Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Microorganisms and Starter Culture Preparation
2.3. Plant-Based Fermentations
2.3.1. Pretreatment of Pulse Materials
2.3.2. Fermentations
2.4. Microbiological Analyses
2.5. Chemical Analyses
2.5.1. Determination of Galacto-Oligosaccharides
2.5.2. Determination of Vicine and Convicine
2.5.3. Determination of Amino Acids
2.6. Sensory Analyses
2.7. Statistical Analyses
3. Results and Discussion
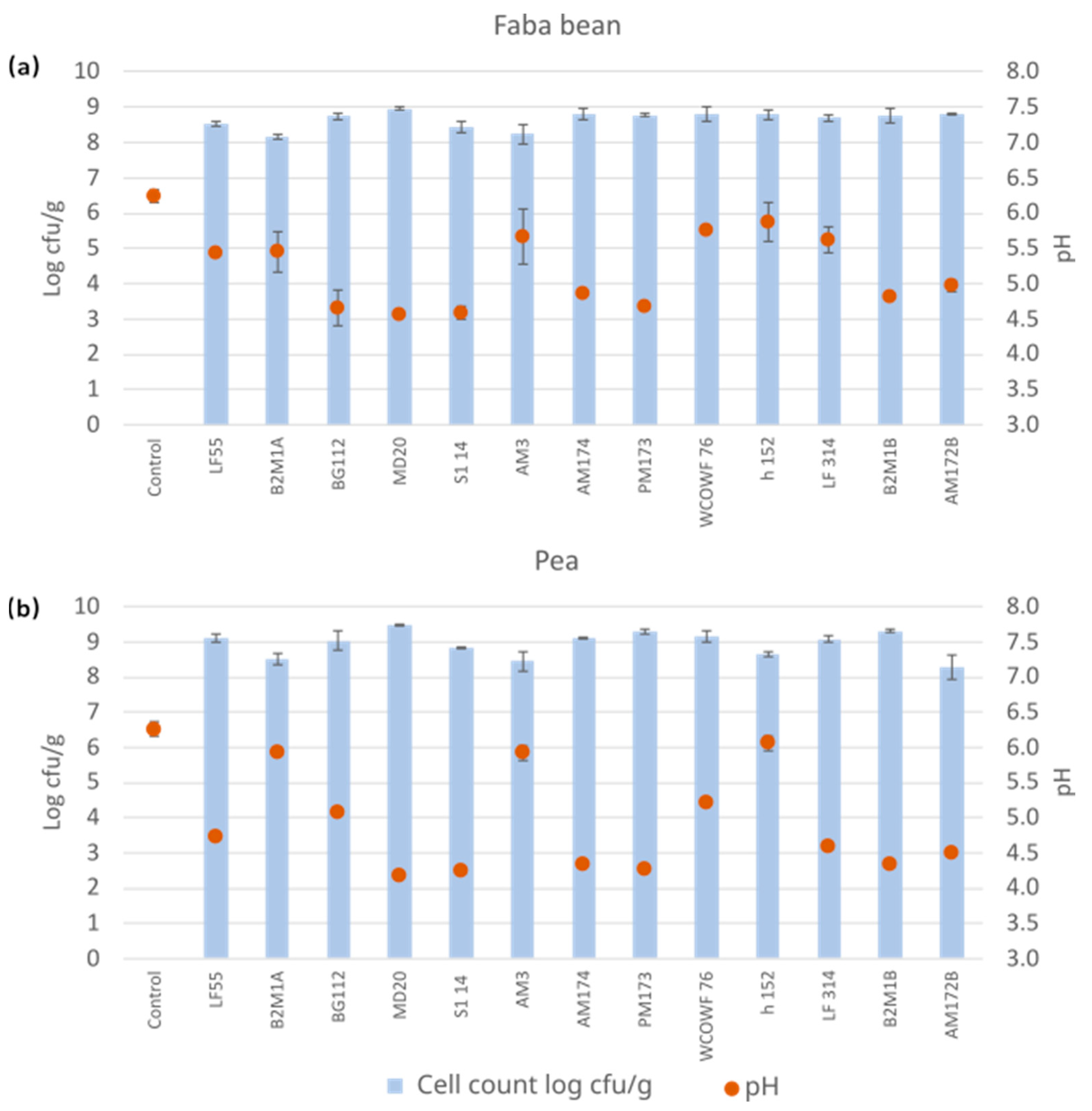
3.1. Cultivations Using a Set of Single LAB Strains
3.2. Antinutrient Levels after Fermentation with Single LAB Strains
3.2.1. Galacto-Oligosaccharides (GOS)
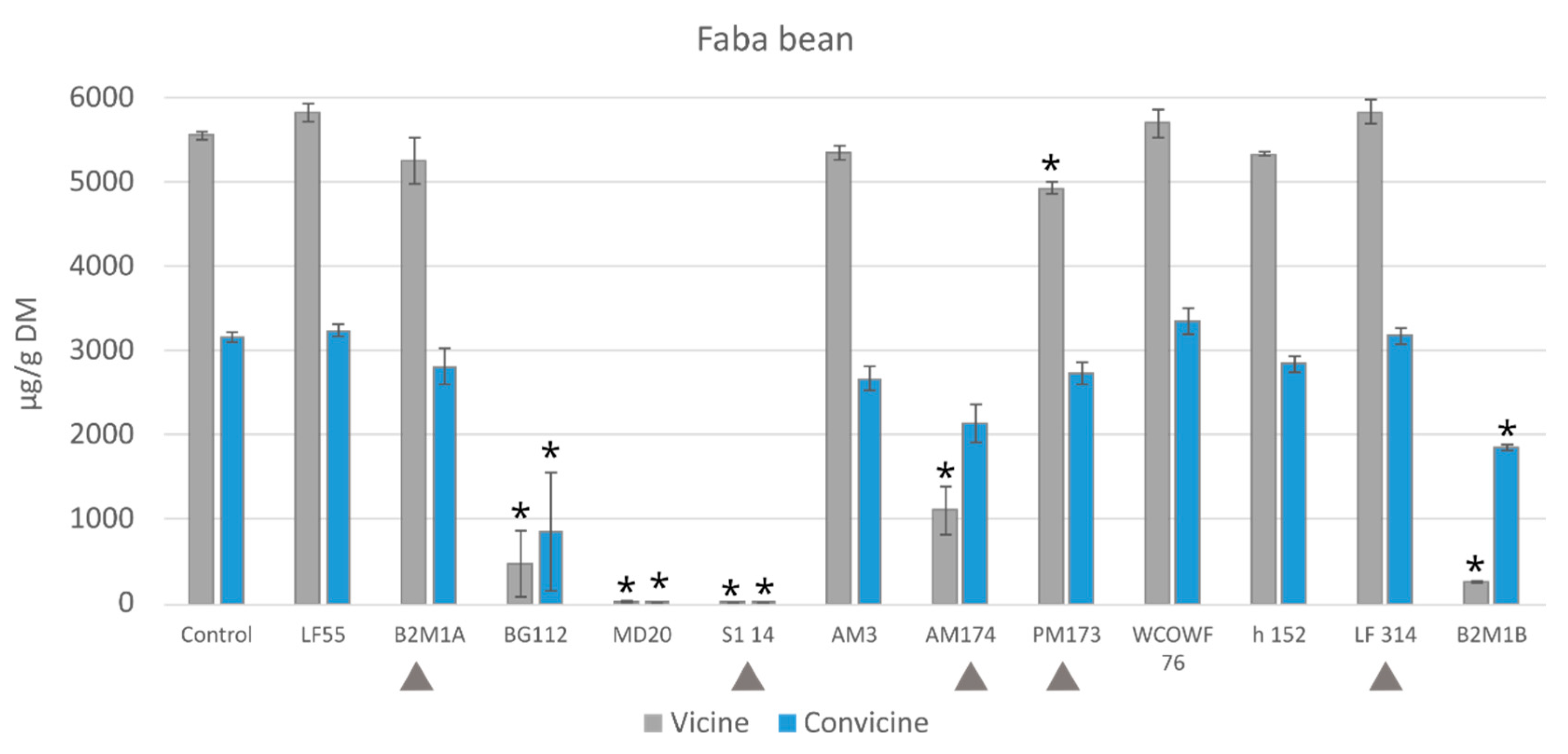
3.2.2. Vicine and Convicine
3.3. Cultivations with the Selected Microbial Mixtures
3.4. Antinutrient Levels after Fermentation with Microbial Starter Mixtures
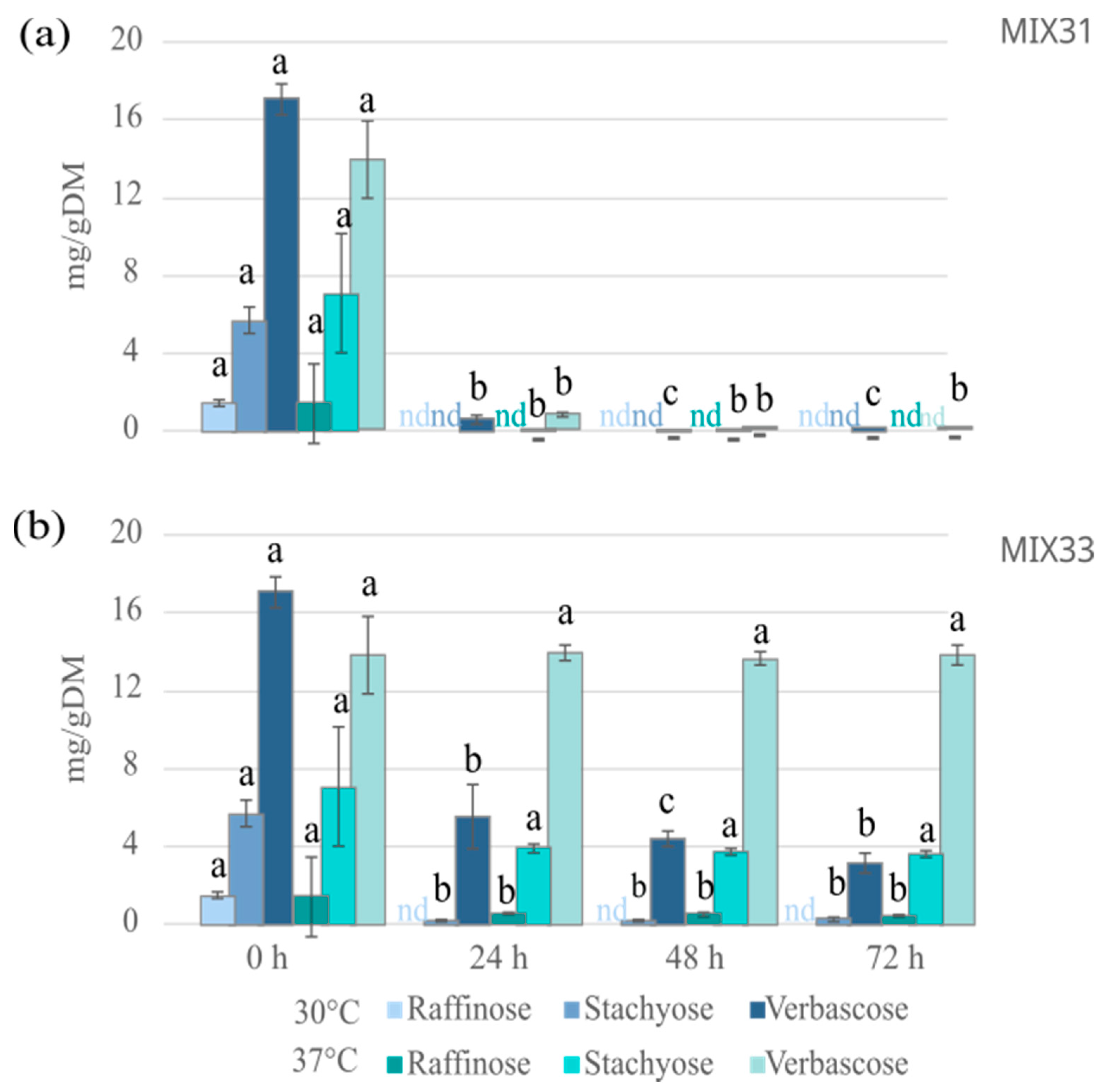
3.4.1. Effect of the Microbial Mixtures on the Level of GOS
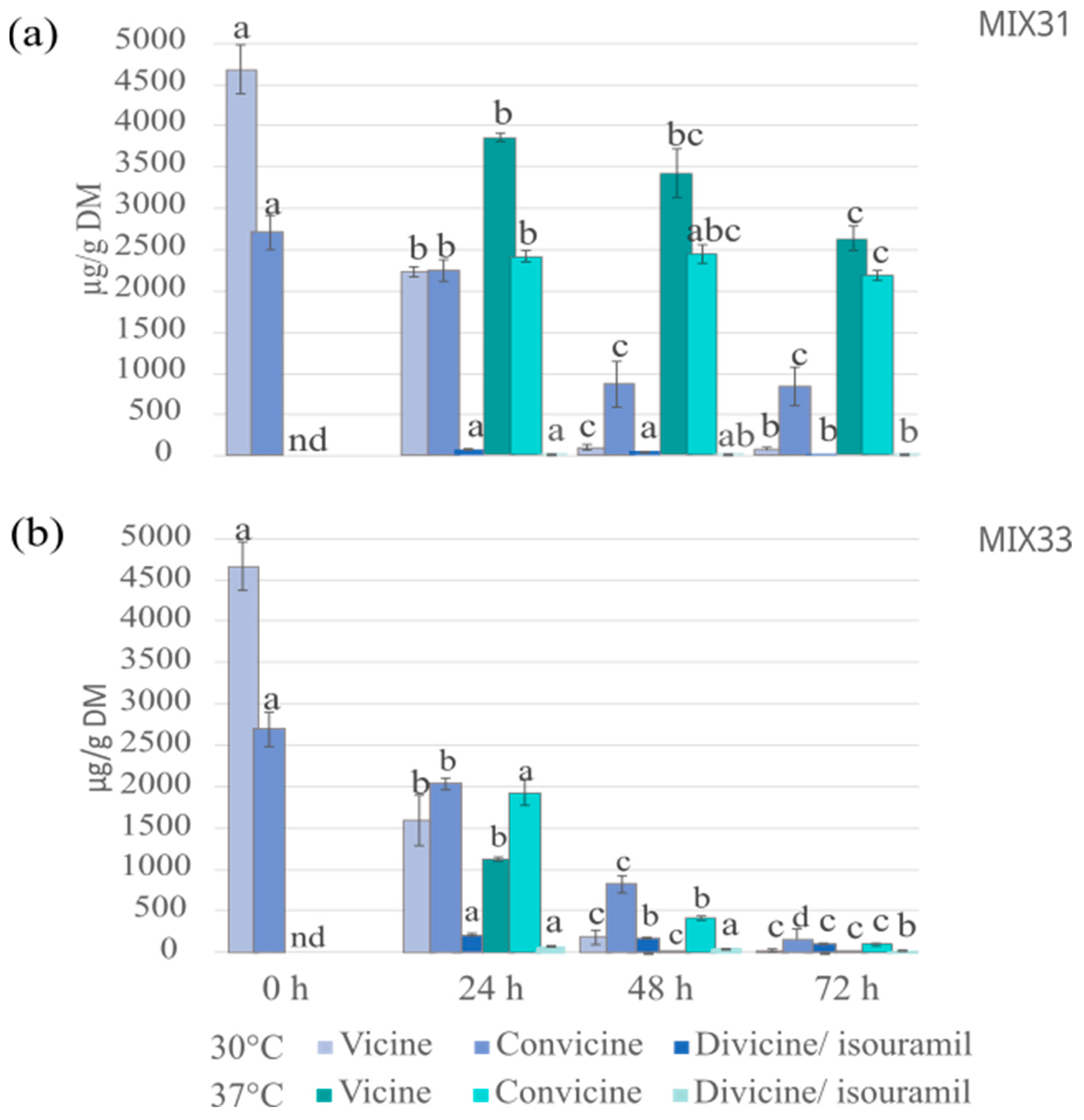
3.4.2. Vicine and Convicine Levels after Fermentation with Microbial Mixtures
3.5. Amino Acid Composition
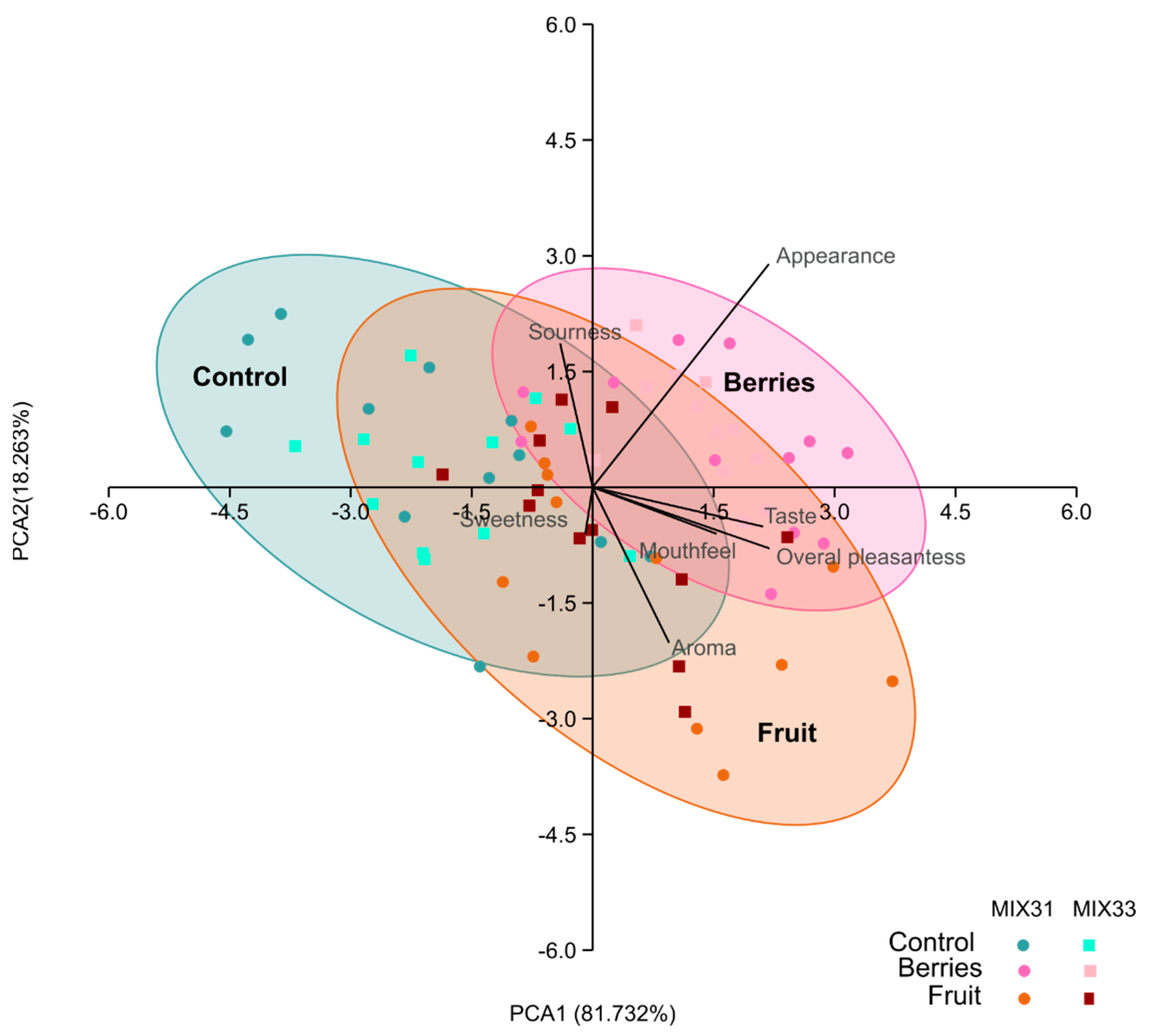
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sozer, N.; Holopainen-Mantila, U.; Poutanen, K. Traditional and New Food Uses of Pulses. Cereal Chem. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Parveen, S.; Jamil, A.; Pasha, I.; Ahmad, F.; Parveen, S.; Jamil, A.; Pasha, I.; Ahmad, F. Pulses: A Potential Source of Valuable Protein for Human Diet. In Legumes Research-Volume 2; IntechOpen: London, UK, 2022; ISBN 978-1-80356-915-4. [Google Scholar]
- Kumar, S.; Pandey, G. Biofortification of Pulses and Legumes to Enhance Nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef] [PubMed]
- Curran, J. The Nutritional Value and Health Benefits of Pulses in Relation to Obesity, Diabetes, Heart Disease and Cancer. Br. J. Nutr. 2012, 108, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A. Proteins in Pulses. J. Nutr. Disord. Ther. 2017, 7, e129. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Neufeld, J.; Frohlich, P.; Young, G.; Malcolmson, L.; House, J.D. Determination of the Protein Quality of Cooked Canadian Pulses. Food Sci. Nutr. 2017, 5, 896–903. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef]
- Bonke, A.; Sieuwerts, S.; Petersen, I.L. Amino Acid Composition of Novel Plant Drinks from Oat, Lentil and Pea. Foods 2020, 9, 429. [Google Scholar] [CrossRef]
- Patterson, C.A.; Curran, J.; Der, T. Effect of Processing on Antinutrient Compounds in Pulses. Cereal Chem. 2017, 94, 2–10. [Google Scholar] [CrossRef]
- Parca, F.; Koca, Y.O.; Unay, A. Nutritional and Antinutritional Factors of Some Pulses Seed and Their Effects on Human Health. Int. J. Second. Metab. 2018, 5, 331–342. [Google Scholar] [CrossRef]
- Hoehnel, A.; Bez, J.; Sahin, A.W.; Coffey, A.; Arendt, E.K.; Zannini, E. Leuconostoc Citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods 2020, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive Compounds in Legumes: Pronutritive and Antinutritive Actions. Implications for Nutrition and Health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef]
- Gutierrez, N.; Avila, C.M.; Duc, G.; Marget, P.; Suso, M.J.; Moreno, M.T.; Torres, A.M. CAPs Markers to Assist Selection for Low Vicine and Convicine Contents in Faba Bean (Vicia Faba L.). Theor. Appl. Genet. 2006, 114, 59–66. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 152/2009 of 27 January 2009 Laying down the Methods of Sampling and Analysis for the Official Control of Feed (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R0152-20170524&from=EL (accessed on 25 October 2023).
- ISO 13903:2005(En), Animal Feeding Stuffs—Determination of Amino Acids Content. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:13903:ed-1:v1:en (accessed on 25 October 2023).
- Abdel-Gawad, A.S. Effect of Domestic Processing on Oligosaccharide Content of Some Dry Legume Seeds. Food Chem. 1993, 46, 25–31. [Google Scholar] [CrossRef]
- Verni, M.; De Mastro, G.; De Cillis, F.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Bacteria Fermentation to Exploit the Nutritional Potential of Mediterranean Faba Bean Local Biotypes. Food Res. Int. 2019, 125, 108571. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of Indigenous Pediococcus Pentosaceus, Leuconostoc Kimchii, Weissella Cibaria and Weissella Confusa for Faba Bean Bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef]
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Nutritional and Functional Effects of the Lactic Acid Bacteria Fermentation on Gelatinized Legume Flours. Int. J. Food Microbiol. 2020, 316, 108426. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating Vicine and Convicine, the Main Anti-Nutritional Factors Restricting Faba Bean Usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Coda, R.; Lampi, A.-M.; Varis, J.; Katina, K.; Piironen, V. Possibilities of Reducing Amounts of Vicine and Convicine in Faba Bean Suspensions and Sourdoughs. Eur. Food Res. Technol. 2019, 245, 1507–1518. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of Air Classification and Fermentation by Lactobacillus Plantarum VTT E-133328 on Faba Bean (Vicia faba L.) Flour Nutritional Properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of Vicine, Convicine and Their Aglycones during Fermentation of Faba Bean Flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Maya-Ocaña, K.; Ortiz-Moreno, A.; Herrera-Cabrera, B.E.; Dávila-Ortiz, G.; Múzquiz, M.; Martín-Pedrosa, M.; Burbano, C.; Cuadrado, C.; Jiménez-Martínez, C. Effect of Roasting and Boiling on the Content of Vicine, Convicine and L-3,4-Dihydroxyphenylalanine in Vicia Faba L. J. Food Qual. 2012, 35, 419–428. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Verni, M.; Koivula, H.; Montemurro, M.; Seppa, L.; Kemell, M.; Katina, K.; Coda, R.; Gobbetti, M. Influence of Fermented Faba Bean Flour on the Nutritional, Technological and Sensory Quality of Fortified Pasta. Food Funct. 2017, 8, 860–871. [Google Scholar] [CrossRef]



| Strain | Species | Origin |
|---|---|---|
| LF55 (Sacco) | Limosilactobacillus fermentum (L. fermentum) | Sacco Srl, Italy |
| B2M1A (Luke) | Levilactobacillus brevis (L. brevis) | Isolated from pulses |
| BG112 (Sacco) | Lactiplantibacillus plantarum (L. plantarum) | Sacco Srl, Italy |
| MD20 (Luke) | Lactiplantibacillus plantarum (L. plantarum) | Isolated from cereals |
| S1 14 (Luke) | Lactiplantibacillus plantarum (L. plantarum) | Isolated from sourdough |
| AM3 (Luke) | Weissella cibaria (W. cibaria) | Isolated from pulses |
| AM174 (Luke) | Pediococcus pentosaceus (P. pentosaceus) | Isolated from pulses |
| PM173 (Luke) | Leuconostoc mesenteroides (Le. mesenteroides) | Isolated from pulses |
| WCOWF 76 (NMBU) | Weissella confusa (W. confusa) | Isolated from soybean/soybean–maize blends |
| h 152 (NMBU) | Levilactobacillus brevis (L. brevis) | Isolated from soybean/soybean–maize blends |
| LF314 (NMBU) | Limosilactobacillus fermentum (L. fermentum) | Isolated from soybean/soybean–maize blends |
| B2M1B (Luke) | Lactococcus lactis (Lc. lactis) | Isolated from pulses |
| AM172B (Luke) | Lactococcus lactis subsp. lactis (Lc. lactis subsp. lactis) | Isolated from pulses |
| Consortium | Strains | Genus and Species |
|---|---|---|
| MIX31 | B2M1A | Levilactobacillus brevis |
| AM174 | Pediococcus pentosaceus | |
| LF 314 | Limosilactobacillus fermentum | |
| MIX33 | S1 14 | Lactiplantibacillus plantarum |
| PM173 | Leuconostoc mesenteroides | |
| B2M1A | Levilactobacillus brevis |
| Faba Bean | Oat | Control FB:Oat | MIX31 | MIX33 | |
|---|---|---|---|---|---|
| Essential amino acids (EAA) | |||||
| Cysteine + Cystine | 0.3 ± 0.01 | 0.50 ± 0.01 | 0.4 ± 0.06 | 0.38 ± 0.02 | 0.41 ± 0.06 |
| Histidine | 0.84 ± 0.02 | 0.35 ± 0.01 | 0.67 ± 0.00 | 0.69 ± 0.00 | 0.72 ± 0.05 |
| Isoleucine | 1.26 ± 0.03 | 0.58 ± 0.01 | 1.01 ± 0.01 | 1.02 ± 0.03 | 1.05 ± 0.01 |
| Leucine | 2.31 ± 0.05 | 1.18 ± 0.02 | 1.90 ± 0.07 | 1.94 ± 0.11 | 1.97 ± 0.05 |
| Lysine | 1.98 ± 0.05 | 0.67 ± 0.01 | 1.57 ± 0.09 | 1.60 ± 0.13 | 1.64 ± 0.02 |
| Methionine | 0.21 ± 0.01 | 0.37 ± 0.01 | 0.24 ± 0.02 | 0.25 ± 0.01 | 0.23 ± 0.05 |
| Phenylalanine | 1.26 ± 0.03 | 0.81 ± 0.01 | 1.08 ± 0.00 | 1.13 ± 0.02 | 1.17 ± 0.05 |
| Threonine | 1.16 ± 0.03 | 0.57 ± 0.01 | 0.97 ± 0.05 | 0.96 ± 0.11 | 1.02 ± 0.02 |
| Tryptophan | 0.32 ± 0.01 | 0.23 ± 0.00 | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.23 ± 0.01 |
| Tyrosine | 1.06 ± 0.03 | 0.62 ± 0.01 | 0.86 ± 0.86 | 0.88 ± 0.88 | 0.94 ± 0.94 |
| Valine | 1.41 ± 0.03 | 0.8 ± 0.01 | 1.19 ± 0.07 | 1.26 ± 0.03 | 1.26 ± 0.04 |
| Non-essential amino acids | |||||
| Alanine | 1.29 ± 0.03 | 0.75 ± 0.01 | 1.08 ± 0.06 | 1.13 ± 0.05 | 1.14 ± 0.05 |
| Arginine | 3.38 ± 0.08 | 1.15 ± 0.02 | 2.27 ± 0.36 | 2.07 ± 0.02 | 2.34 ± 0.06 |
| Aspartic acid | 3.34 ± 0.08 | 1.32 ± 0.02 | 2.68 ± 0.11 | 2.68 ± 0.23 | 2.82 ± 0.18 |
| Glutamic acid | 5.24 ± 0.12 | 3.26 ± 0.06 | 4.39 ± 0.27 | 4.52 ± 0.30 | 4.52 ± 0.13 |
| Glycine | 1.33 ± 0.03 | 0.79 ± 0.01 | 1.12 ± 0.07 | 1.18 ± 0.05 | 1.19 ± 0.04 |
| Proline | 1.26 ± 0.03 | 0.79 ± 0.01 | 1.08 ± 0.13 | 1.20 ± 0.01 | 1.26 ± 0.05 |
| Serine | 1.53 ± 0.04 | 0.83 ± 0.02 | 1.27 ± 0.06 | 1.23 ± 0.17 | 1.31 ± 0.05 |
| Total AA | 29.47 | 15.57 | 24.04 | 24.38 | 25.23 |
| Total EAA | 12.11 | 6.68 | 10.15 | 10.38 | 10.65 |
| Affective Test | JAR Test | ||||||
|---|---|---|---|---|---|---|---|
| Appearance | Aroma | Taste | Mouthfeel | Overall Pleasantness | Sourness | Sweetness | |
| Control MIX31 | 4.1 ± 0.2 a | 3.8 ± 0.3 a | 3.3 ± 0.4 a | 4.3 ± 0.4 abcd | 3.6 ± 0.3 a | 3.7 ± 0.3 ab | |
| Control MIX33 | 4.2 ± 0.2 a | 3.8 ± 0.3 a | 3.6 ± 0.3 ac | 4.3 ± 0.3 a | 3.6 ± 0.3 a | 3.2 ± 0.3 abc | |
| MIX31 berries | 6.0 ± 0.2 b | 4.3 ± 0.3 abc | 5.3 ± 0.3 bc | 5.4 ± 0.2 bcd | 5.3 ± 0.3 b | 3.2 ± 0.2 ab | 2.8 ± 0.2 |
| MIX33 berries | 6.2 ± 0.2 b | 4.4 ± 0.3 abc | 4.8 ± 0.3 bc | 5.4 ± 0.2 bcd | 5.2 ± 0.2 b | 3.3 ± 0.2 ac | 2.6 ± 0.2 |
| MIX31 fruit | 4.4 ± 0.3 a | 5.5 ± 0.4 b | 4.9 ± 0.4 c | 5.4 ± 0.2 bcd | 5.1 ± 0.4 b | 2.6 ± 0.2 bc | 2.9 ± 0.2 |
| MIX33 fruit | 4.6 ± 0.3 a | 4.2 ± 0.3 ac | 4.6 ± 0.3 c | 5.1 ± 0.2 bcd | 4.9 ± 0.3 b | 2.7 ± 0.2 bc | 3.0 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahala, M.; Ikonen, I.; Blasco, L.; Bragge, R.; Pihlava, J.-M.; Nurmi, M.; Pihlanto, A. Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product. Foods 2023, 12, 3922. https://doi.org/10.3390/foods12213922
Kahala M, Ikonen I, Blasco L, Bragge R, Pihlava J-M, Nurmi M, Pihlanto A. Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product. Foods. 2023; 12(21):3922. https://doi.org/10.3390/foods12213922
Chicago/Turabian StyleKahala, Minna, Iida Ikonen, Lucia Blasco, Rina Bragge, Juha-Matti Pihlava, Markus Nurmi, and Anne Pihlanto. 2023. "Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product" Foods 12, no. 21: 3922. https://doi.org/10.3390/foods12213922
APA StyleKahala, M., Ikonen, I., Blasco, L., Bragge, R., Pihlava, J.-M., Nurmi, M., & Pihlanto, A. (2023). Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product. Foods, 12(21), 3922. https://doi.org/10.3390/foods12213922