamic regulation of tumor growth and metastasis by heparan sulfate glycosaminoglycans. Semin. Thromb. Hemost. 2002, 28, 67–78. [Google Scholar] [CrossRef]
- Pomin, V.H. Sulfated glycans in inflammation. Eur. J. Med. Chem. 2015, 92, 353–369. [Google Scholar] [CrossRef]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef]
- Ben Mansour, M.; Majdoub, H.; Bataille, I.; Roudesli, M.S.; Hassine, M.; Ajzenberg, N.; Chaubet, F.; Maaroufi, R.M. Polysaccharides from the Skin of the Ray Raja Radula. Partial Characterization and Anticoagulant Activity. Thromb. Res. 2009, 123, 671–678. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Cesaretti, M.; Luppi, E.; Maccari, F.; Volpi, N. A 96-Well Assay for Uronic Acid Carbazole Reaction. Carbohydr. Polym. 2003, 54, 59–61. [Google Scholar] [CrossRef]
- Lloyd, A.G.; Dodgson, K.S.; Price, R.G.; Rose, F.A.I. Polysaccharide Sulphates. Biochim. Biophys. Acta 1961, 46, 108–115. [Google Scholar] [CrossRef]
- Volpi, N.; Maccari, F. Detection of Submicrogram Quantities of Glycosaminoglycans on Agarose Gels by Sequential Staining with Toluidine Blue and Stains-All. Electrophoresis 2002, 23, 4060–4066. [Google Scholar] [CrossRef]
- Edens, R.E.; Al-Hakim, A.; Weiler, J.M.; Rethwisch, D.G.; Fareed, J.; Linhardt, R.J. Gradient Polyacrylamide Gel Electrophoresis for Determination of Molecular Weights of Heparin Preparations and Low-Molecular-Weight Heparin Derivatives. J. Pharm. Sci. 1992, 81, 823–827. [Google Scholar] [CrossRef]
- Bindia, S.; Diya, D.S.; Gladstone, C.J.; Balaraman, M.; Farhan, Z. A review on an imperative by-product: Glycosaminoglycans- A holistic approach. Carbohydr. Polym. Technol Appl. 2023, 5, 100275. [Google Scholar] [CrossRef]
- Ticar, B.F.; Rohmah, Z.; Neri, T.A.N.; Pahila, I.G.; Vasconcelos, A.; Archer-Hartmann, S.A.; Reiter, C.E.N.; Dobruchowska, J.M.; Choi, B.-D.; Heiss, C.; et al. Biocompatibility and Structural Characterization of Glycosaminoglycans Isolated from Heads of Silver-Banded Whiting (Sillago Argentifasciata Martin & Montalban 1935). Int. J. Biol. Macromol. 2020, 151, 663–676. [Google Scholar] [CrossRef]
- Yang, K.-R.; Tsai, M.-F.; Shieh, C.-J.; Arakawa, O.; Dong, C.-D.; Huang, C.-Y.; Kuo, C.-H. Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods 2021, 10, 2363. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-Products of Scyliorhinus Canicula, Prionace Glauca and Raja Clavata: A Valuable Source of Predominantly 6S Sulfated Chondroitin Sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef]
- Vijayabaskar, P.; Somasundaram, S.T. Studies on molluscan glycosaminoglycans (GAG) from backwater clam Donax cuneatus (Linnaeus). Asian Pac. J. Trop. Biomed. 2012, 2, S519–S525. [Google Scholar] [CrossRef]
- Higashi, K.; Takeuchi, Y.; Mukuno, A.; Tomitori, H.; Miya, M.; Linhardt, R.J.; Toida, T. Composition of glycosaminoglycans in elasmobranchs including several deep-sea sharks: Identification of chondroitin/dermatan sulfate from the dried fins of Isurus oxyrinchus and Prionace glauca. PLoS ONE 2015, 10, e0120860. [Google Scholar] [CrossRef] [PubMed]
- Vijayabaskar, P.; Vaseela, N. In vitro antioxidant properties of sulfated polysaccharide from brown marine algae Sargassum tenerrimum. Asian Pac. J. Trop. Dis. 2012, 2, S890–S896. [Google Scholar] [CrossRef]
- Geetha, V.; Das, M.; Zarei, M.; Mayookha, V.; Harohally, N.V.; Kumar, S.G. Studies on the partial characterization of extracted glycosaminoglycans from fish waste and its potentiality in modulating obesity through in-vitro and in-vivo. Glycoconj. J. 2022, 39, 525–542. [Google Scholar] [CrossRef]
- Urbi, Z.; Azmi, N.S.; Ming, L.C.; Hossain, M.S. A Concise Review of Extraction and Characterization of Chondroitin Sulphate from Fish and Fish Wastes for Pharmacological Application. Curr. Issues Mol. Biol. 2022, 44, 3905–3922. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory Effect of Sulfated Galactans from the Marine Alga Bostrychia Montagnei on Herpes Simplex Virus Replication in Vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Luo, L.; Tao, M.; Chang, X.; Yang, L.; Huang, X.; Hu, L.; Wu, M. A Non-Anticoagulant Heparin-like Snail Glycosaminoglycan Promotes Healing of Diabetic Wound. Carbohydr. Polym. 2020, 247, 116682. [Google Scholar] [CrossRef]
- Dong, F.; Quan, X.; Wang, Q.; Liu, Z.; Cui, T.; Wang, W.; Tang, D.; Zhang, R.; Zhang, C.; Wang, H.; et al. Purification, Structural Characterization, and Anticoagulant Activity Evaluation of Chondroitin Sulfate from Codfish (Gadus Macrocephalus) Bones. Int. J. Biol. Macromol. 2022, 210, 759–767. [Google Scholar] [CrossRef]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of Protein, Chitin, Carotenoids and Glycosaminoglycans from Pacific White Shrimp (Litopenaeus Vannamei) Processing Waste. Process Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Zhang, F.; Linhardt, R.J. Thin Layer Chromatography for the Analysis of Glycosaminoglycan Oligosaccharides. Anal. Biochem 2007, 371, 118–120. [Google Scholar] [CrossRef]
- Talmoudi, N.; Ghariani, N.; Sadok, S. Glycosaminoglycans from Co-Products of «Scyliorhinus canicula»: Extraction and Purification in Reference to the European Pharmacopoeia Requirement. Biol. Proced. Online 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Fujita, H.; Toita, R.; Imazu-Okada, A.; Tsutsumishita-Nakai, N.; Takeda, N.; Nakao, Y.; Wang, H.; Kawano, M.; Matsushita, K.; et al. Amounts and Compositional Analysis of Glycosaminoglycans in the Tissue of Fish. Carbohydr. Res. 2013, 366, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Schnabelrauch, M.; Schiller, J.; Möller, S.; Scharnweber, D.; Hintze, V. Chemically modified glycosaminoglycan derivatives as building blocks for biomaterial coatings and hydrogels. Biol. Chem. 2021, 402, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, M.; González, P.; Serra, J.; Fraguas, J.; Valcarcel, J.; Vázquez, J.A. Chondroitin Sulfate and Hydroxyapatite from Prionace Glauca Shark Jaw: Physicochemical and Structural Characterization. Int. J. Biol. Macromol. 2020, 156, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Analytical Aspects of Pharmaceutical Grade Chondroitin Sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I.; Valcarcel, J. Optimal Isolation and Characterisation of Chondroitin Sulfate from Rabbit Fish (Chimaera Monstrosa). Carbohydr. Polym. 2019, 210, 302–313. [Google Scholar] [CrossRef]
- Xiang, J.; Fangchao, Y.; Bing, W.; Yongqiang, W.; Shuhua, C.; Yuliang, W. Thrombocytopenia induced by lipopolysaccharide may be not related to coagulation and inflammatory response. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015, 27, 754–758. [Google Scholar]
- Dhahri, M.; Sioud, S.; Dridi, R.; Hassine, M.; Boughattas, N.A.; Almulhim, F.; Al Talla, Z.; Jaremko, M.; Emwas, A.M. Extraction, Characterization, and Anticoagulant Activity of a Sulfated Polysaccharide from Bursatella leachii Viscera. ACS Omega 2020, 5, 14786–14795. [Google Scholar] [CrossRef]
- Gui, M.; Song, J.; Zhang, L.; Wang, S.; Wu, R.; Ma, C.; Li, P. Chemical Characteristics and Antithrombotic Effect of Chondroitin Sulfates from Sturgeon Skull and Sturgeon Backbone. Carbohydr. Polym. 2015, 123, 454–460. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yang, Y.; Liu, C.; Zhang, R. Preparation, Structural Characterization and Antioxidant Activity of Water-Soluble Polysaccharides and Purified Fractions from Blackened Jujube by an Activity-Oriented Approach. Food Chem. 2022, 385, 132637. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, N.; Liang, X.; Zhang, H. Dermatan Sulfate and Chondroitin Sulfate from Lophius Litulon Alleviate the Allergy Sensitized by Major Royal Jelly Protein 1. Food Funct. 2022, 13, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G.; Aiello, K.R.M.; Werneck, C.C.; Silva, L.C.F.; Valente, A.-P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A.S. Highly Sulfated Dermatan Sulfates from Ascidians: Structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef] [PubMed]






| Composition (%) | GAGs | Mean ± SD |
|---|---|---|
| Yield | GSB | 3.7 |
| GCB | 1.4 | |
| GHB | 8.1 | |
| Sulfate | GSB | 20 ± 1 |
| GCB | 20 ± 1.5 | |
| GHB | 21 ± 2 | |
| Uronic acid | GSB | 37 ± 3.5 |
| GCB | 35 ± 4 | |
| GHB | 35 ± 4.5 | |
| Total sugar | GSB | 69 ± 4 |
| GCB | 70 ± 8 | |
| GHB | 70 ± 7 |
| Sum of Squares | dF * | Mean of Squares | F Test | p-Value | ||
|---|---|---|---|---|---|---|
| Sulfate | Inter-groups | 0.249 | 2 | 0.124 | 0.049 | 0.952 |
| Intra-groups | 15.201 | 6 | 2.533 | |||
| Total | 15.449 | 8 | ||||
| Uronic Acid | Inter-groups | 7.362 | 2 | 3.681 | 0.225 | 0.805 |
| Intra-groups | 98.286 | 6 | 16.381 | |||
| Total | 105.648 | 8 | ||||
| Total Sugar | Inter-groups | 4.389 | 2 | 2.194 | 0.048 | 0.954 |
| Intra-groups | 274.333 | 6 | 45.722 | |||
| Total | 278.722 | 8 | ||||
| Disaccharide Composition (%) | GSB | GCB | GHB |
|---|---|---|---|
| ΔDi0S (ΔUA-GalNAc) | 5.87 | 4.44 | 9.03 |
| ΔDi6S (ΔUA-GalNAc 6S) | 2.57 | 9.35 | 25.55 |
| ΔDi4S (ΔUA-GalNAc 4S) | 74.78 | 69.22 | 57.17 |
| ΔDi2, 6S (ΔUA2S-GalNAc 6S) | 1.71 | 8.37 | 6.28 |
| ΔDi4, 6S (ΔUA 4S-GalNAc 6S) | 4.14 | 2.05 | 0.37 |
| ΔDi2, 4S (ΔUA2S-GalNAc 4S) | 10.92 | 6.55 | 1.57 |
| Charge density | 1.11 | 1.12 | 0.99 |
| 4S/6S ratio | 29.09 | 7.40 | 2.23 |
| Molecular weight (kDa) | 37.85 | 34.41 | 46.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikha, S.B.; Bougatef, H.; Capitani, F.; Ben Amor, I.; Maccari, F.; Gargouri, J.; Sila, A.; Volpi, N.; Bougatef, A. Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone). Foods 2023, 12, 3887. https://doi.org/10.3390/foods12213887
Chikha SB, Bougatef H, Capitani F, Ben Amor I, Maccari F, Gargouri J, Sila A, Volpi N, Bougatef A. Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone). Foods. 2023; 12(21):3887. https://doi.org/10.3390/foods12213887
Chicago/Turabian StyleChikha, Sawssen Ben, Hajer Bougatef, Federica Capitani, Ikram Ben Amor, Francesca Maccari, Jalel Gargouri, Assaad Sila, Nicola Volpi, and Ali Bougatef. 2023. "Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone)" Foods 12, no. 21: 3887. https://doi.org/10.3390/foods12213887
APA StyleChikha, S. B., Bougatef, H., Capitani, F., Ben Amor, I., Maccari, F., Gargouri, J., Sila, A., Volpi, N., & Bougatef, A. (2023). Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone). Foods, 12(21), 3887. https://doi.org/10.3390/foods12213887








