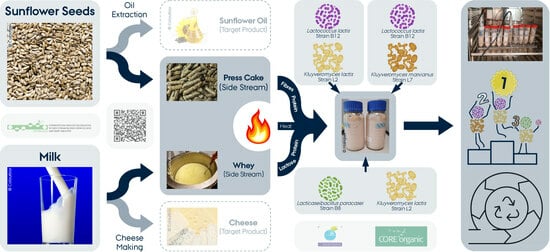
Shaping Future Foods through Fermentation of Side Streams: Microbial, Chemical, and Physical Characteristics of Fermented Blends from Sunflower Seed Press Cake and Cheese Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microbial Analyses
2.2.1. pH Development
2.2.2. Cell Counts
2.3. Chemical Analyses
2.3.1. Degree of Protein Hydrolysis
2.3.2. Gel Electrophoresis
2.3.3. Determination of Sugars
2.3.4. Total Acidity
2.4. Physical Analyses
2.4.1. Rheological Properties
2.4.2. Colour Measurements
2.4.3. Microstructure
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics
3.1.1. Cell Growth of Lactic Acid Bacteria and Yeasts
3.1.2. Acidification Kinetics
3.1.3. Growth of Microbial Contaminants
3.1.4. Metabolisation of Sugars
3.2. Effect of Fermentation on Sunflower Proteins
3.3. Rheological Properties of Fresh Fermented Blends
3.3.1. Small Deformation Properties
3.3.2. Large Deformation Properties
3.4. Visual Appearance and Microstructure
3.5. Storage Stability of the Fermented Blends
4. Conclusions
4.1. Fermentation of Side Stream Blends
4.2. Further Considerations and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lie-Piang, A.; Yang, J.; Schutyser, M.A.I.; Nikiforidis, C.V.; Boom, R.M. Mild Fractionation for More Sustainable Food Ingredients. Annu. Rev. Food Sci. Technol. 2023, 14, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and Product-Related Causes for Food Waste and Implications for the Food Supply Chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Raak, N.; Struck, S.; Jaros, D.; Hernando, I.; Gülseren, İ.; Michalska-Ciechanowska, A.; Foschino, R.; Corredig, M.; Rohm, H. Blending Side Streams. A Potential Solution to Reach a Resource Efficient, Circular, Zero-Waste Food System. Future Foods 2022, 6, 100207. [Google Scholar] [CrossRef]
- Corredig, M.; Young, N.; Dalsgaard, T.K. Food Proteins: Processing Solutions and Challenges. Curr. Opin. Food Sci. 2020, 35, 49–53. [Google Scholar] [CrossRef]
- Karefyllakis, D.; van der Goot, A.J.; Nikiforidis, C.V. Multicomponent Emulsifiers from Sunflower Seeds. Curr. Opin. Food Sci. 2019, 29, 35–41. [Google Scholar] [CrossRef]
- Morejón Caraballo, S.; Rohm, H.; Struck, S. Green Solvents for Deoiling Pumpkin and Sunflower Press Cake: Impact on Composition and Technofunctional Properties. Int. J. Food Sci. Technol. 2023, 58, 1931–1939. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Octaviana, H.; van der Goot, A.J.; Nikiforidis, C.V. The Emulsifying Performance of Mildly Derived Mixtures from Sunflower Seeds. Food Hydrocoll. 2019, 88, 75–85. [Google Scholar] [CrossRef]
- Raak, N.; Corredig, M. Towards Creating Sustainable Foods from Side Streams: Heat-Induced Structure Formation in Blends from Sunflower Seed Press Cakes and Cheese Whey under Moderate Shear. Food Hydrocoll. 2023, 144, 108932. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient Density and Nutritional Value of Milk and Plant-Based Milk Alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Katidi, A.; Xypolitaki, K.; Vlassopoulos, A.; Kapsokefalou, M. Nutritional Quality of Plant-Based Meat and Dairy Imitation Products and Comparison with Animal-Based Counterparts. Nutrients 2023, 15, 401. [Google Scholar] [CrossRef]
- Shaghaghian, S.; McClements, D.J.; Khalesi, M.; Garcia-Vaquero, M.; Mirzapour-Kouhdasht, A. Digestibility and Bioavailability of Plant-Based Proteins Intended for Use in Meat Analogues: A Review. Trends Food Sci. Technol. 2022, 129, 646–656. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavaers, G.M. Mixing Animal and Plant Proteins: Is this a Way to Improve Protein Techno-Functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Tsermoula, P.; Khakimov, B.; Nielsen, J.H.; Engelsen, S.B. WHEY—The Waste-Stream That Became More Valuable than the Food Product. Trends Food Sci. Technol. 2021, 118, 230–241. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey-Ing up the Options—Yesterday, Today and Tomorrow. Int. Dairy J. 2015, 48, 2–14. [Google Scholar] [CrossRef]
- Terefe, N.S.; Augustin, M.A. Fermentation for Tailoring the Technological and Health Related Functionality of Food Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Valtonen, A.; Heikki, A.; Nisov, A.; Nikinmaa, M.; Honkapää, K.; Sozer, N. Synergistic Use of Fermentation and Extrusion Processing to Design Plant Protein-Based Sausages. LWT 2023, 184, 115067. [Google Scholar] [CrossRef]
- Pöri, P.; Lille, M.; Edelmann, M.; Aisala, H.; Santangelo, D.; Coda, R.; Sozer, N. Technological and Sensory Properties of Plant-Based Meat Analogues Containing Fermented Sunflower Protein Concentrate. Future Foods 2023, 8, 100244. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, D. Design of Microbial Consortia for the Fermentation of Pea-Protein-Enriched Emulsions. Int. J. Food Microbiol. 2019, 16, 124–136. [Google Scholar] [CrossRef]
- Mangieri, N.; Ambrosini, D.; Baroffio, S.; Vigentini, I.; Foschino, R.; De Noni, I. Valorisation of Bovine Sweet Whey and Sunflower Press Cake Blend Through Controlled Fermentation as Platform for Innovative Food Materials. Foods 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, N.; Rosciano, G.; Porcellato, D.; Winther, A.R.; De Noni, I.; Fracassetti, D.; Foschino, R.; Vigentini, I. Sustainability of Food Side Streams: A Case Study of Fermented Blends Made with Sour Whey and Sunflower Press Cake Powder Using the Back-Slopping Technique. Front. Sustain. Food Syst. 2023, 7, 1166002. [Google Scholar] [CrossRef]
- Viola, R.; Pelloux, J.; van der Ploeg, A.; Gillespie, T.; Marquis, N.; Roberts, A.G.; Hancock, R.D. Symplastic Connection is Required for Bud Outgrowth Following Dormancy in Potato (Solanum tuberosum L.) tubers. Plant Cell Environ. 2007, 30, 973–983. [Google Scholar] [CrossRef]
- Corker, A.; Ng, H.C.-H.; Poole, R.J.; García-Tuñón, E. 3D Printing with 2D Colloids: Designing Rheology Protocols to Predict ‘Printability’ of Soft-Materials. Soft Mat. 2019, 15, 1444–1456. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–Function Relationships of the Antibacterial Activity of Phenolic Acids and Their Metabolism by Lactic Acid Bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Torriani, S.; Felis, G.E. The Genus Lactobacillus: A Taxonomic Update. Probiotics Antimicro. Prot. 2012, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The Proteolytic System. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic Systems of Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Trappe, V.; Weitz, D.A. Scaling of Viscoelasticity of Weakly Attractive Particles. Phys. Rev. Lett. 2000, 85, 449. [Google Scholar] [CrossRef]
- Raak, N.; Abbate, R.A.; Alkhalaf, M.; Lederer, A.; Rohm, H.; Jaros, D. Concentration-Triggered Liquid-to-Solid Transition of Sodium Caseinate Suspensions as a Function of Temperature and Enzymatic Cross-Linking. Food Hydrocoll. 2020, 101, 105464. [Google Scholar] [CrossRef]
- Raak, N.; Jaros, D.; Rohm, H. Acid-Induced Gelation of Enzymatically Cross-Linked Caseinates: Small and Large Deformation Rheology in Relation to Water Holding Capacity and Micro-Rheological Properties. Coll. Surf. A 2021, 619, 126468. [Google Scholar] [CrossRef]
- Campbell, L.J.; Gu, X.; Dewar, S.J.; Euston, S.R. Effects of Heat Treatment and Glucono-δ-Lactone-induced Acidification on Characteristics of Soy Protein Isolates. Food Hydrocoll. 2009, 23, 344–351. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological Characteristics and Gelation Mechanism of Tofu (Soybean Curd). J. Agric. Food Chem. 1995, 45, 1808–1812. [Google Scholar] [CrossRef]
- Peng, X.; Ren, C.; Guo, S. Particle Formation and Gelation of Soymilk: Effect of Heat. Trends Food Sci. Technol. 2016, 54, 138–147. [Google Scholar] [CrossRef]
- Schuldt, S.; Boden, L.; Schneider, Y.; Rohm, H. Pre-Crack Cutting Properties of Viscoelastic Food Models. J. Food Eng. 2016, 169, 272–277. [Google Scholar] [CrossRef]
- Costanzo, S.; Banc, A.; Louhichi, A.; Chauveau, E.; Wu, B.; Morel, M.-H.; Ramos, L. Tailoring the Viscoelasticity of Polymer Gels of Gluten Proteins through Solvent Quality. Macromolecules 2020, 53, 9470–9479. [Google Scholar] [CrossRef]
- Schlangen, M.; Ribberink, M.A.; Taghian Dinani, S.; Sagis, L.M.C.; van der Goot, A.J. Mechanical and Rheological Effects of Transglutaminase Treatment on Dense Plant Protein Blends. Food Hydrocoll. 2023, 136, 108261. [Google Scholar] [CrossRef]
- Shand, P.J.; Ya, H.; Pietrasik, Z.; Wanasundara, P.K.J.P.D. Transglutaminase Treatment of Pea Proteins: Effect on Physicochemical and Rheological Properties of Heat-Induced Protein Gels. Food Chem. 2008, 107, 692–699. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Sagis, L.M.C.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Mapping the Texture of Plant Protein Blends for Meat Analogues. Food Hydrocoll. 2021, 118, 106753. [Google Scholar] [CrossRef]
- Schlangen, M.; Raak, N.; Dinani, S.T.; Corredig, M.; Van Der Goot, A.J. How Fractionation Procedure of Mung Bean Protein Affects Transglutaminase Crosslinking. Food Hydrocoll. 2023, 145, 109067. [Google Scholar] [CrossRef]
- Çakır, E.; Foegeding, E.A. Combining Protein Micro-Phase Separation and Protein-Polysaccharide Segregative Phase Separation to Produce Gel Structures. Food Hydrocoll. 2011, 25, 1538–1546. [Google Scholar] [CrossRef]
- Ersch, C.; ter Laak, I.; van der Linden, E.; Venema, P.; Martin, A. Modulating Fracture Properties of Mixed Protein Systems. Food Hydrocoll. 2015, 44, 59–65. [Google Scholar] [CrossRef]
- Vaintraub, I.A.; Kratch, V.V. Changes in Free and Bound Chlorogenic Acid and in Polyphenoloxidase Activity during the Industrial Processing of Sunflower Seeds. Food/Nahrung 1989, 33, 95–97. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining Upcycled Food: The Dual Role of Upcycling in Reducing Food Loss and Waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]








| Unfermented | B12L2 | B12L7 | B8L2 | |
|---|---|---|---|---|
| L* (−) | 66.5 ± 3.3 a | 75.0 ± 0.2 b | 72.3 ± 1.8 a,b | 72.8 ± 1.2 a,b |
| a* (−) | 3.4 ± 0.1 a | 0.0 ± 0.4 b | 0.6 ± 1.0 b | 0.6 ± 0.7 b |
| b* (−) | 16.7 ± 0.4 a | 13.7 ± 0.2 b | 14.7 ± 1.2 a,b | 14.6 ± 2.2 a,b |
| Co-Culture | Fermentation Time (h) | pH (−) | Bacterial Contaminants (Log10 CFU/g) | G’ (Pa) | tan δ (−) |
|---|---|---|---|---|---|
| B12L2 | 12 24 48 | 5.23 ± 0.62 4.78 ± 0.35 4.57 ± 0.25 | <2 <2 <2 | 18,490 ± 5890 21,755 ± 7115 23,300 ± 1580 | 0.22 ± 0.01 0.22 ± 0.01 0.22 ± 0.01 |
| B12L7 | 12 24 48 | 5.08 ± 0.21 4.88 ± 0.18 4.65 ± 0.08 | <2 2.48 ± 0.34 * <2 | 19,105 ± 6715 14,540 ± 3800 17,370 ± 4520 | 0.22 ± 0.01 0.22 ± 0.01 0.22 ± 0.01 |
| B8L2 | 12 24 48 | 5.96 ± 0.04 5.50 ± 0.31 4.67 ± 0.21 | 2.96 ± 1.36 * 4.67 ± 0.28 * 4.80 ± 0.28 * | 18,620 ± 1340 * 27,425 ± 2775 * 24,925 ± 3525 | 0.20 ± 0.00 0.21 ± 0.00 * 0.21 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raak, N.; Mangieri, N.; Foschino, R.; Corredig, M. Shaping Future Foods through Fermentation of Side Streams: Microbial, Chemical, and Physical Characteristics of Fermented Blends from Sunflower Seed Press Cake and Cheese Whey. Foods 2023, 12, 4099. https://doi.org/10.3390/foods12224099
Raak N, Mangieri N, Foschino R, Corredig M. Shaping Future Foods through Fermentation of Side Streams: Microbial, Chemical, and Physical Characteristics of Fermented Blends from Sunflower Seed Press Cake and Cheese Whey. Foods. 2023; 12(22):4099. https://doi.org/10.3390/foods12224099
Chicago/Turabian StyleRaak, Norbert, Nicola Mangieri, Roberto Foschino, and Milena Corredig. 2023. "Shaping Future Foods through Fermentation of Side Streams: Microbial, Chemical, and Physical Characteristics of Fermented Blends from Sunflower Seed Press Cake and Cheese Whey" Foods 12, no. 22: 4099. https://doi.org/10.3390/foods12224099
APA StyleRaak, N., Mangieri, N., Foschino, R., & Corredig, M. (2023). Shaping Future Foods through Fermentation of Side Streams: Microbial, Chemical, and Physical Characteristics of Fermented Blends from Sunflower Seed Press Cake and Cheese Whey. Foods, 12(22), 4099. https://doi.org/10.3390/foods12224099