Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry
Abstract
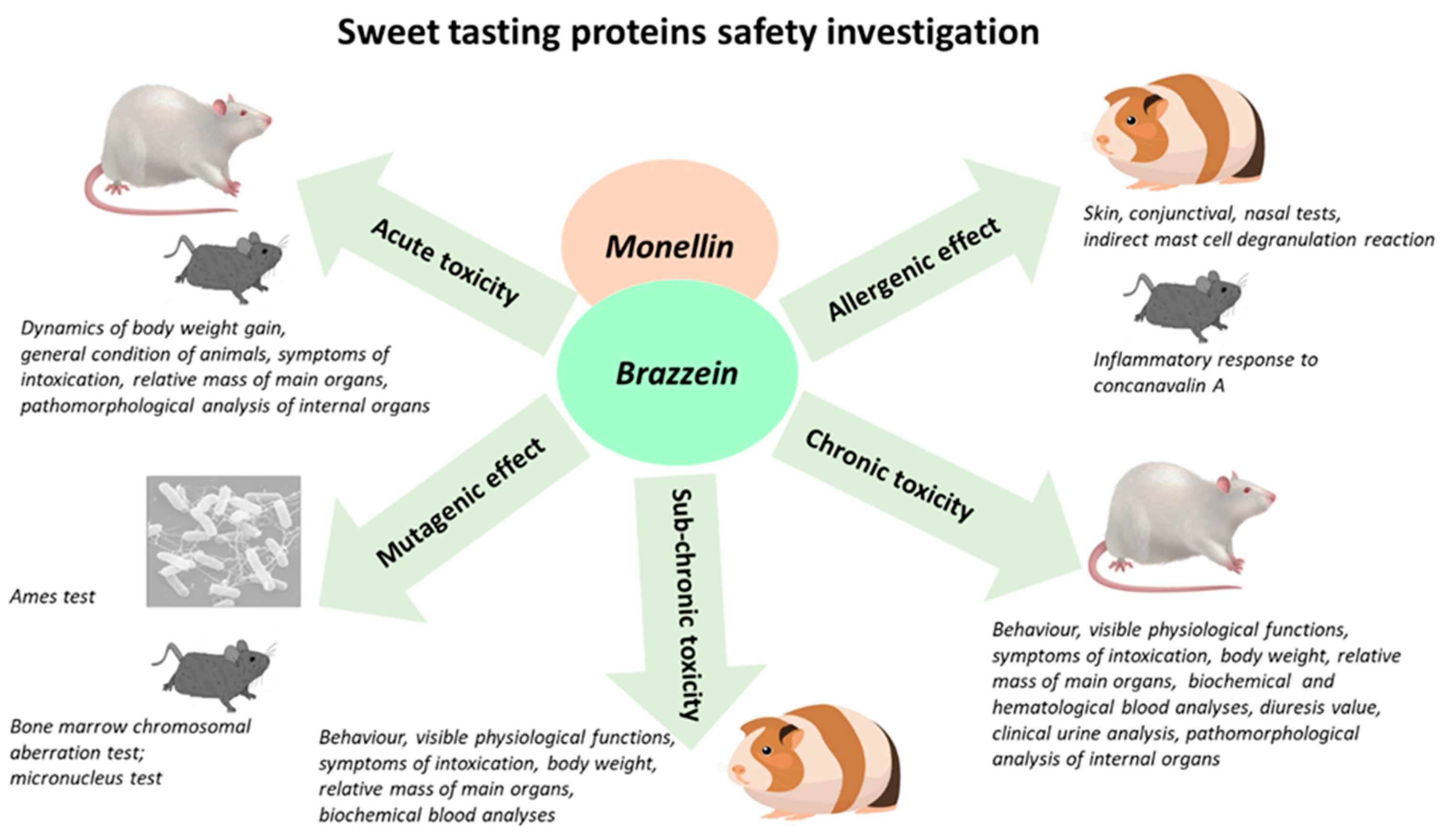
:1. Introduction
2. Materials and Methods
2.1. Preparation of Brazzein and Monellin
2.2. Acute Toxicity
2.3. Subchronic Toxicity
2.4. Chronic Toxicity
2.5. Allergenic Effects
2.6. Mutagenic Effects
2.6.1. Ames Test
2.6.2. Mammalian Bone Marrow Chromosomal Aberration Test and Micronucleus Test
2.7. Statistics
2.8. Regulating Acts and Guidelines
2.9. Bioethics
3. Results
3.1. Acute Toxicity
3.2. Subchronic Toxicity
3.3. Chronic Toxicity
3.4. Allergenic Effects
3.4.1. Skin Test
3.4.2. Conjunctival Test
3.4.3. Nasal Test
3.4.4. Indirect Mast Cell Degranulation Reaction
3.4.5. Inflammatory Response to Concanavalin A
4. Mutagenic Action In Vitro and In Vivo
4.1. Ames Test In Vitro
4.2. Tests In Vivo
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 May 2023).
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose toxicity in ß-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Bassoli, A. Developing new sweeteners from natural compounds. In Optimizing Sweet Taste in Foods; Woodhead Publishing Series in Food Science, Technology and Nutrition; Spillane, W.J., Ed.; Woodhead Publishing: Sawston, UK, 2006; pp. 327–343. [Google Scholar] [CrossRef]
- Mintz, S.W. Sweetness and Power: The Place of Sugar in Modern History; Penguin: New York, NY, USA, 1986. [Google Scholar]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?—Health Be Damned! Pour on the Sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillón, P.; Muñoz-Pareja, M.; Aguilera, M.T.; León-Muñoz, L.M.; Rodríguez-Artalejo, F. Food sources of sodium, saturated fat and added sugar in the Spanish hypertensive and diabetic population. Atherosclerosis 2013, 229, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sahina, A.W.; Zanninia, E.; Coffeyb, A.; Arendt, E.K. Sugar reduction in bakery products: Current strategies and sourdough technology as a potential novel approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef]
- Świąder, K.; Wegner, K.; Piotrowska, A.; Tan, F.-J.; Sadowska, A. Plants as a source of natural high-intensity sweeteners: A review. J. Appl. Bot. Food Qual. 2019, 92, 160–171. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Zheng, Y.; Liu, B. New Insight into the Structure-Activity Relationship of Sweet-Tasting Proteins: Protein Sector and Its Role for Sweet Properties. Front. Nutr. 2021, 8, 691368. [Google Scholar] [CrossRef]
- Farag, M.A.; Rezk, M.M.; Elashal, M.H.; El-Araby, M.; Khalifa, S.A.M.; El-Seedi, H.R. An updated multifaceted overview of sweet proteins and dipeptides as sugar substitutes; the chemistry, health benefits, gut interactions, and safety. Food Res. Int. 2022, 162, 111853. [Google Scholar] [CrossRef]
- Jiang, P.; Ji, Q.; Liu, Z.; Snyder, L.A.; Benard, L.M.J.; Margolskee, R.F.; Max, M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004, 279, 45068–45075. [Google Scholar] [CrossRef]
- Kondo, K.; Miura, Y.; Sone, H.; Kobayashi, K.; Iijima, H. High-level expression of a sweet protein, monellin, in the food yeast Candida utilis. Nat. Biotechnol. 1997, 15, 453–457. [Google Scholar] [CrossRef]
- Tang, N.; Liu, J.; Cheng, Y. Potential improvement of the thermal stability of sweet-tasting proteins by structural calculations. Food Chem. 2021, 345, 128750. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yi, Y.; Liu, L.; He, M.; Deng, S.; Tian, H.; Yao, W.; Gao, X. Design and development of a high temperature stable sweet protein base on monellin. Process Biochem. 2020, 89, 29–36. [Google Scholar] [CrossRef]
- Morris, J.A.; Cagan, R.H. Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim. Biophys. Acta 1972, 261, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Martenson, R.; Deibler, G.; Cagan, R.H. Characterization of monellin, a Protein That Tastes Sweet. J. Biol. Chem. 1973, 248, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Kohmura, M.; Nio, N.; Ariyoshi, Y. Complete Amino Acid Sequence of the Sweet Protein monellin. Agric. Biol. Chem. 1990, 54, 2219–2224. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, C.-H.; Kim, R.; Cho, J.M.; Lee, Y.-B.; Lee, T.-K. Redesigning a sweet protein: Increased stability and renaturability. Protein Eng. Des. Sel. 1989, 2, 571–575. [Google Scholar] [CrossRef]
- Ming, D.; Hellekant, G. Brazzein, a new high-potency thermostable sweet protein from Pentadiplandra brazzeana B. FEBS Lett. 1994, 355, 106–108. [Google Scholar] [CrossRef]
- Assadi-Porter, F.M.; Abildgaard, F.; Blad, H.; Cornilescu, C.C.; Markley, J.L. Brazzein, a Small, Sweet Protein: Effects of Mutations on its Structure, Dynamics and Functional Properties. Chem. Senses 2005, 30 (Suppl. S1), i90–i91. [Google Scholar] [CrossRef]
- Hellekant, G.; Danilova, V. Brazzein a small, sweet protein: Discovery and physiological overview. Chem. Senses 2005, 30 (Suppl. S1), 88–89. [Google Scholar] [CrossRef]
- Barrea, A.; Caze-Subraa, S.; Girondea, C.; Bienvenub, F.; Bienvenub, J.; Rougéa, P. What about the allergenicity of sweet-tasting proteins? Rev. Française D’allergologie 2015, 55, 363–371. [Google Scholar] [CrossRef]
- Chung, J.-H.; Kong, J.-N.; Choi, H.-E.; Kong, K.-H. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018, 267, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kelada, K.D.; Tus´e, D.; Gleba, Y.; McDonald, K.A.; Nandi, S. Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II. Foods 2021, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, S.; Trauth, J.; Bezold, F.; Essen, L.O.; Taxis, C.; Spadaccini, R. Light-induced fermenter production of derivatives of the sweet protein monellin is maximized in prestationary Saccharomyces cerevisiae cultures. Biotechnol. J. 2022, 17, e2100676. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.; Nijkamp, F.; De Rose, V.; Folkerts, G. The guinea pig as an animal model for asthma. Curr. Drug Targets 2008, 9, 452–465. [Google Scholar] [CrossRef] [PubMed]
- OECD Guideline for Testing of Chemicals No. 471.Bacterial Reverse Mutation Test; OECD Organization for Economic Co-Operation and Development (OECD): Paris, France, 1997.
- OECD Guideline for Testing of Chemicals No. 475. Mammalian Bone Marrow Chromosome Aberration Test; OECD Organization for Economic Cooperation and Development (OECD): Paris, France, 1997.
- The Rules of Good Laboratory Practice of the Eurasian Economic Union in the Field of Circulation of Medicines; No. 81; Council of the Eurasian Economic Commission: Moscow, Russia, 2016.
- Khabriev, R.U. Guidelines for the experimental (preclinical) study of new pharmacological substances. Med. Mosc. 2005, 832. [Google Scholar]
- Mironov, A.N. Guidelines for conducting preclinical studies of medicines. Part One Grif K Mosc. 2012, 944. [Google Scholar]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (ETS 123). Strasbourg. 1986. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiArdW0q7OCAxVjcmwGHXeVB2cQFnoECBEQAQ&url=https%3A%2F%2Frm.coe.int%2F168007a67b&usg=AOvVaw1ZyAsm7IDpM6ttzCBEuzxe&opi=89978449 (accessed on 2 November 2023).
- Directive 2010/63/EU of the European Parliament and of the Council of the European Union of 22.09.2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjt9MDXq7OCAxVEUGwGHf-zBMIQFnoECBYQAQ&url=https%3A%2F%2Feur-lex.europa.eu%2FLexUriServ%2FLexUriServ.do%3Furi%3DOJ%3AL%3A2010%3A276%3A0033%3A0079%3Aen%3APDF&usg=AOvVaw3GqFyYVwbZaCsOV3nitPEd&opi=89978449 (accessed on 2 November 2023).
- Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996.
- Rajan, V.; Howard, J.A. Brazzein: A Natural Sweetener. In Sweeteners. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Fortes, C.; Halldorsson, T.; LeBlanc, J.C.; Lindtner, O.; Mortensen, A.; Ntzani, E.; Wallace, H.; Civitella, C.; Horvath, Z.; et al. Re-evaluation of thaumatin (E 957) as food additive. EFSA J. 2021, 19, e06884. [Google Scholar] [CrossRef]
| Administration | Dose | Result (%) (M ± m) |
|---|---|---|
| Brazzein | EDguinea pig | 5.56 ± 0.19 (t = 1.51) |
| Brazzein | 10EDguinea pig | 5.78 ± 0.22 (t = 2.11) |
| Distilled water (control) | 0.5 mL per capita | 5.17 ± 0.14 |
| Administration | Dose | Result (%) (M ± m) |
|---|---|---|
| Monellin | EDguinea pig | 5.50 ± 0.14 (t = 1.51) |
| Monellin | 10EDguinea pig | 5.72 ± 0.22 (t = 1.94) |
| Distilled water (control) | 0.5 mL per capita | 5.17 ± 0.14 |
| Administration | Dose | Result (%) (M ± m) |
|---|---|---|
| Brazzein | EDguinea pig | 17.98 ± 1.53 (t = 0.75) |
| Brazzein | 10EDguinea pig | 18.07 ± 1.01 (t = 0.91) |
| Distilled water (control) | 0.02 mL per capita | 16.20 ± 1.64 |
| Administration | Dose | Result (%) (M ± m) |
|---|---|---|
| Monellin | EDguinea pig | 17.33 ± 1.81 (t = 0.44) |
| Monellin | 10EDguinea pig | 17.48 ± 1.75 (t = 0.50) |
| Distilled water (control) | 0.02 mL per capita | 16.20 ± 1.64 |
| Strain | Metabolic Activation | Agent Concentration, µg/mL | Number of Revertant Colonies per Dish | Xavexp/Xavcon | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Xav | ||||
| TA 100 | MAS- | 50,000 | 115 | 104 | 108 | 109.0 | 0.91 |
| 10,000 | 107 | 119 | 102 | 109.3 | 0.92 | ||
| 2000 | 114 | 110 | 116 | 113.3 | 0.95 | ||
| 400 | 121 | 115 | 111 | 115.7 | 0.97 | ||
| 80 | 119 | 113 | 109 | 113.7 | 0.96 | ||
| H2O | 122 | 108 | 126 | 118.7 | |||
| Sodium azide | 1174 | 1352 | 1430 | 1318.7 | 11.11 * | ||
| MAS+ | 50,000 | 102 | 91 | 114 | 102.3 | 0.92 | |
| 10,000 | 100 | 116 | 110 | 108.7 | 0.98 | ||
| 2000 | 112 | 94 | 104 | 103.3 | 0.93 | ||
| 400 | 114 | 107 | 121 | 114.0 | 1.02 | ||
| 80 | 123 | 111 | 115 | 116.3 | 1.05 | ||
| H2O | 116 | 120 | 98 | 111.3 | |||
| TA 98 | MAS- | 50,000 | 16 | 23 | 24 | 21.0 | 0.89 |
| 10,000 | 24 | 26 | 21 | 23.7 | 1.00 | ||
| 2000 | 30 | 25 | 22 | 25.7 | 1.08 | ||
| 400 | 26 | 27 | 19 | 24.0 | 1.01 | ||
| 80 | 21 | 26 | 20 | 22.3 | 0.94 | ||
| H2O | 23 | 21 | 27 | 23.7 | |||
| DMSO | 22 | 28 | 30 | 26.7 | |||
| 2NF | 148 | 137 | 128 | 137.7 | 5.16 * | ||
| MAS+ | 50,000 | 19 | 23 | 21 | 21.0 | 0.71 | |
| 10,000 | 26 | 21 | 29 | 24.7 | 0.83 | ||
| 2000 | 27 | 23 | 26 | 25.3 | 0.85 | ||
| 400 | 31 | 27 | 22 | 26.7 | 0.90 | ||
| 80 | 18 | 25 | 28 | 23.7 | 0.80 | ||
| H2O | 24 | 32 | 33 | 29.7 | |||
| Ethidium bromide | 226 | 247 | 192 | 221.7 | 7.46 * | ||
| TA 97 | MAS- | 50,000 | 104 | 106 | 97 | 102.3 | 0.78 |
| 10,000 | 113 | 121 | 128 | 120.7 | 0.92 | ||
| 2000 | 124 | 123 | 132 | 126.3 | 0.96 | ||
| 400 | 123 | 120 | 136 | 126.3 | 0.96 | ||
| 80 | 139 | 118 | 134 | 130.3 | 0.99 | ||
| H2O | 135 | 137 | 123 | 131.7 | |||
| DMSO | 122 | 128 | 134 | 128.0 | |||
| 9AA | 592 | 638 | 614 | 614.7 | 4.80 * | ||
| MAS+ | 50,000 | 127 | 144 | 129 | 133.3 | 0.99 | |
| 10,000 | 135 | 148 | 125 | 136.0 | 1.01 | ||
| 2000 | 125 | 131 | 124 | 126.7 | 0.95 | ||
| 400 | 133 | 129 | 130 | 130.7 | 0.98 | ||
| 80 | 144 | 123 | 136 | 134.3 | 1.00 | ||
| H2O | 128 | 142 | 132 | 134.0 | |||
| Strain | Metabolic Activation | Agent Concentration, µg/mL | Number of Revertant Colonies per Dish | Xavexp/Xavcon | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Xav | ||||
| TA 100 | MAS- | 50,000 | 128 | 124 | 118 | 123.3 | 0.99 |
| 10,000 | 125 | 122 | 113 | 120.0 | 0.97 | ||
| 2000 | 117 | 111 | 125 | 117.7 | 0.95 | ||
| 400 | 123 | 117 | 129 | 123.0 | 0.99 | ||
| 80 | 114 | 124 | 119 | 119.0 | 0.96 | ||
| H2O | 125 | 120 | 127 | 124.0 | |||
| Sodium azide | 1270 | 1424 | 1312 | 1335.3 | 10.76 * | ||
| MAS+ | 50,000 | 121 | 123 | 109 | 117.7 | 1.00 | |
| 10,000 | 120 | 118 | 124 | 120.7 | 1.03 | ||
| 2000 | 108 | 127 | 106 | 113.7 | 0.97 | ||
| 400 | 123 | 119 | 112 | 118.0 | 1.01 | ||
| 80 | 126 | 110 | 114 | 116.7 | 0.99 | ||
| H2O | 119 | 111 | 122 | 117.3 | |||
| TA 98 | MAS - | 50,000 | 27 | 25 | 33 | 28.3 | 1.02 |
| 10,000 | 32 | 28 | 20 | 26.7 | 0.96 | ||
| 2000 | 30 | 31 | 23 | 28.0 | 1.01 | ||
| 400 | 33 | 27 | 21 | 27.0 | 0.97 | ||
| 80 | 24 | 24 | 29 | 25.7 | 0.93 | ||
| H2O | 24 | 29 | 30 | 27.7 | |||
| DMSO | 28 | 34 | 26 | 29.3 | |||
| 2NF | 132 | 145 | 151 | 142.7 | 4.87 * | ||
| MAS+ | 50,000 | 30 | 27 | 25 | 27.3 | 0.87 | |
| 10,000 | 34 | 24 | 32 | 30.0 | 0.96 | ||
| 2000 | 34 | 27 | 32 | 31.0 | 0.99 | ||
| 400 | 25 | 23 | 37 | 28.3 | 0.91 | ||
| 80 | 24 | 33 | 29 | 28.0 | 0.89 | ||
| H2O | 27 | 36 | 31 | 31.3 | |||
| Ethidium bromide | 348 | 272 | 315 | 311.7 | 9.96 * | ||
| TA 97 | MAS- | 50,000 | 119 | 129 | 124 | 124.0 | 0.91 |
| 10,000 | 122 | 119 | 125 | 122.0 | 0.89 | ||
| 2000 | 113 | 120 | 126 | 119.7 | 0.87 | ||
| 400 | 128 | 124 | 118 | 123.3 | 0.90 | ||
| 80 | 125 | 130 | 123 | 126.0 | 0.92 | ||
| H2O | 133 | 147 | 131 | 137.0 | |||
| DMSO | 142 | 129 | 132 | 134.3 | |||
| 9AA | 713 | 698 | 647 | 686.0 | 5.01 * | ||
| MAS+ | 50,000 | 122 | 135 | 120 | 125.7 | 0.99 | |
| 10,000 | 133 | 125 | 123 | 127.0 | 1.00 | ||
| 2000 | 124 | 116 | 117 | 119.0 | 0.94 | ||
| 400 | 112 | 124 | 126 | 120.7 | 0.95 | ||
| 80 | 127 | 121 | 129 | 125.7 | 0.99 | ||
| H2O | 130 | 123 | 127 | 126.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novik, T.S.; Koveshnikova, E.I.; Kotlobay, A.A.; Sycheva, L.P.; Kurochkina, K.G.; Averina, O.A.; Belopolskaya, M.V.; Sergiev, P.V.; Dontsova, O.A.; Lazarev, V.N.; et al. Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry. Foods 2023, 12, 4065. https://doi.org/10.3390/foods12224065
Novik TS, Koveshnikova EI, Kotlobay AA, Sycheva LP, Kurochkina KG, Averina OA, Belopolskaya MV, Sergiev PV, Dontsova OA, Lazarev VN, et al. Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry. Foods. 2023; 12(22):4065. https://doi.org/10.3390/foods12224065
Chicago/Turabian StyleNovik, Tamara S., Elena I. Koveshnikova, Anatoly A. Kotlobay, Lyudmila P. Sycheva, Karine G. Kurochkina, Olga A. Averina, Maria V. Belopolskaya, Petr V. Sergiev, Olga A. Dontsova, Vassili N. Lazarev, and et al. 2023. "Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry" Foods 12, no. 22: 4065. https://doi.org/10.3390/foods12224065
APA StyleNovik, T. S., Koveshnikova, E. I., Kotlobay, A. A., Sycheva, L. P., Kurochkina, K. G., Averina, O. A., Belopolskaya, M. V., Sergiev, P. V., Dontsova, O. A., Lazarev, V. N., Maev, I. V., Kostyaeva, M. G., Eremeev, A. V., Chukina, S. I., & Lagarkova, M. A. (2023). Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry. Foods, 12(22), 4065. https://doi.org/10.3390/foods12224065






