Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Edible Insect Samples
2.2. Total Lipids and Lipid Classes
2.3. Fatty Acid Profile and Nutritional Indices
2.4. Amino Acid Composition and Conversion Factor (Kp)
2.5. Amino Acid-Based Nutritional Indices
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Lipid Content and Fatty Acid Composition
3.2. Nutritional Quality of the Lipids
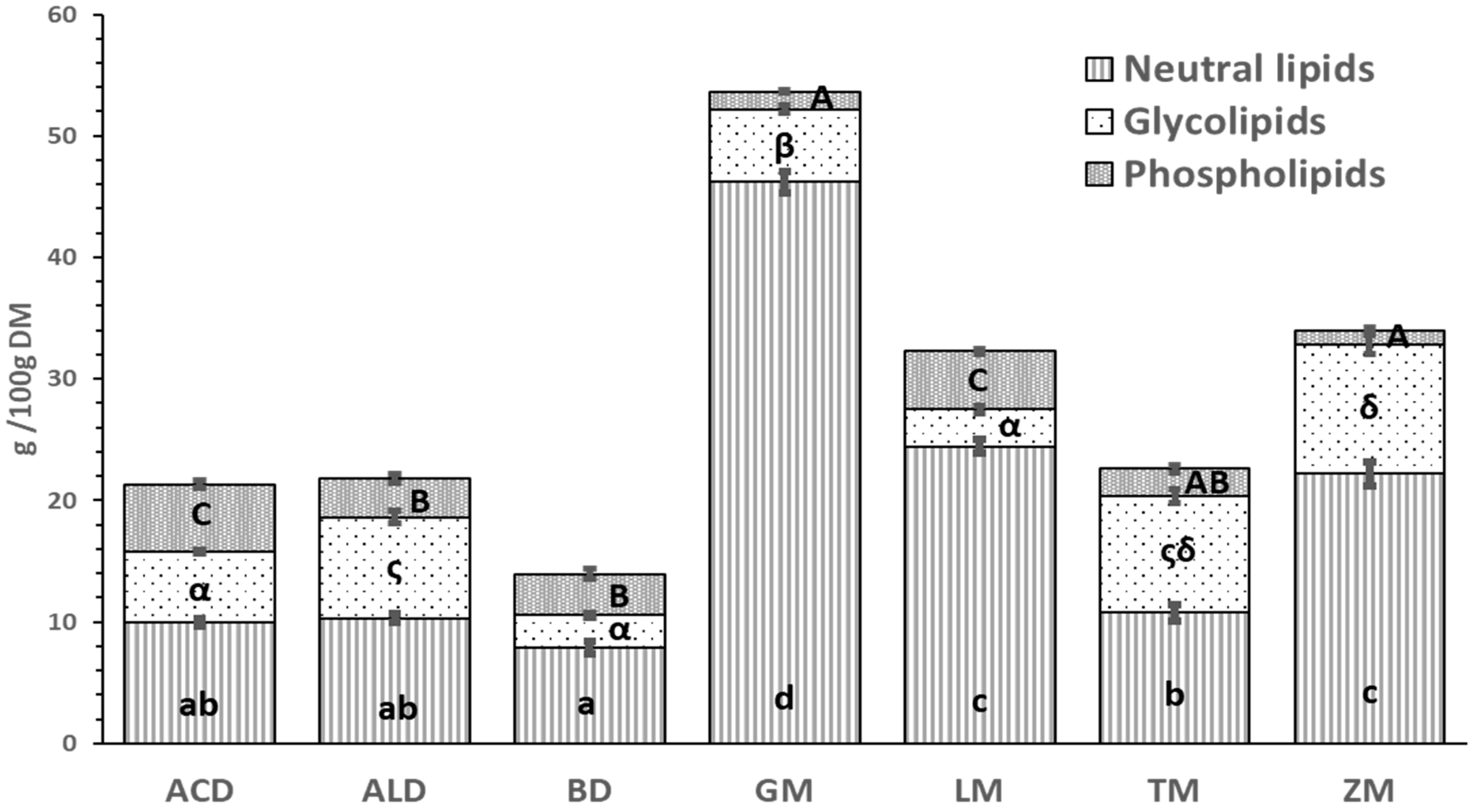
3.3. Analysis of Lipid Classes
3.4. Amino Acid Composition and Estimation of Protein Quality
3.5. True Protein Content
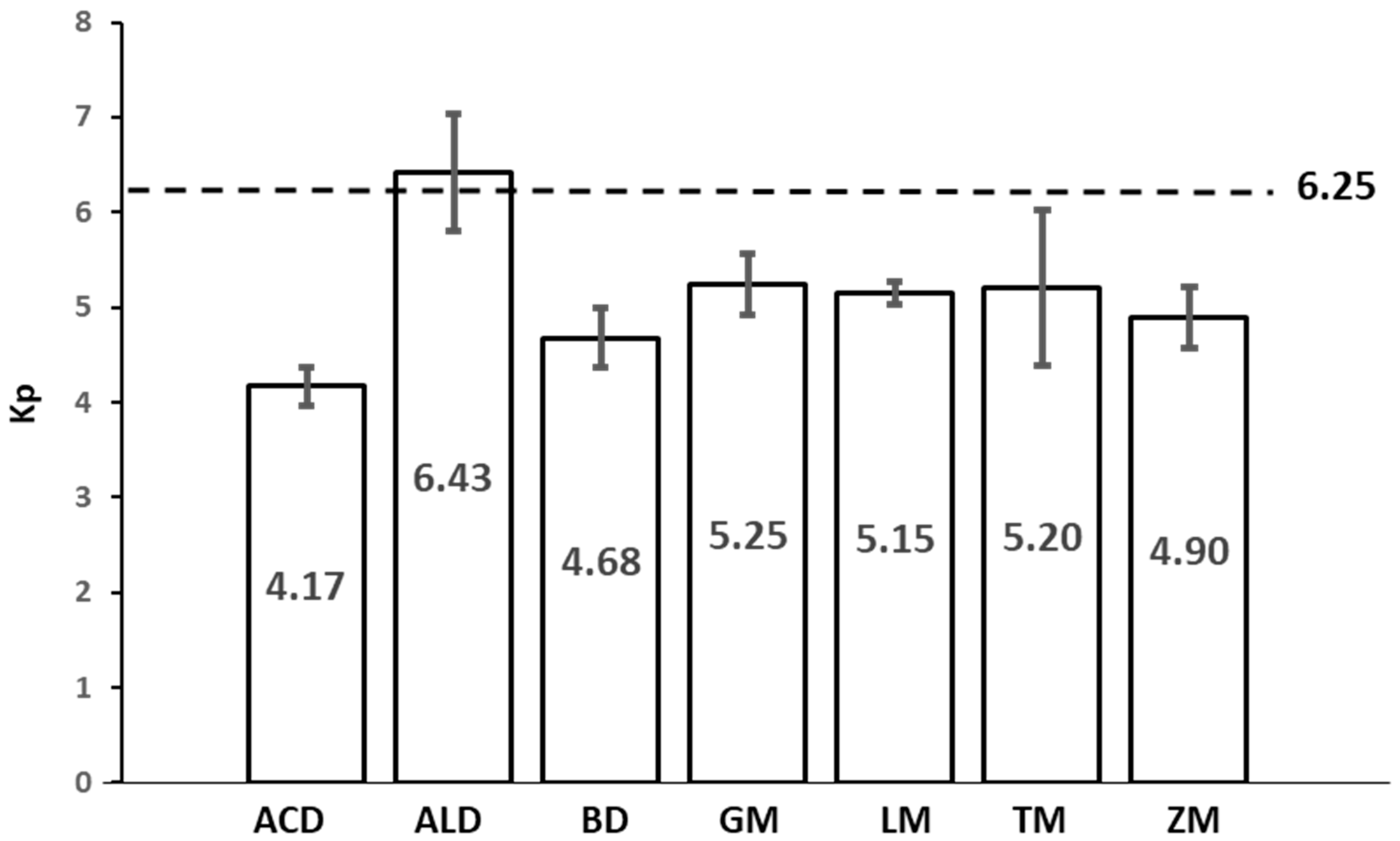
3.6. Protein Conversion Factor (Kp)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Popullation Division. World Population Prospects 2022 Summary of Results; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2022. [Google Scholar]
- Flachowsky, G.; Meyer, U.; Südekum, K.H. Land Use for Edible Protein of Animal Origin—A Review. Animals 2017, 7, 25. [Google Scholar] [CrossRef]
- Baiano, A. Edible Insects: An Overview on Nutritional Characteristics, Safety, Farming, Production Technologies, Regulatory Framework, and Socio-Economic and Ethical Implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food And AgrIculture OrganizatIon of the UnIted NatIons: Rome, Italy, 2013. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional Composition of Five Commercial Edible Insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- European Regulation. European Regulation (EU) 2015/2283 of the European Parliament of and of the Council of 25 November 2015 Concerning Concerning Novel Foods and Novel Food Ingredients. Off. J. Eur. Union 2015, 2015, 1–22. [Google Scholar]
- Giordano, S.; Clodoveo, M.L.; De Gennaro, B.; Corbo, F. Factors Determining Neophobia and Neophilia with Regard to New Technologies Applied to the Food Sector: A Systematic Review. Int. J. Gastron. Food Sci. 2018, 11, 1–19. [Google Scholar] [CrossRef]
- Telfser, K. Creating a Market for a More Sustainable Alternative: Entomophagy Businesses in Europe. Master’s Thesis, Aalto University, Espoo, Finland, 2015. [Google Scholar]
- Finke, M.D. Complete Nutrient Composition of Commercially Raised Invertebrates Used as Food for Insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Kulma, M.; Plachý, V.; Kouřimská, L.; Vrabec, V.; Bubová, T.; Adámková, A.; Hučko, B. Nutritional Value of Three Blattodea Species Used as Feed for Animals. J. Anim. Feed. Sci. 2016, 25, 354–360. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Boekel, M.A.J.S.V. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Smets, R. On the Compositional Analysis and Biorefinery of the Black Soldier Fly (Hermetia illucens). Resolving Uncertainties & Towards More Sustainable Processing, KU LEUVEN, Science, Engineering & Technology. Ph.D. Thesis, Arenberg Doctoral School, Faculty of Engineering Technology, Leuven, Belgium, 2021. [Google Scholar]
- Gowda, S.G.B.; Sasaki, Y.; Hasegawa, E.; Chiba, H.; Hui, S.P. Lipid Fingerprinting of Yellow Mealworm Tenebrio molitor by Untargeted Liquid Chromatography-Mass Spectrometry. J. Insects Food Feed. 2022, 8, 157–168. [Google Scholar] [CrossRef]
- Ochiai, M.; Komiya, Y. Detection of Edible Insect Derived Phospholipids with Polyunsaturated Fatty Acids by Thin-Layer Chromatography, Gas Chromatography, and Enzymatic Methods. J. Food Compos. Anal. 2021, 99, 103869. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect Lipid Profile: Aqueous versus Organic Solvent-Based Extraction Methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Provijn, P.; Brouwers, J.F.; de Meulenaer, B.; Oonincx, D.G.A.B. Lipidome of Cricket Species Used as Food. Food Chem. 2021, 349, 129077. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G. Insects as Food: Fatty Acid Profiles, Lipid Classes, and Sn-2 Fatty Acid Distribution of Lepidoptera Larvae. Eur. J. Lipid Sci. Technol. 2018, 120, 1700391. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.; Lee, S.; Choe, E. Lipid Changes of Freeze-Dried Spinach by Various Kinds of Oxidation. J. Food Sci. 2000, 65, 1290–1295. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sugimura, R.; Shimajiri, J.; Suda, M.; Abe, M.; Hosokawa, M.; Miyashita, K. Oxidative Stability of Glyceroglycolipids Containing Polyunsaturated Fatty Acids. J. Oleo Sci. 2012, 61, 505–513. [Google Scholar] [CrossRef]
- Domoto, N.; Koenen, M.E.; Havenaar, R.; Mikajiri, A.; Chu, B. The Bioaccessibility of Eicosapentaenoic Acid Was Higher from Phospholipid Food Products than from Mono- and Triacylglycerol Food Products in a Dynamic Gastrointestinal Model. Food Sci. Nutr. 2013, 1, 409–415. [Google Scholar] [CrossRef]
- Chinarak, K.; Panpipat, W.; Summpunn, P.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. Insights into the Effects of Dietary Supplements on the Nutritional Composition and Growth Performance of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Food Chem. 2021, 363, 130279. [Google Scholar] [CrossRef]
- Belghit, I.; Lock, E.J.; Fumière, O.; Lecrenier, M.C.; Renard, P.; Dieu, M.; Berntssen, M.H.G.; Palmblad, M.; Rasinger, J.D. Species-Specific Discrimination of Insect Meals for Aquafeeds by Direct Comparison of Tandem Mass Spectra. Animals 2019, 9, 222. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio Molitor, Alphitobius Diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-Protein Conversion Factors for Edible Insects on the Swiss Market: T. Molitor, A. Domesticus, and L. Migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The Nitrogen-to-Protein Conversion Factor of Two Cricket Species-Acheta Domesticus and Gryllus Bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Risk Profile Related to Production and Consumption of Insects as Food and Feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Woodhead Publishing Limited: Cambridge, UK, 2003; Volume 24. [Google Scholar]
- Mohammad Taghi Gharibzahedi, S.; Altintas, Z. Lesser Mealworm (Alphitobius diaperinus L.) Larvae Oils Extracted by Pure and Binary Mixed Organic Solvents: Physicochemical and Antioxidant Properties, Fatty Acid Composition, and Lipid Quality Indices. Food Chem. 2023, 408, 135209. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Lipid Nutritional Indices, Regioisomeric Distribution, and Thermal Properties of Tenebrio molitor and Hermetia illucens Larvae Fat. J. Asia-Pac. Èntomol. 2022, 25, 101951. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.; Santos-Silvã, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino Acid Composition, Protein Content, and Nitrogen-to-Protein Conversion Factors of 21 Seaweed Species from Norwegian Waters. J. Appl. Phycol. 2016, 29, 1001–1009. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kavle, R.R.; Nolan, P.J.; Bekhit, A.E.D.A.; Carne, A.; Morton, J.D.; Agyei, D. Physicochemical Characteristics, Techno-Functionalities, and Amino Acid Profile of Prionoplus Reticularis (Huhu) Larvae and Pupae Protein Extracts. Foods 2023, 12, 417. [Google Scholar] [CrossRef]
- Yu, X.; He, Q.; Wang, D. Dynamic Analysis of Major Components in the Different Developmental Stages of Tenebrio Molitor. Front. Nutr. 2021, 8, 689746. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Nations University. Protein and Amino Acid Requirements in Human Nutrition. World Health Organ Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Chavan, U.D.; Mckenzie, D.B.; Shahidi, F. Functional Properties of Protein Isolates from Beach Pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient Composition of Selected Whole Invertebrates. Zoo Biol. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and Hurdles of Edible Insects for Food and Feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the Proximate Composition and Microbial Diversity of Edible Insects Marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Rutaro, K.; Malinga, G.M.; Lehtovaara, V.J.; Opoke, R.; Valtonen, A.; Kwetegyeka, J.; Nyeko, P.; Roininen, H. The Fatty Acid Composition of Edible Grasshopper Ruspolia Differens (Serville) (Orthoptera: Tettigoniidae) Feeding on Diversifying Diets of Host Plants. Entomol. Res. 2018, 48, 490–498. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty Acids in Insects: Composition, Metabolism, and Biological Significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect Fatty Acids: A Comparison of Lipids from Three Orthopterans and Tenebrio molitor L. Larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty Acid Profiles and Cholesterol Content of Seven Insect Species Assessed by Several Extraction Systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Commercially Available Feeder Insects Fed Enhanced Diets during Growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- Rossi, G.; Mattioli, S.; Rondoni, G.; Bosco, A.D.; Servili, M.; Castellini, C.; Conti, E. Characterisation of Fatty Acid Profiles of Tenebrio molitor Larvae Reared on Diets Enriched with Edible Oils. J. Insects Food Feed. 2022, 8, 901–912. [Google Scholar] [CrossRef]
- Czernichow, S.; Thomas, D.; Bruckert, E. N-6 Fatty Acids and Cardiovascular Health: A Review of the Evidence for Dietary Intake Recommendations. Br. J. Nutr. 2010, 104, 788–796. [Google Scholar] [CrossRef]
- Turley, J.; Thompson, J. Nutrition: Your Life Science; Cengage Learning: London, UK, 2015. [Google Scholar]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the Edible Insects Acheta Domesticus and Tenebrio molitor with Improved Fatty Acid Profile Due to Ultrasound Assisted or Pressurized Liquid Extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Milićević, D.; Vranić, D.; Mašić, Z.; Parunović, N.; Trbović, D.; Nedeljković-Trailović, J.; Petrović, Z. The Role of Total Fats, Saturated/Unsaturated Fatty Acids and Cholesterol Content in Chicken Meat as Cardiovascular Risk Factors. Lipids Health Dis. 2014, 13, 42. [Google Scholar] [CrossRef]
- Cito, A.; Dreassi, E.; Frosinini, R.; Zanfini, A.; Pianigiani, C.; Botta, M.; Francardi, V. The Potential Beneficial Effects of Tenebrio molitor (Coleoptera Tenebrionidae) and Galleria mellonella (Lepidoptera Pyralidae) on Human Health. Redia 2017, 100, 125–133. [Google Scholar] [CrossRef]
- Francardi, V.; Frosinini, R.; Pichini, C.; Botta, M.; Cito, A.; Dreassi, E. Galleria mellonella (Lepidoptera Pyralidae): An Edible Insect of Nutraceutical Interest. Redia 2017, 100, 187–192. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Self-Selection of Two Diet Components by Tenebrio molitor (Coleoptera: Tenebrionidae) Larvae and Its Impact on Fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Homolková, D.; Božik, M.; Plachý, V.; Vrabec, V. Effect of Developmental Stage on the Nutritional Value of Edible Insects. A Case Study with Blaberus Craniifer and Zophobas Morio. J. Food Compos. Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, B.-D.; Oh, H.-M. Rapid Method for the Determination of Lipid from the Green Alga Botryococcus Braunii. Biotechnol. Tech. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Ekpo, K.E.; Onigbinde, A.O.; Asia, I.O. Pharmaceutical Potentials of the Oils of Some Popular Insects Consumed in Southern Nigeria. Afr. J. Pharm. Pharmacol. 2009, 3, 51–057. [Google Scholar]
- Traversier, M.; Gaslondes, T.; Milesi, S.; Michel, S.; Delannay, E. Polar Lipids in Cosmetics: Recent Trends in Extraction, Separation, Analysis and Main Applications. Phytochem. Rev. 2018, 17, 1179–1210. [Google Scholar] [CrossRef]
- Hazahari, N.Y.B.; Hosokawa, M.; Miyashita, K. Comparison of Oxidative Stability of Monogalactosyl Diacylglycerol, Digalactosyl Diacylglycerol, and Triacylglycerol Containing Polyunsaturated Fatty Acids. Food Nutr. Sci. 2018, 09, 221–234. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Bordoni, L.; Petracci, I.; Zhao, F.; Min, W.; Pierella, E.; Assmann, T.S.; Martinez, J.A.; Gabbianelli, R. Nutrigenomics of Dietary Lipids. Antioxidants 2021, 10, 994. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Hirayama, O.; Oido, H. Changes of Lipid and Pigment Compositions in Spinach Leaves during Their Storage. J. Agric. Chem. Soc. Jpn. 1969, 43, 423–428. [Google Scholar]
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Purschke, B.; Tanzmeister, H.; Meinlschmidt, P.; Baumgartner, S.; Lauter, K.; Jäger, H. Recovery of Soluble Proteins from Migratory Locust (Locusta Migratoria) and Characterisation of Their Compositional and Techno-Functional Properties. Food Res. Int. 2018, 106, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.A.; Southgate, D.A.T.; Russel, J. The Composition of Foods; H.M. Stationery Office: London, UK, 1980. [Google Scholar]
- FAO/WHO Energy and Protein Requirements; Report of a Joint FAO/WHO Ad Hoc Expert Committee, Rome, 22 March–2 April 1971 (No. 52); Food and Agriculture Organization: Rome, Italy, 1973.
- Bártová, V.; Bárta, J.; Brabcová, A.; Zdráhal, Z.; Horáčková, V. Amino Acid Composition and Nutritional Value of Four Cultivated South American Potato Species. J. Food Compos. Anal. 2015, 40, 78–85. [Google Scholar] [CrossRef]
- Sommano, S.R.; Bhat, F.M.; Wongkeaw, M.; Sriwichai, T.; Sunanta, P.; Chuttong, B.; Burgett, M. Amino Acid Profiling and Chemometric Relations of Black Dwarf Honey and Bee Pollen. Front. Nutr. 2020, 7, 558579. [Google Scholar] [CrossRef]
- Yang, F.; Huang, X.; Zhang, C.; Zhang, M.; Huang, C.; Yang, H. Amino Acid Composition and Nutritional Value Evaluation of Chinese Chestnut (Castanea mollissima Blume) and Its Protein Subunit. RSC Adv. 2018, 8, 2653–2659. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein Quality Evaluation Twenty Years after the Introduction of the Protein Digestibility Corrected Amino Acid Score Method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Wang, P.; Huang, J.; Sun, J.; Liu, R.; Jiang, T.; Sun, G. Evaluating the Nutritional Properties of Food: A Scoping Review. Nutrients 2022, 14, 2352. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.J.I.; Chen, J.; Benjamin, O. Extraction, Characterization and Functional Properties of Soluble Proteins from Edible Grasshopper (Schistocerca Gregaria) and Honey Bee (Apis Mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
| FA | ACD | ALD | BD | GM | LM | TM | ZM |
|---|---|---|---|---|---|---|---|
| C8:0 | - | - | - | - | - | - | tr |
| C9:0 | tr | tr | tr | tr | tr | tr | tr |
| C10:0 | tr | tr | tr | tr | tr | tr | tr |
| C11:1 | - | - | - | - | - | - | tr |
| C12:0 | tr | tr | tr | tr | tr | tr | tr |
| C13:0 | - | - | - | - | - | tr | - |
| C13:1 | - | - | tr | - | tr | - | tr |
| C14:0 | tr | tr | tr | tr | 1.67 ± 0.01 a | 3.57 ± 0.01 b | tr |
| C14:1 | - | - | tr | - | tr | tr | tr |
| C15:0 | tr | tr | tr | tr | tr | tr | tr |
| C16:0 | 24.26 ± 0.03 c | 24.29 ± 0.16 c | 15.95 ± 0.03 a | 32.86 ± 0.56 e | 28.44 ± 0.17 d | 18.62 ± 0.33 b | 28.24 ± 0.10 d |
| C16:1 | tr | tr | 2.90 ± 0.05 b | tr | tr | 1.32 ± 0.00 a | tr |
| C17:0 | tr | tr | tr | tr | tr | tr | tr |
| C16:3/C17:1 | - | tr | tr | tr | tr | tr | tr |
| C18:0 | 9.19 ± 0.06 g | 8.70 ± 0.12 f | 6.02 ± 0.09 c | 1.52 ± 0.01 a | 8.27 ± 0.14 e | 5.33 ± 0.24 b | 7.34 ± 0.09 d |
| C18:1 | 23.30 ± 0.11 a | 37.16 ± 0.09 d | 54.26 ± 0.10 f | 42.90 ± 0.54 e | 31.60 ± 0.17 b | 35.63 ± 0.07 c | 36.11 ± 0.05 c |
| C18:2 | 36.67 ± 0.14 e | 22.57 ± 0.10 c | 15.66 ± 0.13 a | 17.32 ± 0.25 b | 16.96 ± 0.08 b | 28.23 ± 0.36 d | 22.36 ± 0.27 c |
| C19:0 | - | tr | tr | - | - | tr | - |
| C18:3n − 3 | 2.55 ± 0.01 b | tr | tr | 1.23 ± 0.01 a | 10.47 ± 0.11 c | 1.18 ± 0.31 a | 1.23 ± 0.03 a |
| C20:0 | tr | tr | tr | tr | tr | tr | tr |
| C20:1 | tr | tr | tr | 2.36 ± 1.38 | tr | tr | - |
| C20:2 | tr | tr | tr | - | - | tr | - |
| C20:3n − 6 | - | tr | - | - | - | - | - |
| C20:4n − 6 | - | tr | tr | - | - | - | - |
| C20:3n − 3 | - | tr | tr | - | - | tr | - |
| C20:5n − 3 | tr | tr | - | - | tr | - | - |
| C22:0 | tr | tr | tr | tr | tr | tr | - |
| C22:1 | tr | tr | tr | tr | tr | tr | - |
| C22:2 | - | - | tr | - | - | - | - |
| C22:5n − 6 | tr | tr | tr | - | tr | tr | - |
| C22:4 | tr | - | - | - | - | - | - |
| C22:5n − 3 | - | tr | tr | tr | tr | - | - |
| C24:0/C22:6n − 3 | - | - | - | tr | tr | tr | - |
| C24:1 | tr | tr | tr | tr | tr | 1.08 ± 0.08 | tr |
| SFA | 35.18 ± 0.09 c | 36.41 ± 0.11 d | 24.64 ± 0.13 a | 34.79 ± 0.58 c | 39.21 ± 0.01 f | 31.17 ± 0.34 b | 38.11 ± 0.31 e |
| MUFA | 25.06 ± 0.05 a | 39.21 ± 0.06 d | 58.28 ± 0.06 f | 46.57 ± 0.85 e | 33.02 ± 0.21 b | 38.42 ± 0.03 cd | 37.95 ± 0.13 c |
| PUFA | 39.76 ± 0.14 f | 24.38 ± 0.16 c | 17.08 ± 0.16 a | 18.64 ± 0.26 b | 27.77 ± 0.21 d | 30.41 ± 0.32 e | 23.94 ± 0.27 c |
| PUFA/SFA | 1.13 ± 0.01 f | 0.67 ± 0.01 c | 0.69 ± 0.01 cd | 0.54 ± 0.00 a | 0.71 ± 0.01 d | 0.98 ± 0.02 e | 0.63 ± 0.01 b |
| n − 3 | 2.75 ± 0.01 d | 1.15 ± 0.05 b | 0.89 ± 0.00 a | 1.26 ± 0.02 bc | 10.67 ± 0.15 e | 1.49 ± 0.16 c | 1.23 ± 0.03 b |
| n − 6 | 37.01 ± 0.13 f | 23.09 ± 0.10 d | 16.05 ± 0.15 a | 17.32 ± 0.25 b | 16.97 ± 0.09 b | 28.77 ± 0.15 e | 22.36 ± 0.27 c |
| n − 6/n − 3 | 13.47 ± 0.02 b | 20.16 ± 0.76 d | 18.04 ± 0.18 c | 13.76 ± 0.12 b | 1.59 ± 0.02 a | 19.43 ± 1.92 cd | 18.24 ± 0.53 cd |
| IA | 0.41 ± 0.00 b | 0.44 ± 0.00 c | 0.26 ± 0.00 a | 0.51 ± 0.01 e | 0.58 ± 0.00 f | 0.48 ± 0.01 d | 0.52 ± 0.00 e |
| IT | 0.86 ± 0.00 d | 0.98 ± 0.01 e | 0.57 ± 0.00 a | 0.96 ± 0.02 e | 0.67 ± 0.00 b | 0.72 ± 0.03 c | 1.08 ± 0.01 f |
| h/H | 2.54 ± 0.01 e | 2.44 ± 0.02 d | 4.20 ± 0.01 g | 1.86 ± 0.01 a | 1.96 ± 0.01 b | 2.94 ± 0.05 f | 2.06 ± 0.01 c |
| FA | ACD | ALD | BD | GM | LM | TM | ZM |
|---|---|---|---|---|---|---|---|
| C8:0 | - | - | - | - | - | - | tr |
| C9:0 | tr | tr | tr | tr | tr | tr | tr |
| C10:0 | tr | tr | tr | tr | tr | tr | tr |
| C11:1 | - | - | - | - | - | - | tr |
| C12:0 | tr | - | tr | - | tr | tr | tr |
| C13:0 | - | - | - | - | - | tr | - |
| C13:1 | - | - | - | - | - | - | tr |
| C14:0 | tr | tr | tr | tr | 1.88 ± 0.02 a | 2.68 ± 0.07 b | tr |
| C14:1 | - | - | tr | - | tr | - | - |
| C15:0 | tr | tr | tr | tr | tr | tr | tr |
| C16:0 | 25.91 ± 0.09 d | 22.66 ± 0.25 c | 17.99 ± 0.04 b | 33.93 ± 0.59 | 30.61 ± 0.23 f | 16.13 ± 0.02 a | 27.95 ± 0.08 e |
| C16:1 | tr | tr | 3.36 ± 0.04 b | tr | tr | 1.28 ± 0.01 a | tr |
| C17:0 | tr | tr | tr | tr | tr | tr | tr |
| C16:3/C17:1 | - | tr | tr | tr | tr | tr | tr |
| C18:0 | 7.35 ± 0.00 e | 7.69 ± 0.06 f | 4.34 ± 0.05 c | 1.01 ± 0.01 a | 6.87 ± 0.19 d | 3.74 ± 0.12 b | 6.94 ± 0.03 d |
| C18:1 | 29.28 ± 0.17 a | 42.48 ± 0.26 e | 58.11 ± 0.14 | 43.85 ± 0.61 f | 34.18 ± 0.25 b | 41.42 ± 0.24 d | 37.87 ± 0.07 c |
| C18:2 | 30.59 ± 0.08 e | 21.68 ± 0.22 d | 12.03 ± 0.06 a | 16.01 ± 0.27 c | 15.08 ± 0.10 b | 31.46 ± 0.07 f | 21.69 ± 0.04 d |
| C19:0 | - | - | - | - | - | - | - |
| C18:3n − 3 | 2.54 ± 0.01 c | tr | tr | 1.00 ± 0.02 ab | 9.21 ± 0.12 d | tr | 1.19 ± 0.04 b |
| C20:0 | - | tr | tr | - | - | tr | - |
| C20:1 | tr | tr | - | 2.53 ± 1.52 | - | - | - |
| C20:2 | - | - | - | - | - | - | - |
| C20:3n − 6 | - | tr | - | - | - | - | - |
| C20:4n − 6 | - | - | - | - | - | - | - |
| C20:3n − 3 | - | - | - | - | - | - | - |
| C20:5n − 3 | - | tr | - | - | - | - | - |
| C22:0 | - | - | - | - | - | - | - |
| C22:1 | - | - | - | - | - | - | - |
| C22:2 | - | - | - | - | - | - | - |
| C22:5n − 6 | tr | tr | tr | - | - | tr | - |
| C22:4 | tr | - | - | - | - | - | - |
| C22:5n − 3 | - | tr | - | - | - | - | - |
| C24:0/C22:6n − 3 | - | - | - | - | - | - | - |
| C24:1 | 1.43 ± 0.04 | tr | tr | tr | tr | tr | tr |
| SFA | 34.58 ± 0.03 c | 32.08 ± 0.11 b | 24.17 ± 0.11 a | 35.26 ± 0.61 c | 39.92 ± 0.09 e | 23.68 ± 0.11 a | 36.91 ± 0.24 d |
| MUFA | 31.85 ± 0.10 a | 44.27 ± 0.25 d | 62.48 ± 0.11 f | 47.67 ± 0.90 e | 35.68 ± 0.29 b | 43.58 ± 0.24 d | 39.87 ± 0.20 c |
| PUFA | 33.57 ± 0.07 f | 23.64 ± 0.19 c | 13.35 ± 0.10 a | 17.07 ± 0.29 b | 24.40 ± 0.25 d | 32.74 ± 0.13 e | 23.22 ± 0.04 c |
| FA | ACD | ALD | BD | GM | LM | TM | ZM |
|---|---|---|---|---|---|---|---|
| C8:0 | - | - | - | - | - | - | tr |
| C9:0 | tr | - | tr | - | tr | tr | tr |
| C10:0 | - | tr | - | tr | tr | - | tr |
| C11:1 | - | - | - | - | - | - | - |
| C12:0 | tr | tr | tr | tr | tr | tr | - |
| C13:0 | - | - | - | - | - | tr | - |
| C13:1 | - | - | tr | - | tr | - | - |
| C14:0 | tr | 1.13 ± 0.00 a | 1.18 ± 0.01 a | tr | 2.11 ± 0.01 c | 4.82 ± 0.05 d | 1.29 ± 0.03 b |
| C14:1 | - | - | tr | - | - | tr | tr |
| C15:0 | tr | tr | 1.01 ± 0.04 | tr | 1.20 ± 0.04 | tr | tr |
| C16:0 | 28.88 ± 0.13 d | 26.17 ± 0.03 c | 16.94 ± 0.05 a | 25.92 ± 0.31 c | 29.45 ± 0.10 e | 19.64 ± 0.05 b | 28.65 ± 0.29 d |
| C16:1 | tr | 1.20 ± 0.00 b | 3.89 ± 0.02 d | tr | tr | 1.67 ± 0.00 c | 1.06 ± 0.06 a |
| C17:0 | tr | tr | tr | tr | - | tr | tr |
| C16:3/C17:1 | - | tr | tr | tr | tr | tr | tr |
| C18:0 | 7.08 ± 0.18 d | 6.47 ± 0.02 c | 5.43 ± 0.05 b | 3.60 ± 0.12 a | 8.08 ± 0.03 e | 3.45 ± 0.01 a | 6.53 ± 0.23 c |
| C18:1 | 23.92 ± 0.22 a | 37.96 ± 0.12 e | 50.87 ± 0.14 g | 39.21 ± 0.20 f | 30.48 ± 0.09 b | 36.41 ± 0.09 d | 34.72 ± 0.36 c |
| C18:2 | 33.45 ± 0.24 f | 23.57 ± 0.03 c | 16.96 ± 0.06 b | 25.49 ± 0.23 d | 16.08 ± 0.10 a | 30.39 ± 0.03 e | 23.18 ± 0.21 c |
| C19:0 | - | - | - | - | - | - | - |
| C18:3n − 3 | 3.31 ± 0.02 d | tr | 1.01 ± 0.02 a | 2.42 ± 0.03 c | 9.59 ± 0.07 e | tr | 1.40 ± 0.03 b |
| C20:0 | tr | tr | tr | - | tr | - | - |
| C20:1 | - | tr | - | 1.39 ± 0.62 | tr | - | - |
| C20:2 | - | - | - | - | - | - | - |
| C20:3n − 6 | - | - | - | - | - | - | - |
| C20:4n − 6 | - | tr | - | - | - | - | - |
| C20:3n − 3 | - | - | - | - | - | - | - |
| C20:5n − 3 | tr | tr | - | - | tr | - | - |
| C22:0 | - | tr | - | - | tr | - | - |
| C22:1 | - | - | - | - | tr | - | - |
| C22:2 | - | - | tr | - | - | - | - |
| C22:5n − 6 | - | - | - | - | - | - | - |
| C22:4 | - | - | - | - | - | - | - |
| C22:5n − 3 | - | - | tr | - | tr | - | - |
| C24:0/C22:6n − 3 | - | - | - | - | - | - | - |
| C24:1 | tr | tr | 1.00 ± 0.11 | tr | tr | tr | tr |
| SFA | 37.93 ± 0.05 e | 34.98 ± 0.15 d | 25.50 ± 0.02 a | 30.09 ± 0.23 c | 41.75 ± 0.06 g | 29.60 ± 0.12 b | 38.71 ± 0.08 f |
| MUFA | 25.15 ± 0.22 a | 39.88 ± 0.11 e | 55.91 ± 0.09 g | 41.95 ± 0.45 f | 32.23 ± 0.11 b | 38.91 ± 0.12 d | 36.27 ± 0.31 c |
| PUFA | 36.92 ± 0.27 f | 25.15 ± 0.05 b | 18.59 ± 0.11 a | 27.96 ± 0.23 d | 26.02 ± 0.17 c | 31.50 ± 0.01 e | 25.02 ± 0.23 b |
| FA | ACD | ALD | BD | GM | LM | TM | ZM |
|---|---|---|---|---|---|---|---|
| C8:0 | - | - | - | - | - | - | - |
| C9:0 | tr | - | tr | - | - | 5.00 ± 3.92 | - |
| C10:0 | tr | - | tr | - | tr | 1.66 ± 0.90 | - |
| C11:1 | - | - | - | - | - | - | - |
| C12:0 | - | tr | - | tr | - | 1.02 ± 0.34 | - |
| C13:0 | - | - | - | - | - | - | - |
| C13:1 | - | - | - | - | - | - | - |
| C14:0 | tr | tr | tr | tr | tr | 2.50 ± 0.37 | - |
| C14:1 | - | - | - | - | - | - | - |
| C15:0 | tr | 1.78 ± 0.07 a | tr | tr | tr | 3.78 ± 0.22 b | tr |
| C16:0 | 16.27 ± 0.18 a | 24.63 ± 1.28 b | 10.53 ± 0.02 a | 27.31 ± 2.02 b | 16.66 ± 0.21 a | 26.09 ± 2.62 b | 29.98 ± 5.02 b |
| C16:1 | tr | tr | 1.07 ± 0.15 | tr | tr | - | - |
| C17:0 | tr | tr | tr | tr | - | 1.17 ± 0.15 | tr |
| C16:3/C17:1 | - | - | - | - | tr | - | - |
| C18:0 | 14.86 ± 0.17 b | 17.85 ± 0.96 c | 10.31 ± 0.27 a | 9.10 ± 0.71 a | 15.59 ± 0.04 b | 21.28 ± 1.23 d | 21.91 ± 0.54 d |
| C18:1 | 11.91 ± 0.06 b | 17.74 ± 0.71 cd | 48.03 ± 0.17 f | 27.86 ± 1.41 e | 19.15 ± 0.10 d | 4.13 ± 0.26 a | 15.83 ± 2.02 c |
| C18:2 | 50.86 ± 0.47 c | 22.84 ± 0.57 b | 22.99 ± 0.35 b | 25.59 ± 1.19 b | 27.13 ± 0.04 b | 4.77 ± 4.35 a | 27.40 ± 5.28 b |
| C19:0 | - | tr | tr | - | - | tr | - |
| C18:3n − 3 | 1.73 ± 0.04 a | tr | tr | 3.81 ± 0.28 a | 17.47 ± 0.10 b | 2.31 ± 3.69 a | tr |
| C20:0 | tr | 4.53 ± 0.23 b | 1.35 ± 0.03 a | tr | tr | 7.99 ± 0.61 c | 1.67 ± 1.53 a |
| C20:1 | - | tr | tr | tr | - | tr | - |
| C20:2 | tr | tr | tr | - | - | 2.93 ± 4.54 | - |
| C20:3n − 6 | - | - | - | - | - | - | - |
| C20:4n − 6 | - | tr | tr | - | - | - | - |
| C20:3n − 3 | - | tr | tr | - | - | tr | - |
| C20:5n − 3 | tr | tr | - | - | - | - | - |
| C22:0 | tr | 3.47 ± 2.82 | tr | tr | tr | 4.48 ± 0.37 | - |
| C22:1 | tr | tr | tr | tr | tr | 2.77 ± 0.36 | - |
| C22:2 | - | - | - | - | - | - | - |
| C22:5n − 6 | - | - | - | - | tr | - | - |
| C22:4 | - | - | - | - | - | - | - |
| C22:5n − 3 | - | - | - | tr | tr | - | - |
| C24:0/C22:6n − 3 | - | - | - | tr | tr | 2.97 ± 0.83 | - |
| C24:1 | tr | 1.74 ± 0.32 ab | 1.03 ± 0.01 a | tr | tr | 3.23 ± 0.53 b | 1.22 ± 1.11 a |
| SFA | 33.34 ± 0.45 b | 54.26 ± 0.98 c | 25.04 ± 0.28 a | 39.28 ± 2.88 b | 33.92 ± 0.44 b | 75.76 ± 2.94 d | 55.20 ± 5.24 c |
| MUFA | 12.96 ± 0.05 a | 20.93 ± 0.47 b | 50.49 ± 0.07 d | 30.35 ± 1.88 c | 19.94 ± 0.08 b | 10.91 ± 0.67 a | 17.05 ± 2.83 b |
| PUFA | 53.70 ± 0.50 d | 24.81 ± 0.52 b | 24.47 ± 0.35 b | 30.37 ± 1.00 b | 46.14 ± 0.44 c | 13.33 ± 3.06 a | 27.76 ± 5.05 b |
| Amino Acid | ACD | ALD | BD | GM | LM | TM | ZM | FAO/WHO (2007) Standard |
|---|---|---|---|---|---|---|---|---|
| Met | 2.20 ± 0.13 b | 1.71 ± 0.08 a | 1.57 ± 0.06 a | 1.64 ± 0.25 a | 1.42 ± 0.21 a | 1.75 ± 0.27 ab | 1.64 ± 0.03 a | |
| Cyss + cys | 1.46 ± 0.25 b | 0.84 ± 0.04 a | 1.21 ± 0.08 ab | 0.73 ± 0.11 a | 0.87 ± 0.19 a | 1.15 ± 0.10 ab | 0.90 ± 0.36 a | |
| Total sulphur AA | 3.65 ± 0.38 b | 2.56 ± 0.11 a | 2.78 ± 0.03 ab | 2.37 ± 0.36 a | 2.29 ± 0.39 a | 2.90 ± 0.37 ab | 2.55 ± 0.35 a | 1.6 |
| Tyr | 6.26 ± 0.57 ab | 6.16 ± 0.38 ab | 7.28 ± 0.78 bc | 6.47 ± 0.66 ab | 5.64 ± 0.28 a | 8.11 ± 0.48 cd | 9.23 ± 0.35 d | |
| Phe | 4.07 ± 0.13 abcd | 4.22 ± 0.29 bcd | 3.69 ± 0.09 abc | 3.36 ± 0.61 ab | 3.23 ± 0.09 a | 4.39 ± 0.50 cd | 4.88 ± 0.10 d | |
| Total aromatic AA | 10.33 ± 0.70 ab | 10.38 ± 0.67 ab | 10.97 ± 0.77 ab | 9.83 ± 1.25 a | 8.87 ± 0.20 a | 12.49 ± 0.98 bc | 14.11 ± 0.45 c | 3.8 |
| Ile | 4.46 ± 0.07 b | 4.77 ± 0.03 bc | 3.95 ± 0.19 a | 3.97 ± 0.34 a | 4.66 ± 0.10 bc | 4.94 ± 0.06 c | 4.91 ± 0.06 c | 3 |
| Leu | 7.79 ± 0.02 cd | 7.36 ± 0.06 bc | 6.99 ± 0.16 ab | 6.55 ± 0.57 a | 8.44 ± 0.03 d | 7.88 ± 0.09 cd | 7.56 ± 0.13 bc | 5.9 |
| Lys | 5.20 ± 0.66 a | 7.35 ± 0.42 b | 6.03 ± 0.65 ab | 5.67 ± 0.89 a | 5.01 ± 0.31 a | 5.20 ± 0.44 a | 5.09 ± 0.22 a | 4.5 |
| Thr | 4.07 ± 0.14 ab | 4.42 ± 0.01 b | 4.20 ± 0.11 ab | 3.70 ± 0.53 a | 3.82 ± 0.10 ab | 4.28 ± 0.04 ab | 4.27 ± 0.04 ab | 2.3 |
| Val | 6.59 ± 0.24 b | 6.40 ± 0.01 b | 7.01 ± 0.37 b | 5.44 ± 0.52 a | 7.10 ± 0.33 b | 7.13 ± 0.13 b | 6.99 ± 0.05 b | 3.9 |
| His | 2.92 ± 0.20 b | 3.21 ± 0.19 bc | 3.14 ± 0.30 bc | 2.04 ± 0.40 a | 2.87 ± 0.27 b | 3.55 ± 0.19 bc | 3.80 ± 0.10 c | 1.5 |
| Total EAA | 45.01 ± 0.02 bc | 46.44 ± 0.61 bc | 45.07 ± 0.34 bc | 39.57 ± 4.83 a | 43.06 ± 0.54 ab | 48.37 ± 1.24 bc | 49.28 ± 0.34 c | |
| Ser | 5.36 ± 0.27 ab | 4.66 ± 0.03 ab | 4.66 ± 0.09 ab | 10.09 ± 5.42 b | 4.08 ± 0.10 a | 5.05 ± 0.06 ab | 4.84 ± 0.04 ab | |
| Arg | 7.78 ± 0.15 c | 5.91 ± 0.22 ab | 5.71 ± 0.19 ab | 5.01 ± 0.74 a | 6.20 ± 0.17 b | 5.94 ± 0.19 b | 5.70 ± 0.11 ab | |
| Gly | 6.17 ± 0.16 abc | 5.02 ± 0.15 a | 6.97 ± 0.51 bc | 6.36 ± 1.54 abc | 7.34 ± 0.38 c | 5.96 ± 0.03 abc | 5.53 ± 0.09 ab | |
| Asx | 8.28 ± 0.28 ab | 9.47 ± 0.41 c | 9.31 ± 0.68 bc | 10.14 ± 0.36 c | 7.21 ± 0.31 a | 7.88 ± 0.36 a | 8.16 ± 0.21 a | |
| Glx | 11.72 ± 0.89 | 13.43 ± 0.44 | 12.05 ± 1.05 | 13.74 ± 1.69 | 11.28 ± 0.55 | 11.49 ± 0.43 | 13.24 ± 0.33 | |
| Ala | 9.17 ± 0.46 bc | 7.95 ± 0.25 ab | 9.68 ± 0.88 c | 7.32 ± 0.79 a | 12.73 ± 0.31 d | 7.65 ± 0.47 a | 7.25 ± 0.05 a | |
| Pro | 6.52 ± 0.38 ab | 7.13 ± 0.09 bc | 6.56 ± 0.28 ab | 7.77 ± 0.83 c | 8.09 ± 0.31 c | 7.66 ± 0.11 c | 6.00 ± 0.06 a | |
| Total NEAA | 54.99 ± 0.02 ab | 53.56 ± 0.61 ab | 54.93 ± 0.34 ab | 60.43 ± 4.83 c | 56.94 ± 0.54 bc | 51.63 ± 1.24 ab | 50.72 ± 0.34 a | |
| E/T (%) | 45.01 ± 0.02 bc | 46.44 ± 0.61 bc | 45.07 ± 0.34 bc | 39.57 ± 4.83 a | 43.06 ± 0.54 ab | 48.37 ± 1.24 bc | 49.28 ± 0.34 c | |
| EAAI | 2.48 ± 0.00 bc | 2.48 ± 0.01 bc | 2.48 ± 0.00 bc | 2.43 ± 0.04 a | 2.46 ± 0.01 ab | 2.50 ± 0.01 bc | 2.51 ± 0.00 c | |
| AAS (%) | 132.09 ± 0.41 | 124.75 ± 0.96 | 118.54 ± 2.71 | 111.02 ± 9.71 | 111.30 ± 6.82 | 115.49 ± 9.85 | 159.28 ± 21.71 | |
| Limiting AA | Lys | Leu | Leu | Leu | Lys | Lys | Met + Cys | |
| PER−1 | 2.56 ± 0.02 cd | 2.34 ± 0.02 bc | 2.20 ± 0.09 ab | 1.94 ± 0.22 a | 2.78 ± 0.03 d | 2.55 ± 0.04 cd | 2.48 ± 0.06 c | |
| PER−2 | 2.41 ± 0.06 d | 2.23 ± 0.03 bcd | 1.94 ± 0.15 ab | 1.83 ± 0.20 a | 2.77 ± 0.04 e | 2.26 ± 0.04 cd | 1.99 ± 0.09 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. https://doi.org/10.3390/foods12224090
Perez-Santaescolastica C, de Pril I, van de Voorde I, Fraeye I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods. 2023; 12(22):4090. https://doi.org/10.3390/foods12224090
Chicago/Turabian StylePerez-Santaescolastica, Cristina, Ilse de Pril, Ilse van de Voorde, and Ilse Fraeye. 2023. "Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors" Foods 12, no. 22: 4090. https://doi.org/10.3390/foods12224090
APA StylePerez-Santaescolastica, C., de Pril, I., van de Voorde, I., & Fraeye, I. (2023). Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods, 12(22), 4090. https://doi.org/10.3390/foods12224090






