Physicochemical Characteristics, Antioxidant Properties, Aroma Profile, and Sensory Qualities of Value-Added Wheat Breads Fortified with Post-Distillation Solid Wastes of Aromatic Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals and Reagents
2.3. Breadmaking Procedure
2.4. Physicochemical Parameters of Breads
2.5. Surface Coverage of Breads by Mold during Storage
2.6. Preparation of Free and Bound Phenolic Extracts
2.7. Spectroscopic Determination of Total Phenolics, Total Flavonoids, and Antioxidant Activity of Fortified Breads with SWAP
2.7.1. Determination of Total Phenolic Content (TPC)
2.7.2. Determination of Total Flavonoid Content (TFC)
2.7.3. ABTS Radical Scavenging Assay
2.7.4. DPPH Radical Scavenging Assay
2.7.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. HPLC-DAD-MS Analysis of Phenolic Compounds
2.9. Aroma Profile Analysis Using SPME-GC/MS
2.10. Sensory Analysis
2.11. Statistical Data Analysis
3. Results and Discussion
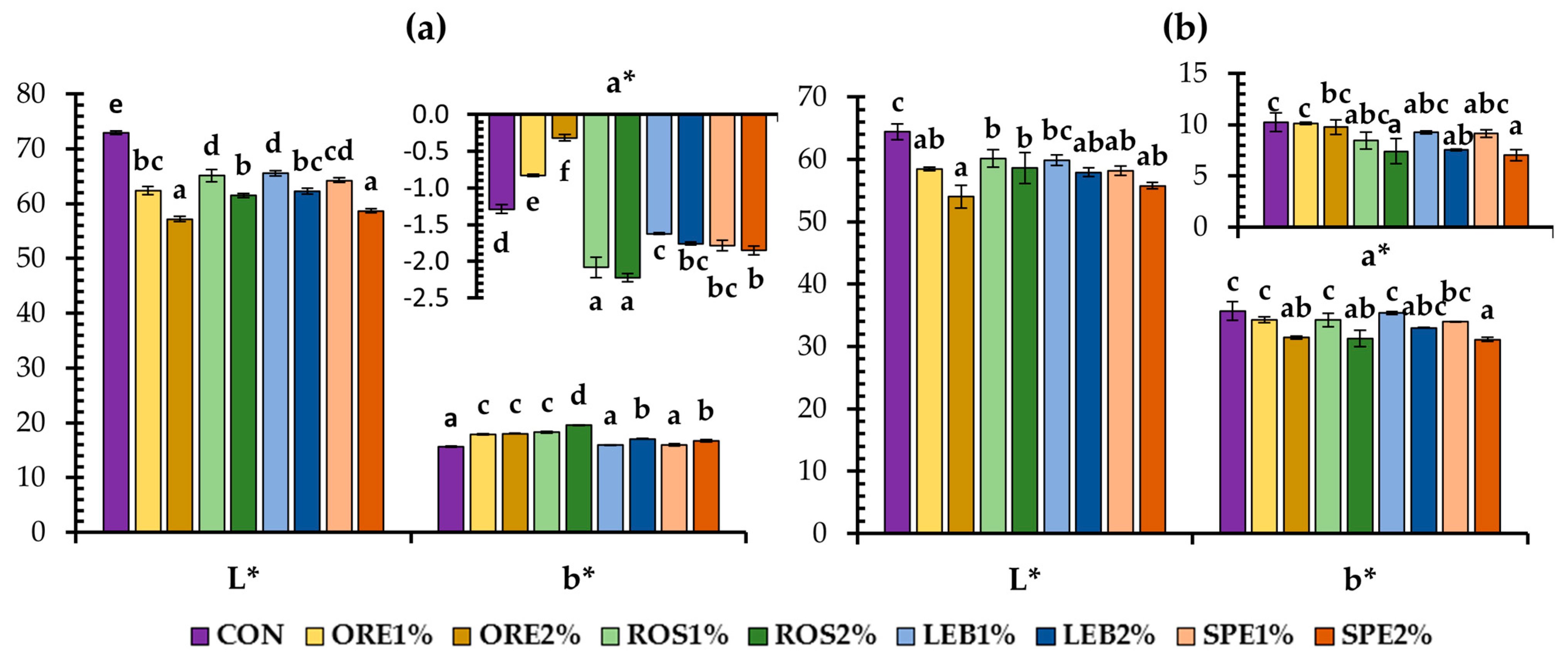
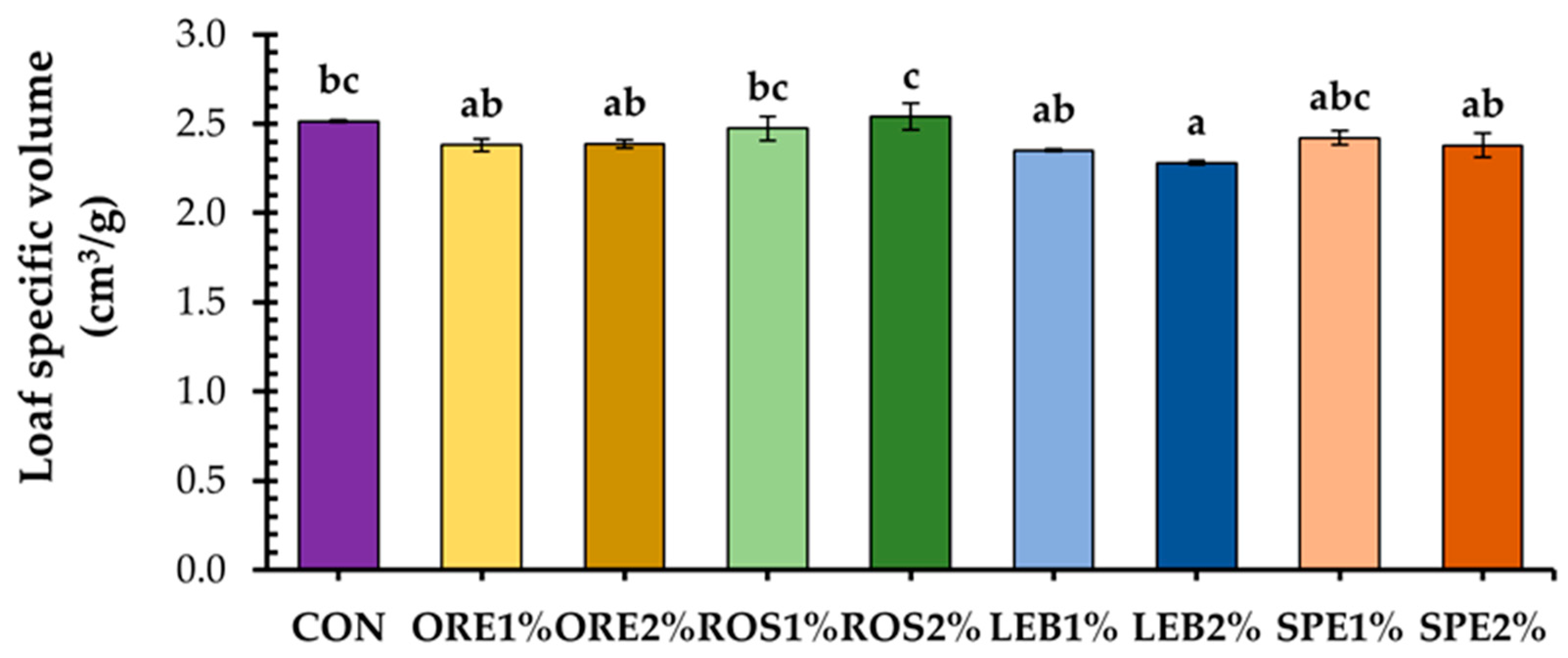
3.1. Physical Characteristics of Fortified Wheat Breads with SWAP
3.2. Shelf-Life of Wheat Breads Fortified with SWAP
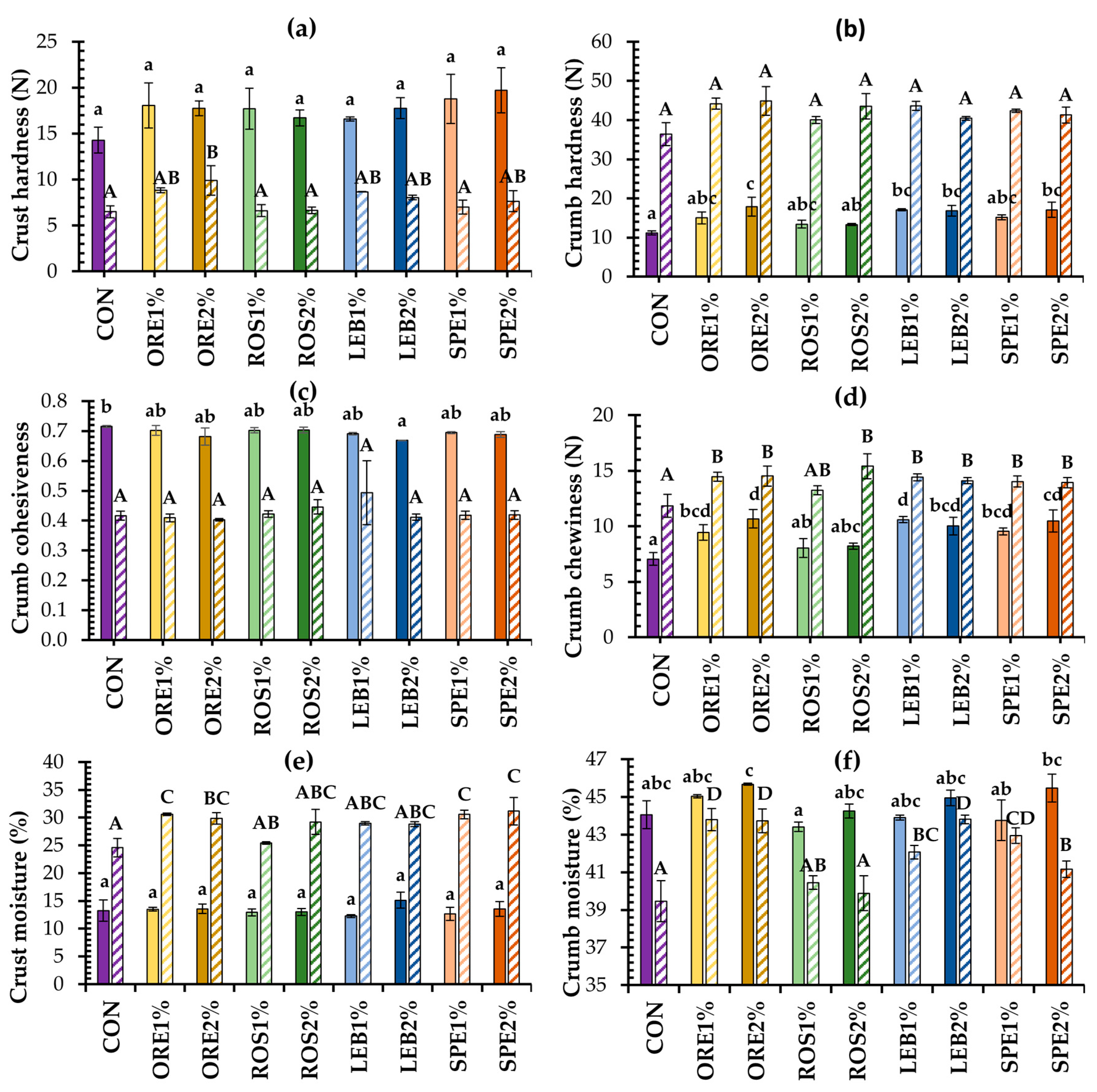
3.2.1. Changes in Bread Texture
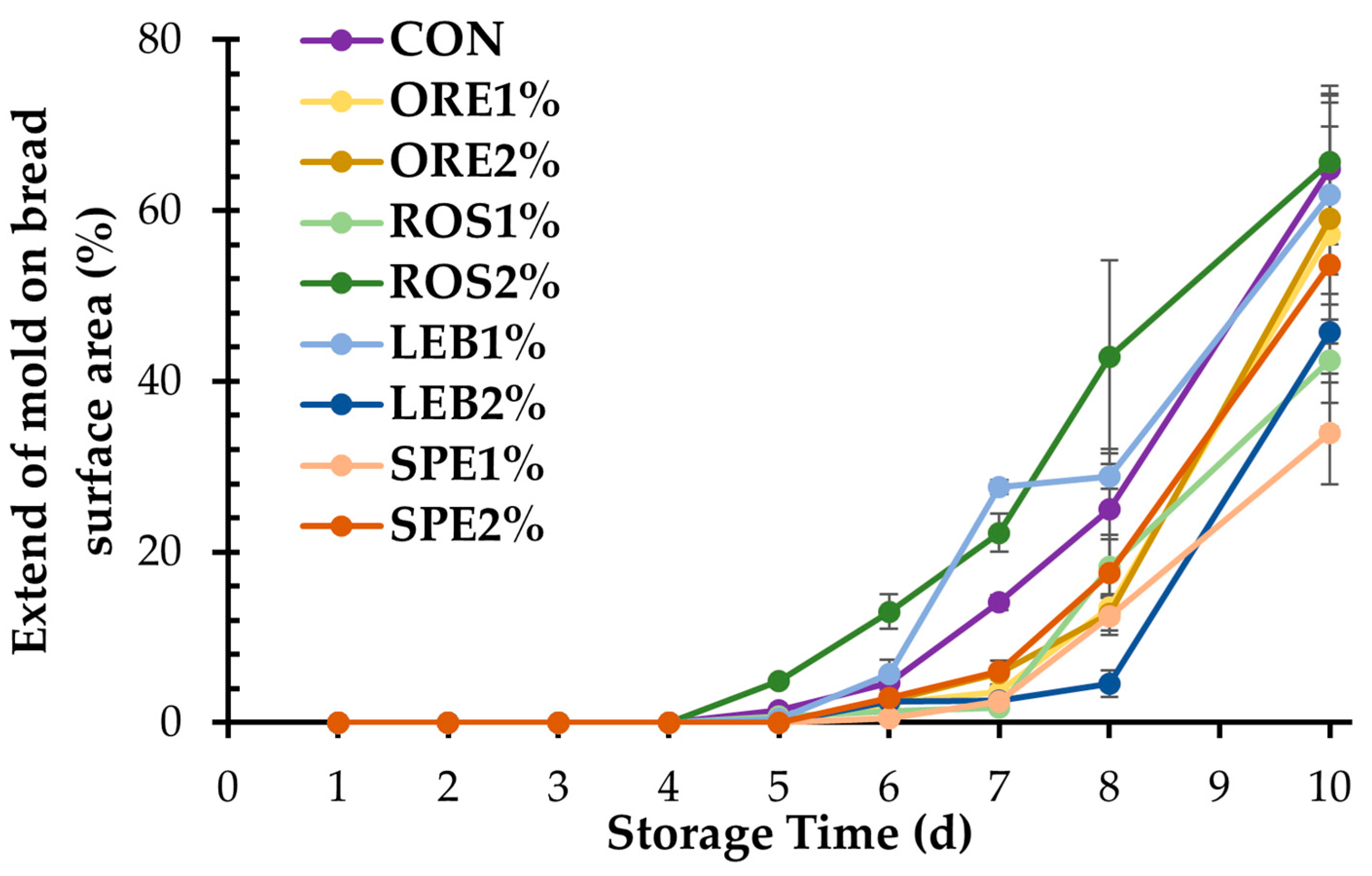
3.2.2. Mold Growth in Breads
3.3. TPC, TFC, and Antioxidant Activity of SWAP and Fortified Wheat Breads
3.4. Phenolic Profiles of SWAP Materials and Fortified Wheat Breads
3.5. Aroma Profile of Fortified Wheat Breads with SWAP
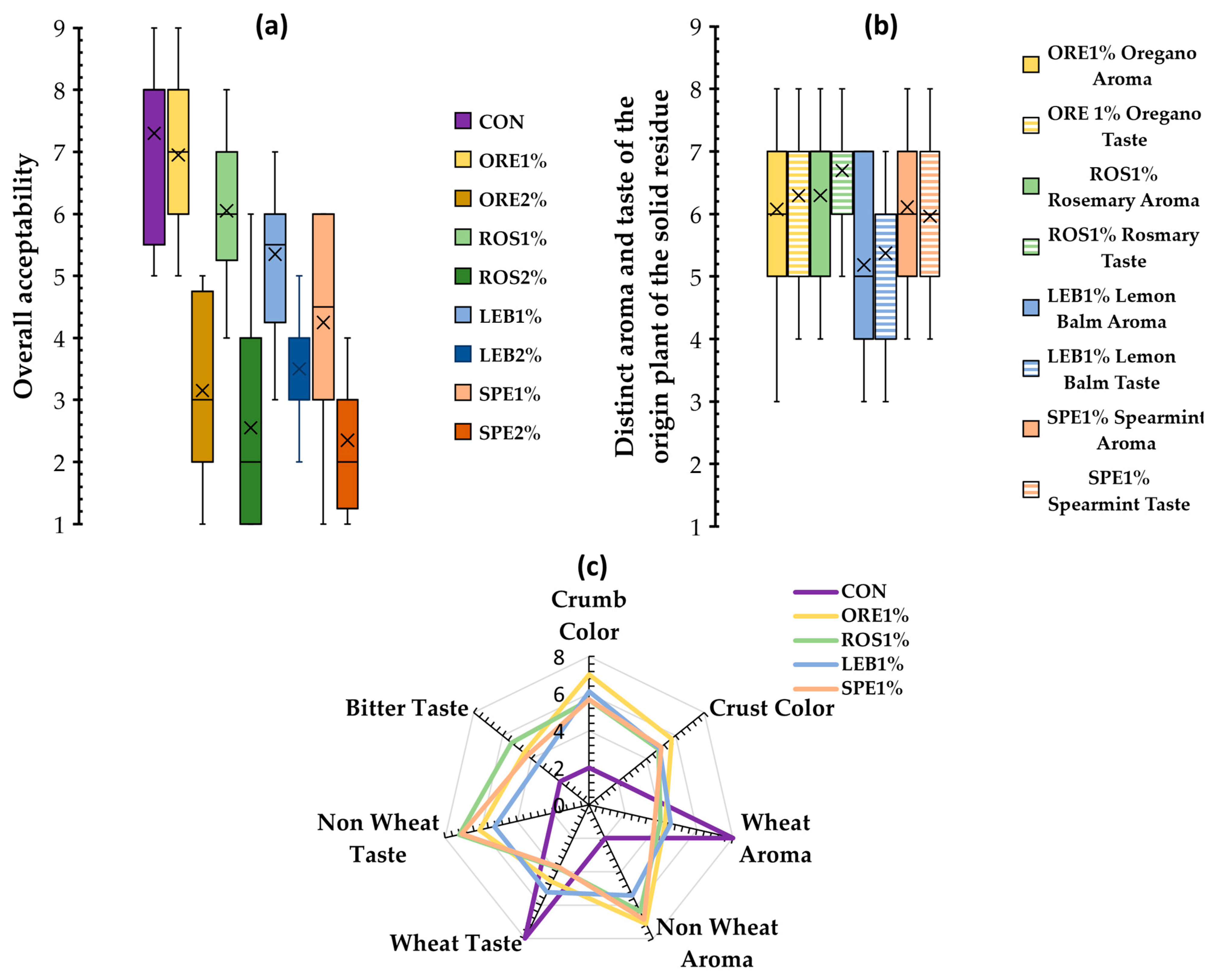
3.6. Sensory Analysis of Fortified Wheat Breads with SWAP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins, and carbohydrates—Influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-González, Y.A.; Alvarez-Parrilla, E.; del Rocío Martínez-Ruiz, N.; Vázquez-Flores, A.A.; Gaytán-Martínez, M.; de la Rosa, L.A. Addition of phenolic compounds to bread: Antioxidant benefits and impact on food structure and sensory characteristics. Food Prod. Process. Nutr. 2021, 3, 25. [Google Scholar] [CrossRef]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Amoah, I.; Taarji, N.; Johnson, P.-N.T.; Barrett, J.; Cairncross, C.; Rush, E. Plant-based food by-products: Prospects for valorisation in functional bread development. Sustainability 2020, 12, 7785. [Google Scholar] [CrossRef]
- Rahaie, S.; Gharibzahedi, S.M.T.; Razavi, S.H.; Jafari, S.M. Recent developments on new formulations based on nutrient-dense ingredients for the production of healthy-functional bread: A review. J. Food Sci. Technol. 2014, 51, 2896–2906. [Google Scholar] [CrossRef]
- Amoah, I.; Cairncross, C.; Osei, E.O.; Yeboah, J.A.; Cobbinah, J.C.; Rush, E. Bioactive properties of bread formulated with plant-based functional ingredients before consumption and possible links with health outcomes after consumption—A review. Plant Foods Hum. Nutr. 2022, 77, 329–339. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Bouloumpasi, E.; Biliaderis, C.G. Phenolic extracts from solid wastes of the aromatic plant essential oil industry: Potential uses in food applications. Food Chem. Adv. 2022, 1, 100065. [Google Scholar] [CrossRef]
- Filipčev, B. Chapter 16—The effects of aromatic plants and their extracts in food products. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 279–294. [Google Scholar] [CrossRef]
- Skendi, A.; Katsantonis, D.Ν.; Chatzopoulou, P.; Irakli, M.; Papageorgiou, M. Antifungal activity of aromatic plants of the Lamiaceae family in bread. Foods 2020, 9, 1642. [Google Scholar] [CrossRef]
- Dhillon, G.; Kaur, A.; Bhise, S.; Ahluwalia, P. Synergistic effect of spices and herbs on rheological and bread making properties of wheat flour. J. Pure Appl. Microbiol. 2016, 10, 1099–1107. [Google Scholar]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem. 2019, 43, e13020. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crops Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crops Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Marcelino, S.; Gaspar, P.D.; Paço, A. Sustainable waste management in the production of medicinal and aromatic plants—A systematic review. Sustainability 2023, 15, 13333. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Antibacterial and antioxidant properties of oregano and rosemary essential oil distillation by-products. Biol. Life Sci. Forum. 2021, 6, 47. [Google Scholar] [CrossRef]
- Sui, X.; Yap, P.Y.; Zhou, W. Anthocyanins during baking: Their degradation kinetics and impacts on color and antioxidant capacity of bread. Food Bioproc. Techn. 2015, 8, 983–994. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of volatile compounds of lupin protein isolate-enriched wheat flour bread. Food Res. Intern. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Jensen, S.; Ostdal, H.; Skibsted, L.H.; Thybo, A.K. Antioxidants and shelf life of whole wheat bread. J. Cereal Sci. 2011, 53, 291–297. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Piechowska, P.; Waśkiewicz, A.; Zawirska-Wojtasiak, R. Effects of polyphenols on volatile profile and acrylamide formation in a model wheat bread system. Food Chem. 2019, 297, 125008. [Google Scholar] [CrossRef] [PubMed]
- AACC International 44-15.02 (Moisture Content) Methods. In Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists International: St. Paul, MN, USA, 2010.
- Nouska, C.; Hatzikamari, M.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Enhancement of textural and sensory characteristics of wheat bread using a chickpea sourdough fermented with a selected autochthonous microorganism. Foods 2023, 12, 3112. [Google Scholar] [CrossRef] [PubMed]
- Armero, E.; Collar, C. Texture properties of formulated wheat doughs—Relationships with dough and bread technological quality. Z. Leb. Forsch. A 1997, 204, 136–145. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Bao, J.S.; Cai, Y.; Sun, M.; Wang, G.Y.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–23. [Google Scholar] [CrossRef]
- Das, L.; Raychaudhuri, U.; Chakraborty, R. Herbal fortification of bread with fennel seeds. Food Techn. Biotechn. 2013, 51, 434–440. [Google Scholar]
- Kotsiou, K.; Sacharidis, D.D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 2022, 124, 107322. [Google Scholar] [CrossRef]
- Baik, M.-Y.; Chinachoti, P. Moisture redistribution and phase transitions during bread staling. Cereal Chem. 2000, 77, 484–488. [Google Scholar] [CrossRef]
- Gray, J.A.; Bemiller, J.N. Bread staling: Molecular basis and control. Compr. Rev. Food Sci. F 2003, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ronda, F.; Roos, Y.H. Staling of fresh and frozen gluten-free bread. J. Cereal Sci. 2011, 53, 340–346. [Google Scholar] [CrossRef]
- Kotsiou, K.; Palassaros, G.; Matsakidou, A.; Mouzakitis, C.K.; Biliaderis, C.G.; Lazaridou, A. Roasted-sprouted lentil flour as a novel ingredient for wheat flour substitution in breads: Impact on dough properties and quality attributes. Food Hydrocoll. 2023, 145, 109164. [Google Scholar] [CrossRef]
- Algboory, H.L.; Kadum, H.; Muhialdin, B.J. Shelf-life assessment of bread containing Cyperus rotundus rhizome aqueous extract with antimicrobial compounds identified by 1H-NMR. LWT 2021, 140, 110823. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Intern. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef]
- Alice, G.; Bubueanu, C.; Pirvu, L.; Sultana, N.; Bazdoaca, C.; Dobre, N. Polyphenol content dynamics in hydrodistillation water residues of Lamiaceae species. J. Essent. Oil-Bear. Plants 2019, 22, 858–864. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Rabalski, I. Changes in phenolic acids and antioxidant properties during baking of bread and muffin made from blends of hairless canary seed, wheat, and corn. Antioxidants 2022, 11, 1059. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Phenolic compounds during production of bread from purple wheat grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.Y.; Perera, C.O. Properties of bread dough with added fibre polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saltivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Rahman, M.J.; Malunga, L.N.; Eskin, M.; Eck, P.; Thandapilly, S.J.; Thiyam-Hollander, U. Valorization of heat-treated brewers’ spent grain through the identification of bioactive phenolics by UPLC-PDA and evaluation of their antioxidant activities. Front. Nutr. 2021, 8, 634519. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Bryngelsson, S.; Dimberg, L.H.; Kamal-Eldin, A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.). J. Agric. Food Chem. 2002, 50, 1890–1896. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Quílez, J.; Ruiz, J.A.; Romero, M.P. Relationships between sensory flavor evaluation and volatile and nonvolatile compounds in commercial wheat bread type baguette. J. Food Sci. 2006, 71, S423–S427. [Google Scholar] [CrossRef]
- De Luca, L.; Aiello, A.; Pizzolongo, F.; Blaiotta, G.; Aponte, M.; Romano, R. Volatile organic compounds in breads prepared with different sourdoughs. Appl. Sci. 2021, 11, 1330. [Google Scholar] [CrossRef]
- Pico, J.; Antolín, B.; Roman, L.; Gomez, M.; Bernal, J. Analysis of volatile compounds in gluten-free bread crusts with an optimised and validated SPME-GC/QTOF methodology. Food Res. Intern. 2018, 106, 686–695. [Google Scholar] [CrossRef] [PubMed]
- The Good Scents Company. Available online: http://www.thegoodscentscompany.com/ (accessed on 28 September 2023).
- Yeddes, W.; Aidi Wannes, W.; Hammami, M.; Smida, M.; Chebbi, A.; Marzouk, B.; Saidani Tounsi, M. Effect of environmental conditions on the chemical composition and antioxidant activity of essential oils from Rosmarinus officinalis L. growing wild in Tunisia. J. Essent. Oil-Bear. Plants 2018, 21, 972–986. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Müller, J. Changes of essential oil content and composition during convective drying of lemon balm (Melissa officinalis L.). Ind. Crops Prod. 2014, 52, 118–124. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Dina, E.; Vontzalidou, A.; Cheilari, A.; Bagatzounis, P.; Agapidou, E.; Giannenas, I.; Grigoriadou, K.; Aligiannis, N. Sustainable use of Greek herbs by-products, as an alternative source of biologically active ingredients for innovative products. Front. Nutr. 2022, 9, 867666. [Google Scholar] [CrossRef]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Forster, C.; Leidecker, F.; Wiesea, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2013; pp. 495–508. [Google Scholar]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Chapter 35—Carvone (Mentha spicata L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: SanDiego, CA, USA, 2016; pp. 309–316. [Google Scholar] [CrossRef]
- Shori, A.B.; Kee, L.A.; Baba, A.S. Total phenols, antioxidant activity and sensory evaluation of bread fortified with spearmint. Arab. J. Sci. Eng. 2021, 46, 5257–5264. [Google Scholar] [CrossRef]


| Sample Symbol | Wheat Flour (g) | Solid Waste of Aromatic Plant (SWAP) | Water (mL) | Baker’s Yeast (g) | Salt (g) |
|---|---|---|---|---|---|
| CON | 100 | - | 58.5 | 1 | 2 |
| ORE1% | 99 | 1 g ORE | 60.0 | 1 | 2 |
| ORE2% | 98 | 2 g ORE | 61.0 | 1 | 2 |
| ROS1% | 99 | 1 g ROS | 59.5 | 1 | 2 |
| ROS2% | 98 | 2 g ROS | 60.5 | 1 | 2 |
| LEB1% | 99 | 1 g LEB | 61.5 | 1 | 2 |
| LEB2% | 98 | 2 g LEB | 62.0 | 1 | 2 |
| SPE1% | 99 | 1 g SPE | 60.0 | 1 | 2 |
| SPE2% | 98 | 2 g SPE | 62.0 | 1 | 2 |
| Fraction | Bread Sample | TPC (mg GAE/100 g Bread d.b.) | TFC (mg CATE/100 g Bread d.b.) | Antioxidant Activity (mg TE/100 g Bread d.b.) | ||
|---|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | ||||
| Free | CON | 21.8 ± 1.20a | 14.29 ± 2.18a | 26.13 ± 0.5a | 5.77 ± 0.18a | 29.95 ± 0.35a |
| ORE1% | 59.39 ± 1.16b | 23.29 ± 0.82b | 70.27 ± 5.76b | 37.25 ± 0.91b | 75.27 ± 1.02b | |
| ORE2% | 91.27 ± 1.43e | 35.65 ± 0.47c | 139.70 ± 1.64g | 66.27 ± 0.09e | 86.34 ± 1.56c | |
| ROS1% | 70.8 ± 0.69c | 38.20 ± 3.04c | 90.29 ± 0.81d | 54.42 ± 0.57d | 89.43 ± 1.99c | |
| ROS2% | 97.75 ± 2.04f | 48.29 ± 0.03d | 140.79 ± 3.06g | 99.53 ± 2.45g | 96.39 ± 0.52d | |
| LEB1% | 78.29 ± 0.89d | 53.28 ± 1.15e | 109.50 ± 2.38e | 76.26 ± 0.39f | 119.78 ± 0.43f | |
| LEB2% | 121.34 ± 2.49g | 81.54 ± 0.20f | 190.18 ± 1.96h | 131.67 ± 0.78h | 134.66 ± 1.81g | |
| SPE1% | 63.17 ± 0.35b | 38.96 ± 3.25c | 81.89 ± 1.35c | 50.18 ± 1.56c | 89.35 ± 1.32c | |
| SPE2% | 99.94 ± 3.20f | 79.92 ± 2.68f | 133.59 ± 0.08f | 100.69 ± 0.16g | 102.16 ± 0.83e | |
| Total | CON | 98.73 ± 4.71a | 26.49 ± 0.91a | 135.66 ± 12.93a | 13.63 ± 1.14a | 66.77 ± 6.11a |
| ORE1% | 144.63 ± 1.22b | 54.18 ± 2.91b | 283.83 ± 7.52c | 83.21 ± 2.20b | 191.27 ± 0.71c | |
| ORE2% | 201.03 ± 2.75cd | 92.78 ± 3.66d | 416.63 ± 14.61e | 129.78 ± 0.52d | 267.34 ± 7.08d | |
| ROS1% | 144.57 ± 2.33b | 64.08 ± 1.00c | 226.80 ± 12.08b | 119.23 ± 2.43c | 160.28 ± 10.88b | |
| ROS2% | 202.68 ± 2.80cd | 62.67 ± 2.77bc | 275.44 ± 7.93bc | 186.78 ± 7.36g | 166.21 ± 1.07b | |
| LEB1% | 210.84 ± 5.51d | 109.00 ± 4.76e | 363.75 ± 56.31d | 176.05 ± 5.78f | 287.81 ± 6.03e | |
| LEB2% | 285.5 ± 6.12f | 160.29 ± 2.56f | 593.65 ± 25.43f | 285.42 ± 8.80h | 390.99 ± 0.36f | |
| SPE1% | 197.36 ± 5.03c | 54.82 ± 8.10b | 275.67 ± 15.54bc | 87.70 ± 3.05b | 157.39 ± 0.86b | |
| SPE2% | 252.00 ± 5.60e | 103.14 ± 0.25e | 342.38 ± 8.57d | 150.32 ± 0.44e | 200.24 ± 4.16c | |
| Factor | Two-Factor ANOVA—p values | |||||
| Free | plant | 0.090 | 0.000 | 0.013 | 0.000 | 0.000 |
| level | 0.018 | 0.006 | 0.017 | 0.001 | 0.059 | |
| plant × level | 0.991 | 0.371 | 0.537 | 0.684 | 0.675 | |
| Total | plant | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| level | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| plant × level | 0.036 | 0.000 | 0.003 | 0.000 | 0.000 | |
| Analytes | ORE | ROS | LEB | SPE |
|---|---|---|---|---|
| CA | 0.25 ± 0.00 | 0.28 ± 0.06 | 0.28 ± 0.03 | 0.25 ± 0.03 |
| RMA | 17.42 ± 0.81 | 21.29 ± 0.47 | 54.10 ± 2.26 | 25.02 ± 2.98 |
| SALB | 4.80 ± 0.03 | - | - | 0.19 ± 0.02 |
| SALA | 1.11 ± 0.07 | 0.90 ± 0.05 | 0.32 ± 0.03 | 2.56 ± 0.09 |
| LITHA | - | - | 5.82 ± 0.46 | 0.24 ± 0.01 |
| SALI | - | - | - | 4.04 ± 0.13 |
| Total PAs | 23.58a | 22.47a | 60.52c | 31.30b |
| CARV | 26.00 ± 0.92 | - | - | - |
| CARO | - | 23.95 ± 0.17 | - | - |
| CARA | 2.48 ± 0.04 | 203.00 ± 1.06 | 1.98 ± 0.07 | 2.05 ± 0.07 |
| Total PTs | 28.45b | 226.95c | 1.98a | 2.05a |
| VIC | 2.68 ± 0.07 | 0.14 ± 0.01 | 0.07 ± 0.02 | 0.12 ± 0.01 |
| API | 0.04 ± 0.00 | - | - | - |
| LUTGL | 0.09 ± 0.01 | 0.04 ± 0.00 | 0.08 ± 0.02 | 0.93 ± 0.12 |
| LUTRU | - | - | - | 1.30 ± 0.14 |
| NAR | 0.22 ± 0.09 | - | - | - |
| ERD | 0.49 ± 0.01 | - | - | - |
| HESP | - | 0.14 ± 0.01 | 0.03 ± 0.00 | 0.85 ± 0.16 |
| TAX | 0.62 ± 0.02 | - | - | - |
| VER | 0.24 ± 0.07 | 0.21 ± 0.00 | nd | 0.74 ± 0.06 |
| Total FLAVs | 4.37c | 0.87a | 0.89a | 3.94b |
| Total PCs | 56.41b | 250.29d | 63.39c | 35.25a |
| Analytes | Fraction | CON | ORE1% | ORE2% | ROS1% | ROS2% | LEB1% | LEB2% | SPE1% | SPE2% |
|---|---|---|---|---|---|---|---|---|---|---|
| t-FA | F | 0.27 ± 0.02 | 0.24 ± 0.01 | 0.22 ± 0.01 | 0.29 ± 0.01 | 0.28 ± 0.03 | 0.22 ± 0.02 | 0.20 ± 0.03 | 0.28 ± 0.04 | 0.25 ± 0.04 |
| T | 7.24 ± 0.29 | 5.71 ± 0.11 | 5.77 ± 0.37 | 5.77 ± 0.16 | 5.78 ± 0.45 | 6.53 ± 0.4 | 7.35 ± 0.52 | 6.60 ± 0.4 | 7.12 ± 0.56 | |
| c-FA | F | 0.23 ± 0.01 | 0.19 ± 0.04 | 0.19 ± 0.05 | 0.22 ± 0.02 | 0.20 ± 0.03 | 0.24 ± 0.01 | 0.24 ± 0.03 | 0.24 ± 0.06 | 0.24 ± 0.06 |
| T | 2.13 ± 0.23 | 2.00 ± 0.13 | 2.41 ± 0.21 | 2.16 ± 0.30 | 2.13 ± 0.36 | 1.94 ± 0.01 | 2.05 ± 0.13 | 1.34 ± 0.18 | 1.27 ± 0.30 | |
| pCA | F | 0.06 ± 0.01 | 0.99 ± 0.04 | 2.63 ± 0.04 | 0.03 ± 0.00 | 0.04 ± 0.00 | 1.13 ± 0.16 | 0.11 ± 0.00 | 0.06 ± 0.01 | 0.09 ± 0.01 |
| T | 0.47 ± 0.08 | 1.46 ± 0.02 | 3.15 ± 0.07 | 0.66 ± 0.07 | 0.67 ± 0.09 | 1.79 ± 0.24 | 0.77 ± 0.02 | 0.45 ± 0.08 | 0.44 ± 0.08 | |
| CA | F | 0.08 ± 0.01 | 0.39 ± 0.00 | 0.55 ± 0.01 | 0.43 ± 0.01 | 0.64 ± 0.02 | 0.49 ± 0.00 | 0.78 ± 0.02 | 0.47 ± 0.01 | 0.65 ± 0.02 |
| T | 0.28 ± 0.01 | 1.02 ± 0.06 | 2.90 ± 0.26 | 0.78 ± 0.04 | 1.24 ± 0.04 | 6.14 ± 0.61 | 9.95 ± 0.44 | 0.71 ± 0.01 | 0.93 ± 0.03 | |
| RMA | F | - | 15.16 ± 0.40 | 27.03 ± 0.24 | 22.46 ± 0.25 | 36.28 ± 0.88 | 58.10 ± 3.54 | 92.90 ± 3.87 | 35.32 ± 0.96 | 50.91 ± 0.10 |
| SALB | F | - | 1.75 ± 0.04 | 2.74 ± 0.16 | - | - | 0.31 ± 0.02 | 0.65 ± 0.04 | 0.16 ± 0.02 | 0.38 ± 0.01 |
| SALA | F | - | 0.76 ± 0.04 | 1.42 ± 0.01 | 0.82 ± 0.02 | 1.44 ± 0.02 | 0.25 ± 0.01 | 0.35 ± 0.04 | 2.20 ± 0.08 | 5.29 ± 0.21 |
| LITHA | F | - | - | - | - | - | 2.62 ± 0.05 | 5.24 ± 0.16 | 0.31 ± 0.01 | 0.64 ± 0.03 |
| SALI | F | - | - | - | - | - | - | - | 2.30 ± 0.34 | 3.54 ± 0.28 |
| Total PAs | F | 0.63a | 19.47b | 34.76d | 24.24c | 38.88d | 63.35e | 100.46f | 41.33d | 61.97e |
| T | 10.12a | 27.84b | 45.4c | 32.64b | 47.54c | 77.66e | 119.25f | 49.38c | 70.51d | |
| CARV | F | - | 13.50 ± 2.57 | 30.15 ± 2.75 | - | - | - | - | - | - |
| CARO | F | - | - | - | 41.78 ± 0.22 | 65.70 ± 3.15 | - | - | - | - |
| CARA | F | - | 4.22 ± 0.31 | 5.40 ± 0.07 | 111.54 ± 5.04 | 187.71 ± 2.92 | 4.61 ± 0.15 | 3.03 ± 0.01 | 2.11 ± 0.16 | 3.17 ± 0.05 |
| Total PTs | F | - | 17.72b | 35.55c | 153.32d | 253.41e | 4.61a | 3.03a | 2.11a | 3.17a |
| VIC | F | - | 3.70 ± 0.17 | 6.30 ± 0.03 | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.28 ± 0.11 | 0.16 ± 0.01 | 0.21 ± 0.02 | 0.28 ± 0.01 |
| API | F | - | 0.14 ± 0.01 | 0.26 ± 0.00 | - | - | - | - | - | - |
| LUTGL | F | - | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.00 | 0.20 ± 0.00 | 0.32 ± 0.04 | 0.54 ± 0.01 |
| LUTRU | F | - | - | - | - | - | - | - | 3.18 ± 0.05 | 5.23 ± 0.05 |
| NAR | F | - | 0.32 ± 0.00 | 0.63 ± 0.01 | - | - | - | - | - | - |
| ERD | F | - | 0.61 ± 0.00 | 1.14 ± 0.05 | - | - | - | - | - | - |
| HESP | F | - | - | - | 0.51 ± 0.05 | 0.94 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.78 ± 0.04 | 2.94 ± 0.04 |
| TAX | F | - | 0.78 ± 0.03 | 0.82 ± 0.06 | - | - | - | - | - | - |
| VER | F | - | 0.31± 0.01 | 0.33 ± 0.01 | 0.46 ± 0.04 | 0.27 ± 0.00 | - | - | 1.40 ± 0.14 | 2.64 ± 0.05 |
| Total FLAVs | F | - | 5.95d | 9.59f | 1.30b | 1.66c | 0.46a | 0.37a | 6.89e | 11.60g |
| Total PCs | F | 0.63a | 43.14b | 79.89cd | 178.41f | 293.95g | 68.41c | 103.85e | 50.33b | 76.78c |
| T | 10.12a | 51.51b | 90.53d | 187.26f | 302.61g | 82.7c | 122.64e | 58.38b | 85.41c |
| Volatile Compound | Distilled Solid Wastes | Breads | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORE | ROS | LEB | SPE | CON | ORE1% | ORE2% | ROS1% | ROS2% | LEB1% | LEB2% | SPE1% | SPE2% | |
| Aldehydes | |||||||||||||
| butanal-2-methyl | - | - | - | - | 0.54 | 0.23 | 0.25 | 0.77 | 0.45 | 0.66 | 0.72 | 0.51 | 0.28 |
| pentanal | - | - | 0.63 | - | 0.76 | - | - | - | - | 0.99 | 1.05 | 0.71 | 0.36 |
| hexanal | - | - | - | - | 7.70 | 0.75 | 0.21 | 6.55 | 4.11 | 9.42 | 7.55 | 4.66 | 2.48 |
| heptanal | - | - | - | - | 4.06 | 0.27 | - | 2.82 | 1.61 | 3.27 | 3.03 | 1.88 | 1.01 |
| 2-heptenal | - | - | - | - | - | 0.22 | 0.36 | 3.59 | 1.75 | 12.39 | 8.17 | 2.62 | 3.60 |
| octanal | - | - | - | - | 1.23 | - | - | - | - | - | - | - | - |
| 2-hexenal | - | - | - | - | - | - | - | 0.97 | 0.64 | 1.66 | 1.56 | 0.46 | 0.57 |
| benzaldehyde | - | - | - | - | 2.64 | 0.53 | 0.16 | 1.68 | 1.19 | - | - | 0.81 | 0.45 |
| nonanal | - | - | - | - | 1.75 | - | - | - | 0.50 | - | - | - | - |
| Alcohols | |||||||||||||
| 1-butanol-3-methyl | - | - | - | - | 26.96 | 1.97 | 0.58 | 20.38 | 9.95 | 28.32 | 26.67 | 10.63 | 6.24 |
| 1-butanol-2-methyl | - | - | - | - | 9.07 | 0.97 | 0.30 | 9.35 | 4.84 | 11.28 | 10.86 | 4.78 | 2.66 |
| amylol | - | - | - | - | 1.42 | - | - | - | - | - | - | 0.62 | 0.44 |
| hexanol | - | - | - | - | 10.32 | - | - | 6.24 | 2.84 | 8.06 | 7.38 | 3.07 | 2.10 |
| heptanol | - | - | - | - | 1.28 | - | - | - | - | - | - | - | - |
| 1-octen-3-ol | - | - | - | - | 1.19 | - | 0.16 | 1.21 | 0.77 | 2.80 | 3.19 | 0.93 | 1.39 |
| 1-dodecanol | - | - | - | - | - | 0.27 | 0.04 | 1.50 | 1.25 | 1.63 | 1.85 | - | - |
| furfuryl alcohol | - | - | - | - | 2.32 | - | - | - | - | - | - | - | - |
| phenethyl alcohol | - | - | - | - | 5.50 | - | - | - | - | - | - | - | - |
| Ketones | |||||||||||||
| 2-heptanone | - | - | - | - | 1.20 | - | - | - | - | - | - | 0.71 | 0.47 |
| 3-heptanone | - | - | - | - | - | - | - | - | - | 3.30 | 3.55 | 1.11 | 1.59 |
| Furans | |||||||||||||
| 2-amylfuran | - | - | - | - | 3.91 | 0.28 | 0.15 | 1.53 | 1.20 | 1.19 | 2.19 | 0.88 | 0.49 |
| Esters | |||||||||||||
| propyl phenylacetate | - | - | - | - | 1.32 | 0.26 | 0.07 | 1.20 | 0.79 | 1.67 | 1.59 | 1.27 | 0.75 |
| 1-hexyl acetate | - | - | - | - | - | - | - | - | 2.94 | 1.30 | - | - | - |
| Terpenoids | |||||||||||||
| α-pinene | - | 1.56 | - | - | - | - | - | 2.38 | 2.67 | - | - | - | - |
| β-pinene | - | 1.40 | 0.65 | 0.28 | - | - | - | 1.72 | 1.93 | - | - | - | - |
| β-myrcene | - | 1.16 | 0.44 | - | - | - | - | - | - | - | - | - | - |
| α-terpinene | - | - | - | - | - | - | - | - | 1.03 | - | - | - | - |
| p-cymene | - | 2.66 | - | 0.23 | - | 0.65 | 0.27 | 1.05 | 1.48 | - | - | - | - |
| limonene | - | 2.03 | 0.83 | - | - | - | - | 0.55 | 0.90 | - | - | - | - |
| β-phellandrene | - | - | 0.38 | - | - | - | - | - | - | - | - | - | - |
| 1,8-cineole | - | 16.98 | - | 4.62 | - | - | - | 4.63 | 4.34 | - | - | - | - |
| γ-terpinene | - | - | 0.20 | - | - | - | - | - | - | - | - | - | - |
| linalool | - | 1.77 | 0.18 | 9.93 | - | - | - | - | - | - | - | - | - |
| citronellal | - | - | 0.50 | ||||||||||
| borneol | - | 3.77 | - | 0.78 | - | - | - | - | - | - | - | - | - |
| terpinen-4-ol | 0.52 | 1.41 | - | 1.08 | - | - | - | - | - | - | - | - | - |
| α-terpineol | - | 5.54 | - | 1.08 | - | - | - | 1.07 | 2.17 | - | - | - | - |
| neral | - | - | 2.76 | ||||||||||
| carvone | - | - | - | 35.14 | - | - | - | - | - | - | - | 54.80 | 58.88 |
| geraniol | - | - | 0.80 | - | - | - | - | - | - | - | - | - | - |
| bornyl acetate | - | 6.04 | - | 1.18 | - | - | - | 4.81 | 6.88 | - | - | - | - |
| geranial | - | - | 5.50 | - | - | - | - | - | - | - | - | - | - |
| thymoquinone | - | - | - | - | - | 46.08 | 45.29 | - | - | - | - | - | - |
| thymol | 8.37 | - | - | - | - | - | - | - | - | - | - | - | |
| carvacrol | 77.78 | - | - | - | - | 43.82 | 49.47 | - | - | - | - | - | - |
| α-copaene | - | - | 4.36 | - | - | - | - | - | - | - | - | - | - |
| β- bourbonene | - | - | 1.63 | 5.07 | - | - | - | - | - | - | - | 6.26 | 8.21 |
| α-cubebene | - | - | 0.62 | - | - | - | - | - | - | - | - | - | - |
| β-elemene | - | - | 1.70 | - | - | - | - | - | - | - | - | - | - |
| β-caryophyllene | 3.05 | 37.51 | 39.00 | 3.34 | - | 1.50 | 1.16 | 23.48 | 40.97 | 6.38 | 13.57 | 0.64 | 0.69 |
| β-copaene | - | - | 0.58 | - | - | - | - | - | - | - | - | - | - |
| α-bergamotene | - | - | 1.10 | 5.07 | - | - | - | - | - | - | - | - | - |
| aromadendrene | - | - | - | 1.07 | - | - | - | - | - | - | - | - | - |
| α-humulene | 0.35 | 2.51 | 3.86 | - | - | - | - | - | - | - | - | - | - |
| β-farnesene | - | - | - | 2.22 | - | - | - | - | - | - | - | - | - |
| cis-muurola-4(15),5-diene | - | - | - | 2.00 | - | - | - | - | - | - | - | 0.78 | 0.54 |
| γ-muurolene | - | - | 1.40 | - | - | - | - | - | - | - | - | - | - |
| germacrene D | - | - | 20.60 | 6.07 | - | - | - | - | - | 1.12 | 3.40 | 0.89 | 1.94 |
| α-muurolene | - | - | 1.70 | - | - | - | - | - | - | - | - | - | - |
| γ-cadinene | - | - | 1.53 | 2.07 | - | - | - | - | - | - | - | - | - |
| δ-cadinene | - | - | 5.80 | - | - | - | - | - | - | - | - | - | - |
| α-cadinene | - | - | 1.50 | - | - | - | - | - | - | - | - | - | - |
| caryophyllene oxide | 1.96 | 3.71 | 1.40 | - | - | 0.41 | 0.41 | 0.88 | 1.90 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouska, C.; Irakli, M.; Georgiou, M.; Lytou, A.E.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G.; Lazaridou, A. Physicochemical Characteristics, Antioxidant Properties, Aroma Profile, and Sensory Qualities of Value-Added Wheat Breads Fortified with Post-Distillation Solid Wastes of Aromatic Plants. Foods 2023, 12, 4007. https://doi.org/10.3390/foods12214007
Nouska C, Irakli M, Georgiou M, Lytou AE, Skendi A, Bouloumpasi E, Chatzopoulou P, Biliaderis CG, Lazaridou A. Physicochemical Characteristics, Antioxidant Properties, Aroma Profile, and Sensory Qualities of Value-Added Wheat Breads Fortified with Post-Distillation Solid Wastes of Aromatic Plants. Foods. 2023; 12(21):4007. https://doi.org/10.3390/foods12214007
Chicago/Turabian StyleNouska, Chrysanthi, Maria Irakli, Miltiadis Georgiou, Anastasia E. Lytou, Adriana Skendi, Elisavet Bouloumpasi, Paschalina Chatzopoulou, Costas G. Biliaderis, and Athina Lazaridou. 2023. "Physicochemical Characteristics, Antioxidant Properties, Aroma Profile, and Sensory Qualities of Value-Added Wheat Breads Fortified with Post-Distillation Solid Wastes of Aromatic Plants" Foods 12, no. 21: 4007. https://doi.org/10.3390/foods12214007







