Recent Advances in Non-Targeted Screening of Compounds in Plastic-Based/Paper-Based Food Contact Materials
Abstract
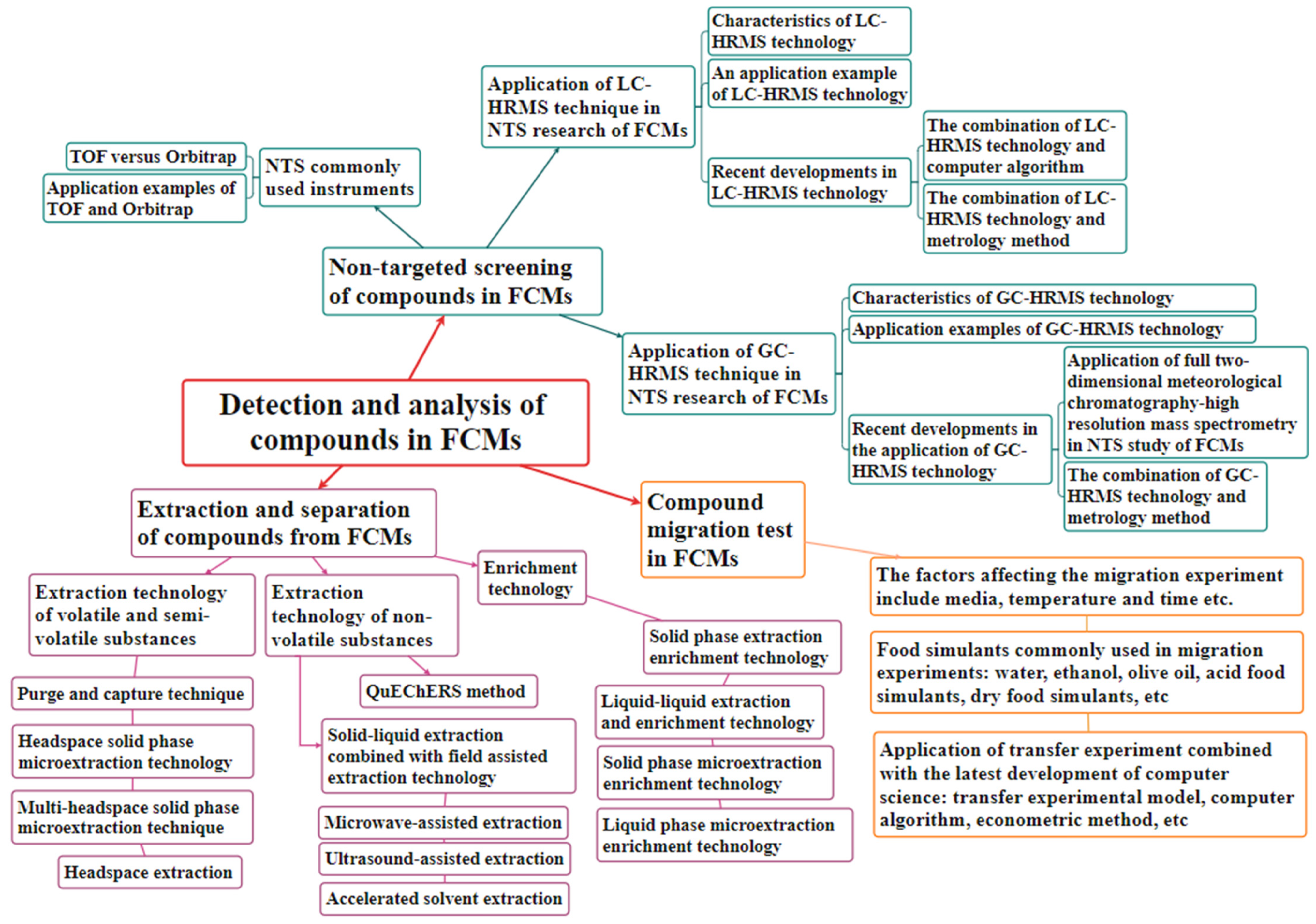
:1. Introduction
2. Separation, Extraction, and Migration Experiments of Chemical Substances in Plastic/Paper-Based FCMs
2.1. Separation and Extraction of Chemical Compounds in Plastic/Paper-Based FCMs
2.2. Migration Experiment
2.3. Recent Developments and Applications of Migration Experiments
3. NTS MS Analysis of Compounds in Plastic/Paper-Based FCMs
3.1. TOF Compares Orbitrap and Its Application Instance
3.2. Application of LC-HRMS Technology in FCM Detection
3.3. The Development of LC-HRMS Combined with Computer Algorithms and Metrology Methods
3.4. Application of GC-HRMS Technology in FCM Detection
3.5. Development of Full Two-Dimensional Meteorological Chromatography (GC × GC)-HRMS for NTS Detection of FCMs
3.6. Development of Analytical Methods for GC-HRMS
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASE | accelerated solvent extraction |
| APGC | Atmospheric-pressure gas chromatography |
| ASAP | Ambient solid analysis probe |
| BP | benzophenone |
| BFRs | brominated flame retardants |
| EI | electron impact |
| FCM | food contact material |
| GC | gas chromatography |
| GC × GC | full two-dimensional meteorological chromatography |
| GC-MS/MS | gas chromatography-tandem mass spectrometry |
| HRMS | high-resolution mass spectrometry |
| HS | headspace extraction |
| HS-SPME | headspace solid-phase microextraction technology |
| IMS | ion mobility spectroscopy |
| LC | liquid chromatography |
| LDPE | low-density polyethylene |
| LLE | liquid–liquid extraction |
| LPME | Liquid-phase microextraction |
| MAE | microwave-assisted extraction |
| MHS-SPME | multi-headspace solid-phase microextraction technique |
| MOAH | Mineral oil aromatic hydrocarbons |
| MS | mass spectrometry |
| NIAs | non-intentionally added materials |
| NTS | non-targeted screening |
| PA | polyamide |
| PAAs | Primary aromatic amines |
| PAPs | polyfluoroalkyl phosphate |
| PE | polyethylene |
| PET | polyester |
| PFCAs/PFSAs | Perfluorocarboxylic acid/sulfonic acid |
| PFAs | Polyfluoroalkyl substances |
| PTOHs | fluoropolyols |
| qTOF | quadruply-time-of-flight |
| rPET | recovered polyethylene terephthalate |
| RSD | relative standard deviation |
| SPE | solid-phase extraction |
| SPME | solid-phase microextraction |
| SVOCs | semi-volatile organic compounds |
| TOF | time of flight |
| UAE | ultrasound-assisted extraction |
| UPLC | ultra-performance liquid chromatography |
| VCs | volatile contaminants |
| VOCs | volatile organic compounds |
| 2,4-DTBP | 2,4-di-tert-butylphenol |
| 2-BP | 2-hydroxybenzophenone |
| 4-BP | 4-hydroxybenzophenone |
References
- Dueñas-Mas, M.J.; Ballesteros-Gómez, A.; de Boer, J. Determination of several PFAS groups in food packaging material from fast-food restaurants in France. Chemosphere 2023, 339, 139734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, K.Y.; Jung, J.S.; Sin, H.S.; Lee, H.G.; Jang, D.Y.; Lee, S.H.; Lim, K.M.; Choi, D. Comparison of migration and cumulative risk assessment of antioxidants, antioxidant degradation products, and other non-intentionally added substances from plastic food contact materials. Food Packag. Shelf Life 2023, 35, 101037. [Google Scholar] [CrossRef]
- Pack, E.C.; Lee, K.Y.; Jung, J.S.; Jang, D.Y.; Kim, H.S.; Koo, Y.J.; Lee, H.G.; Kim, Y.S.; Lim, K.M.; Lee, S.H.; et al. Determination of the migration of plastic additives and non-intentionally added substances into food simulants and the assessment of health risks from convenience food packaging. Food Packag. Shelf Life 2021, 30, 100736. [Google Scholar] [CrossRef]
- Guan, M.-Y.; Hu, C.-Y.; Peng, Q.-S.; Zeng, Y.; Wei, W.A.; Wu, Z.-C.; Wang, Z.-W.; Zhong, H.-N. Formation and migration of 5-hydroxymethylfurfural and furfural from food contact bamboo sticks during heating and their safety evaluation. J. Food Compos. Anal. 2023, 117, 105146. [Google Scholar] [CrossRef]
- Schwartz-Narbonne, H.; Xia, C.; Shalin, A.; Whitehead, H.D.; Yang, D.; Peaslee, G.F.; Wang, Z.; Wu, Y.; Peng, H.; Blum, A.; et al. Per- and Polyfluoroalkyl Substances in Canadian Fast Food Packaging. Environ. Sci. Technol. Lett. 2023, 10, 343–349. [Google Scholar] [CrossRef]
- Ma, X.; Sui, H.; Sun, X.; Ali, M.M.; Debrah, A.A.; Du, Z. A risk classification strategy for migrants of food contact material combined with three (Q)SAR tools in silico. J. Hazard. Mater. 2021, 419, 126422. [Google Scholar] [CrossRef]
- Luo, R.-j.; Lin, Q.-b.; Zhu, L.; Yan, J.-w.; Li, Z. Detection of primary aromatic amines content in food packaging ink and migration from printed plastic bags. Food Packag. Shelf Life 2022, 32, 100820. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Hernando, M.D.; Uclés, S.; Rajski, L.; Cimmino, S.; Fernández-Alba, A.R. Identification of non-intentionally added substances in food packaging nano films by gas and liquid chromatography coupled to orbitrap mass spectrometry. Talanta 2017, 172, 68–77. [Google Scholar] [CrossRef]
- Nerín, C.; Wrona, M. Polymers | food contact and packaging materials—Analytical aspects—Sciencedirect. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 350–359. [Google Scholar] [CrossRef]
- Cavazza, A.; Mattarozzi, M.; Franzoni, A.; Careri, M. A spotlight on analytical prospects in food allergens: From emerging allergens and novel foods to bioplastics and plant-based sustainable food contact materials. Food Chem. 2022, 388, 132951. [Google Scholar] [CrossRef]
- Lin, J.; Wu, W.-L.; Zhong, A.-H.; Xian, Y.-P.; Zhong, H.-N.; Dong, B.; Liang, M.; Hu, J.-P.; Wu, Y.-N.; Yang, X.-F.; et al. Non-targeted analysis and risk assessment of intentionally and non-intentionally added substances migrating from the emerging biodegradable food contact material poly(butylene adipate-co-terephthalate)/modified starch blend film. Food Packag. Shelf Life 2023, 40, 101190. [Google Scholar] [CrossRef]
- Sirot, V.; Rivière, G.; Leconte, S.; Leblanc, J.-C.; Kolf-Clauw, M.; Vasseur, P.; Cravedi, J.-P.; Hulin, M. Infant total diet study in France: Exposure to substances migrating from food contact materials. Environ. Int. 2021, 149, 106393. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Rybak, K.; Wiktor, A.; Mika, A.; Boruszewski, P.; Woch, J.; Przybysz, K.; Witrowa-Rajchert, D. The quality and safety of food contact materials—Paper and cardboard coated with paraffin emulsion. Food Control. 2018, 93, 183–190. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Barhoumi, B.; Sander, S.G.; Tolosa, I. A review on per- and polyfluorinated alkyl substances (PFASs) in microplastic and food-contact materials. Environ. Res. 2022, 206, 112595. [Google Scholar] [CrossRef] [PubMed]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeno, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; Groeneveld, I.; Sanchez, P.L.; Gebbink, W.; Gersen, A.; de Nijs, M.; van Leeuwen, S.P.J. Review of analytical approaches for the identification of non-intentionally added substances in paper and board food contact materials. Trends Food Sci. Technol. 2019, 85, 44–54. [Google Scholar] [CrossRef]
- Ouyang, X.Y.; Lu, Z.C.; Hu, Y.L.; Xie, Z.H.; Li, G.K. Research progress on sample pretreatment methods for migrating substances from food contact materials. J. Sep. Sci. 2021, 44, 879–894. [Google Scholar] [CrossRef]
- Paiva, A.C.; Crucello, J.; de Aguiar Porto, N.; Hantao, L.W. Fundamentals of and recent advances in sorbent-based headspace extractions. TrAC Trends Anal. Chem. 2021, 139, 116252. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Lin, Q.-B.; Song, X.-C.; Chen, S.; Zhong, H.-N.; Nerin, C. Discrimination of Virgin and Recycled Polyethylene Based on Volatile Organic Compounds Using a Headspace GC-MS Coupled with Chemometrics Approach. Food Packag. Shelf Life 2020, 26, 100553. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Niri, V.H.; Pawliszyn, J. Development of a syringe pump assisted dynamic headspace sampling technique for needle trap device. J. Chromatogr. A 2008, 1196–1197, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Heynderickx, P.M. Dynamic headspace analysis using online measurements: Modeling of average and initial concentration. Talanta 2019, 198, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace solid-phase microextraction: Fundamentals and recent advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Parigoridi, I.-E.; Tsoumani, E.; Demertzis, P.G.; Akrida-Demertzi, K. Development of a reliable extraction method for the identification and quantification of 7 plasticizers in recycled paperboard materials intended for food contact applications. Sustain. Chem. Pharm. 2023, 31, 100941. [Google Scholar] [CrossRef]
- Žnideršič, L.; Mlakar, A.; Prosen, H. Development of a SPME-GC-MS/MS method for the determination of some contaminants from food contact material in beverages. Food Chem. Toxicol. 2019, 134, 110829. [Google Scholar] [CrossRef]
- Federico, S.; Pilar, S.B.M.D.; Carlo, B.; Sonia, B.; Maria, C.F.R.; Lourdes, M.M.; Reichenbach, S.E.; James, M.; Daniela, P.; Chiara, C. Delineating the extra-virgin olive oil aroma blueprint by multiple headspace solid phase microextraction and differential-flow modulated comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2021, 1650, 462232. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative determination of volatile organic compounds formed during Polylactide processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- Moreno-Gordaliza, E.; Dolores Marazuela, M.; Milagros Gómez-Gómez, M. Risk assessment of silver and microplastics release from antibacterial food containers under conventional use and microwave heating. Food Chem. 2023, 420, 136097. [Google Scholar] [CrossRef]
- Hawash, H.B.; Hagar, M.; Elkady, M.F.; Moneer, A.A.; Galhoum, A.A.; Attia, N.F.; Kassem, T.S. Synthesis and functionalization of cross-linked molecularly imprinted polymer (MIP) microwave-assisted for recognition and selective extraction of lead (II) and arsenic (V) from water: Isotherms modeling and integrative mechanisms. Chem. Eng. J. 2023, 457, 146019. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Enascuta, C.-E.; Radu, E.; Ciltea-Udrescu, M.; Lavric, V. Does the ultrasonic field improve the extraction productivity compared to classical methods—Maceration and reflux distillation? Chem. Eng. Process.—Process Intensif. 2022, 179, 109082. [Google Scholar] [CrossRef]
- Dorival–García, N.; Galbiati, F.; Kruell, R.; Kovasy, R.; Dunne, S.O.; D’Silva, K.; Bones, J. Identification of additives in polymers from single-use bioprocessing bags by accelerated solvent extraction and ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry. Talanta 2020, 219, 121198. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Bera, G.; Gómez-Ríos, G.A.; Wang, M.; Lu, D.; Rubero, A.; Srinivasan, K.; Al-Esawi, H.; Liu, Y. Novel fully automated and parallel gas assisted dynamic accelerated solvent extractor and parallel solvent evaporator for analysis of solid and semi-solid samples. Adv. Sample Prep. 2023, 6, 100073. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wu, W.; Wang, Z.; Chu, Y.; Chen, X. Hollow porous dummy molecularly imprinted polymer as a sorbent of solid-phase extraction combined with accelerated solvent extraction for determination of eight bisphenols in plastic products. Microchem. J. 2019, 145, 1176–1184. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical strategies for organic food packaging contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef]
- Diamantidou, D.; Tsochatzis, E.; Kalogiannis, S.; Lopes, J.A.; Theodoridis, G.; Gika, H. Analysis of Migrant Cyclic PET Oligomers in Olive Oil and Food Simulants Using UHPLC-qTOF-MS. Foods 2023, 12, 2739. [Google Scholar] [CrossRef] [PubMed]
- Sznajder-Katarzyńska, K.; Surma, M.; Wiczkowski, W.; Piskuła, M. Determination of perfluoroalkyl substances (PFASs) in fats and oils by QuEChERS/micro-HPLC-MS/MS. Food Res. Int. 2020, 137, 109583. [Google Scholar] [CrossRef]
- Zhu, W.; Jin, P.; Yang, H.; Li, F.; Wang, C.; Li, T.; Fan, J. A green extraction strategy for the detection of antioxidants in food simulants and beverages migrated from plastic packaging materials. Food Chem. 2023, 406, 135060. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, X.; Zhang, S.; Hu, Y.; Chen, H. Migration detection of six aromatic amines in polyamide food contact materials by HPLC after molecularly imprinted polymer pipette tip solid phase extraction. Food Packag. Shelf Life 2023, 36, 101029. [Google Scholar] [CrossRef]
- Suseela, M.N.L.; Viswanadh, M.K.; Mehata, A.K.; Priya, V.; Vikas; Setia, A.; Malik, A.K.; Gokul, P.; Selvin, J.; Muthu, M.S. Advances in solid-phase extraction techniques: Role of nanosorbents for the enrichment of antibiotics for analytical quantification. J. Chromatogr. A 2023, 1695, 463937. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Guan, X.; Chen, F.; Li, C.; Zhu, L.; Xue, M.; Yuan, D.; Valtchev, V.; Yan, Y.; et al. Three-Dimensional Large-Pore Covalent Organic Framework with stp Topology. J. Am. Chem. Soc. 2020, 142, 13334–13338. [Google Scholar] [CrossRef] [PubMed]
- Klemes, M.J.; Ling, Y.; Ching, C.; Wu, C.; Xiao, L.; Helbling, D.E.; Dichtel, W.R. Reduction of a Tetrafluoroterephthalonitrile-β-Cyclodextrin Polymer to Remove Anionic Micropollutants and Perfluorinated Alkyl Substances from Water. Angew. Chem. Int. Ed. 2019, 58, 12049–12053. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hu, Y.; Lin, J.; Li, G.; Zhong, Q. Calix[4]arene-based covalent organic frameworks with host-guest recognition for selective adsorption of six per- and polyfluoroalkyl substances in food followed by UHPLC-MS/MS detection. J. Hazard. Mater. 2023, 459, 132198. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Mieth, A.; Alberto Lopes, J.; Simoneau, C. A Salting-out Liquid-Liquid extraction (SALLE) for the analysis of caprolactam and 2,4-di-tert butyl phenol in water and food simulants. Study of the salinity effect to specific migration from food contact materials. J. Chromatogr. B 2020, 1156, 122301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, S.; Tian, H.; Sun, B. Research progress in the use of liquid-liquid extraction for food flavour analysis. Trends Food Sci. Technol. 2023, 132, 138–149. [Google Scholar] [CrossRef]
- SzabÓ, B.S.; Jakab, P.P.; HegedŰS, J.; Kirchkeszner, C.; Petrovics, N.; Nyiri, Z.; Bodai, Z.; Rikker, T.; Eke, Z. Determination of 24 primary aromatic amines in aqueous food simulants by combining solid phase extraction and salting-out assisted liquid–liquid extraction with liquid chromatography tandem mass spectrometry. Microchem. J. 2021, 164, 105927. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Ouladsmane, M.A.; Aouak, T.A.; Alothman, Z.A.; Badjah Hadj Ahmed, A.Y. Determination of phthalates in bottled waters using solid-phase microextraction and gas chromatography tandem mass spectrometry. Chemosphere 2022, 304, 291–297. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Guo, Y.; Chen, H.; Ding, Q.; Zhang, L. Oxygenated carbon nanotubes cages coated solid-phase microextraction fiber for selective extraction of migrated aromatic amines from food contact materials. J. Chromatogr. A 2021, 1646, 462031. [Google Scholar] [CrossRef]
- Murtada, K.; Bowman, D.; Edwards, M.; Pawliszyn, J. Thin-film microextraction combined with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry screening for presence of multiclass organic pollutants in drinking water samples. Talanta 2022, 242, 123301. [Google Scholar] [CrossRef]
- De Tandt, E.; Demuytere, C.; Van Asbroeck, E.; Moerman, H.; Mys, N.; Vyncke, G.; Delva, L.; Vermeulen, A.; Ragaert, P.; De Meester, S.; et al. A recycler’s perspective on the implications of REACH and food contact material (FCM) regulations for the mechanical recycling of FCM plastics. Waste Manag. 2021, 119, 315–329. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Yang, Q.; Zhang, S.; Chang, G.; Zang, X.; Wang, C.; Wang, Z. Covalent triazine-based frameworks for efficient solid-phase microextraction of phthalic acid esters from food-contacted plastics. J. Chromatogr. A 2022, 1681, 463474. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Liang, R.; Hu, Y.; Li, G.; Hu, C.; Zhong, Q. Hollow tube covalent organic framework for syringe filter-based extraction of ultraviolet stabilizer in food contact materials. J. Chromatogr. A 2021, 1656, 462538. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Pacheco-Fernández, I.; Taima-Mancera, I.; Díaz, J.H.A.; Pino, V. Evolution and current advances in sorbent-based microextraction configurations. J. Chromatogr. A 2020, 1634, 461670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, X.; Dang, X.; Chen, H.; Hu, Y. Adsorption mechanism of polycyclic aromatic hydrocarbons on polythiophene-graphene covalent complex and its analytical application in food contact materials. Microchem. J. 2021, 171, 106767. [Google Scholar] [CrossRef]
- Li, T.; Song, Y.; Li, J.; Zhang, M.; Shi, Y.; Fan, J. New low viscous hydrophobic deep eutectic solvents in vortex-assisted liquid-liquid microextraction for the determination of phthalate esters from food-contacted plastics. Food Chem. 2020, 309, 125752. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-González, J.A.; Aranda-Merino, N.; Pérez-Bernal, J.L.; Ramos-Payán, M. Solid supports and supported liquid membranes for different liquid phase microextraction and electromembrane extraction configurations. A review. J. Chromatogr. A 2023, 1691, 463825. [Google Scholar] [CrossRef]
- Snigur, D.; Azooz, E.A.; Zhukovetska, O.; Guzenko, O.; Mortada, W. Low-density solvent-based liquid-liquid microextraction for separation of trace concentrations of different analytes. Trends Anal. Chem. 2023, 167, 117260. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Lemos, V.A.; Teixeira, L.S.G. Dynamic reversed-phase liquid-liquid microextraction for the determination of Cd, Cr, Mn, and Ni in vegetable oils by energy dispersive X-ray fluorescence spectrometry. J. Food Compos. Anal. 2023, 117, 105098. [Google Scholar] [CrossRef]
- Santos, L.B.; Assis, R.d.S.d.; Silva, U.N.; Lemos, V.A. Switchable-hydrophilicity solvent-based liquid-phase microextraction in an on-line system: Cobalt determination in food and water samples. Talanta 2022, 238, 123038. [Google Scholar] [CrossRef]
- GB 5009.156-2016; National Food Safety Standard—General Principle of Migration Test Pre-Treatment Method of Food Contact Materials and Their Products. National Health and Family Planning Commission, People’s Republic of China: Beijing, China, 2016.
- GB 31604.1-2015; National Food Safety Standard—General Principle for the Migration Test of Food Contact Materials and Their Products. National Health and Family Planning Commission, People’s Republic of China: Beijing, China, 2015.
- EU No.10/2011; COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food (Text with EEA Relevance). The European Commission: Brussels, Belgium, 2011.
- Dittmann, B.; Schmid, P.; Kemmer, D. Role of food contact materials in the safety assessment of potentially hazardous substances and in the dietary exposure of infants. Glob. Pediatr. 2022, 2, 100013. [Google Scholar] [CrossRef]
- Sonego, E.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Bannò, A.; Simonetti, G.; Buiarelli, F. Occurrence and migration study of chemicals from baking paper and aluminium foil. Food Chem. 2023, 409, 135260. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, M.P.; Aparicio, J.L.; Rincón, M. Interpretation of the migration of benzophenone type photoinitiators into different food simulants and foodstuffs in terms of the physicochemical properties of the migrants. Food Packag. Shelf Life 2020, 23, 100444. [Google Scholar] [CrossRef]
- Marangoni, L.; Fávaro Perez, M.Â.; Torres, C.D.; Cristianini, M.; Massaharu Kiyataka, P.H.; Albino, A.C.; Padula, M.; Rodrigues Anjos, C.A. Effect of high-pressure processing on the migration of ε-caprolactam from multilayer polyamide packaging in contact with food simulants. Food Packag. Shelf Life 2020, 26, 100576. [Google Scholar] [CrossRef]
- Jaén, J.; Domeño, C.; Úbeda, S.; Aznar, M.; Nerín, C. Migration of mineral oil aromatic hydrocarbons (MOAH) from cardboard containers to dry food and prediction tool. Food Control. 2022, 138, 109016. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, H.; Lu, L.; Lv, X.; Ju, G.; Zhao, J.; Sun, F.; Wang, Y.; Yu, W. Simultaneous Determination and Exposure Assessment of Antioxidants in Food-Contact Plastic Materials by HPLC-MS/MS. J. Food Prot. 2023, 86, 100121. [Google Scholar] [CrossRef]
- Fengler, R.; Gruber, L. Migration and permeation of mineral oil components from paper-based food contact materials into foods—A critical comparison of analytical methods. Food Packag. Shelf Life 2020, 25, 100537. [Google Scholar] [CrossRef]
- Szabó, B.S.; Petrovics, N.; Kirchkeszner, C.; Nyiri, Z.; Bodai, Z.; Eke, Z. Stability study of primary aromatic amines in aqueous food simulants under storage conditions of food contact material migration studies. Food Packag. Shelf Life 2022, 33, 100909. [Google Scholar] [CrossRef]
- Lerch, M.; Nguyen, K.H.; Granby, K. Is the use of paper food contact materials treated with per- and polyfluorinated alkyl substances safe for high-temperature applications?—Migration study in real food and food simulants. Food Chem. 2022, 393, 133375. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Zabaleta, I.; Prieto, A.; Olivares, M.; Zuloaga, O.; Elizalde, M.P. Migration of photoinitiators, phthalates and plasticizers from paper and cardboard materials into different simulants and foodstuffs. Food Chem. 2021, 344, 128597. [Google Scholar] [CrossRef]
- Zabaleta, I.; Blanco-Zubiaguirre, L.; Baharli, E.N.; Olivares, M.; Prieto, A.; Zuloaga, O.; Elizalde, M.P. Occurrence of per- and polyfluorinated compounds in paper and board packaging materials and migration to food simulants and foodstuffs. Food Chem. 2020, 321, 126746. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; De Jager, L.; Begley, T.H. Migration of phenolic brominated flame retardants from contaminated food contact articles into food simulants and foods. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Acquaviva, L.; Buiarelli, F.; Riccardi, C.; Pomata, D.; Di Filippo, P. A Survey on Bio-based Food Packaging Material About the Presence and Migration of Flame Retardants. Curr. Anal. Chem. 2023, 19, 417–427. [Google Scholar] [CrossRef]
- Lerch, M.; Fengler, R.; Mbog, G.-R.; Nguyen, K.H.; Granby, K. Food simulants and real food—What do we know about the migration of PFAS from paper based food contact materials? Food Packag. Shelf Life 2023, 35, 100992. [Google Scholar] [CrossRef]
- Nasiri, A.; Gastaldi, E.; Gontard, N.; Peyron, S. Multi-faceted migration in food contact polyethylene-based nanocomposite packaging. Appl. Clay Sci. 2020, 198, 105803. [Google Scholar] [CrossRef]
- Petrovics, N.; Kirchkeszner, C.; Patkó, A.; Tábi, T.; Magyar, N.; Kovácsné Székely, I.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Effect of crystallinity on the migration of plastic additives from polylactic acid-based food contact plastics. Food Packag. Shelf Life 2023, 36, 101054. [Google Scholar] [CrossRef]
- Petrovics, N.; Kirchkeszner, C.; Tábi, T.; Magyar, N.; Kovácsné Székely, I.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Effect of temperature and plasticizer content of polypropylene and polylactic acid on migration kinetics into isooctane and 95 v/v% ethanol as alternative fatty food simulants. Food Packag. Shelf Life 2022, 33, 100916. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Dreolin, N.; Goshawk, J.; Nerín, C. The analysis of the migration of per and poly fluoroalkyl substances (PFAS) from food contact materials using ultrahigh performance liquid chromatography coupled to ion-mobility quadrupole time-of-flight mass spectrometry (UPLC- IMS-QTOF). Talanta 2024, 266, 124999. [Google Scholar] [CrossRef]
- Harmon, P.; Otter, R. A review of common non-ortho-phthalate plasticizers for use in food contact materials. Food Chem. Toxicol. 2022, 164, 112984. [Google Scholar] [CrossRef]
- Zhou, R.; Geng, J.; Jiang, J.; Lin, L.; Zhang, J.; Yang, Y.; Wang, X.; Niu, Y.; Shao, B. Occurrence and migration of organophosphite and organophosphate esters into food simulants from single-use food packaging in China. Environ. Pollut. 2023, 330, 121782. [Google Scholar] [CrossRef]
- Kirchkeszner, C.; Petrovics, N.; Tábi, T.; Magyar, N.; Kovács, J.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Swelling as a promoter of migration of plastic additives in the interaction of fatty food simulants with polylactic acid- and polypropylene-based plastics. Food Control. 2022, 132, 108354. [Google Scholar] [CrossRef]
- Petrosino, F.; Coppola, G.; Chakraborty, S.; Curcio, S. Modeling of specific migration from food contact materials. J. Food Eng. 2023, 357, 111652. [Google Scholar] [CrossRef]
- Huang, K.; Wu, H.-L.; Wang, T.; Dong, M.-Y.; Yan, X.-Q.; Yu, R.-Q. Chemometrics-assisted excitation-emission matrix fluorescence spectroscopy for real-time migration monitoring of multiple polycyclic aromatic hydrocarbons from plastic products to food simulants. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2023, 304, 123360. [Google Scholar] [CrossRef]
- Ciffroy, P.; Mertens, B.; Van Hoeck, E.; Van Overmeire, I.; Johansson, E.; Alfonso, B.; Baderna, D.; Selvestrel, G.; Benfenati, E. Modeling the migration of chemicals from food contact materials to food: The MERLIN-expo/VERMEER toolbox. Food Chem. Toxicol. 2022, 166, 113118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Lin, P.; Wang, C.-C.; Lin, Y.-C.; Tung, C.-W. Machine learning for predicting chemical migration from food packaging materials to foods. Food Chem. Toxicol. 2023, 178, 113942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Liu, B.; Lin, Q.; Xia, Y. Identification of chemicals in a polyvinyl chloride/polyethylene multilayer film by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry and their migration into solution. J. Chromatogr. A 2020, 1625, 461274. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Pineda, A.; Coscolla, C. Analysis of unknowns in recycled LDPE plastic by LC-Orbitrap Tribrid HRMS using MS3 with an intelligent data acquisition mode. Microchem. J. 2020, 158, 105256. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Zabaleta, I.; Usobiaga, A.; Prieto, A.; Olivares, M.; Zuloaga, O.; Elizalde, M.P. Target and suspect screening of substances liable to migrate from food contact paper and cardboard materials using liquid chromatography-high resolution tandem mass spectrometry. Talanta 2020, 208, 120394. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikova, Y.; Nuñez, A. Non-targeted analysis with liquid chromatography—High resolution mass spectrometry for the identification of food packaging migrants. J. Chromatogr. A 2022, 1676, 463215. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, Z.; Sun, X.; Ma, X.; Song, J.; Sui, H.; Debrah, A.A. Non-targeted analysis and risk assessment of non-volatile compounds in polyamide food contact materials. Food Chem. 2021, 345, 128625. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Lopes, J.A.; Kappenstein, O. Study of the ionic strength effect on the migration of polyamide 6 and 66 oligomers into liquid simulants by a LC-qTOF-MS method. Food Packag. Shelf Life 2023, 35, 101015. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Nuñez, A.; Johnston, J. Screening of chemicals migrating from plastic food contact materials for oven and microwave applications by liquid and gas chromatography—Orbitrap mass spectrometry. J. Chromatogr. A 2021, 1651, 462261. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, Y.; Coscollà, C.; Yusà, V. Comprehensive analysis of photoinitiators and primary aromatic amines in food contact materials using liquid chromatography High-Resolution Mass Spectrometry. Talanta 2019, 191, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Meng, W.; Su, G. Organophosphate esters (OPEs) in plastic food packaging: Non-target recognition, and migration behavior assessment. Environ. Int. 2023, 177, 108010. [Google Scholar] [CrossRef] [PubMed]
- He, P.-x.; Ling, Y.; Yong, W.; Yao, M.-y.; Zhang, Y.-j.; Feng, X.-s.; Zhang, Y.; Zhang, F. Determination of 22 alternative plasticizers in wrap film by solid phase extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J. Chromatogr. A 2022, 1669, 462916. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. Ion mobility quadrupole time-of-flight mass spectrometry for the identification of non-intentionally added substances in UV varnishes applied on food contact materials. A safety by design study. Talanta 2019, 205, 120103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chughtai, H.; Tian, L.; Liu, L.; Roy, J.-F.; Bayen, S. Development of quantitative structure-retention relationship models to improve the identification of leachables in food packaging using non-targeted analysis. Talanta 2023, 253, 123861. [Google Scholar] [CrossRef]
- Fisher, C.M.; Croley, T.R.; Knolhoff, A.M. Data processing strategies for non-targeted analysis of foods using liquid chromatography/high-resolution mass spectrometry. TrAC Trends Anal. Chem. 2021, 136, 116188. [Google Scholar] [CrossRef]
- Osorio, J.; Dreolin, N.; Aznar, M.; Nerín, C.; Hancock, P. Determination of volatile non intentionally added substances coming from a starch-based biopolymer intended for food contact by different gas chromatography-mass spectrometry approaches. J. Chromatogr. A 2019, 1599, 215–222. [Google Scholar] [CrossRef]
- Su, Q.-Z.; Vera, P.; Van de Wiele, C.; Nerín, C.; Lin, Q.-B.; Zhong, H.-N. Non-target screening of (semi-)volatiles in food-grade polymers by comparison of atmospheric pressure gas chromatography quadrupole time-of-flight and electron ionization mass spectrometry. Talanta 2019, 202, 285–296. [Google Scholar] [CrossRef]
- Jaén, J.; Domeño, C.; Alfaro, P.; Nerín, C. Atmospheric Solids Analysis Probe (ASAP) and Atmospheric Pressure Gas Chromatography (APGC) coupled to Quadrupole Time of Flight Mass Spectrometry (QTOF-MS) as alternative techniques to trace aromatic markers of mineral oils in food packaging. Talanta 2021, 227, 122079. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Non-targeted screening of chemicals migrating from paper-based food packaging by GC-Orbitrap mass spectrometry. Talanta 2021, 226, 122120. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of volatile compounds and their sensory impact in a biopolymer based on polylactic acid (PLA) and polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Cabrera, J.F.; Contreras-Llin, A.; Moyano, E.; Santos, F.J. A novel methodology for the determination of neutral perfluoroalkyl and polyfluoroalkyl substances in water by gas chromatography-atmospheric pressure photoionisation-high resolution mass spectrometry. Anal. Chim. Acta 2020, 1100, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kirchkeszner, C.; Petrovics, N.; Nyiri, Z.; Sámuel Szabó, B.; Eke, Z. Role of gas chromatography–single quadrupole mass spectrometry in the identification of compounds migrating from polypropylene-based food contact plastics. Microchem. J. 2022, 181, 107772. [Google Scholar] [CrossRef]
- Carrero-Carralero, C.; Escobar-Arnanz, J.; Ros, M.; Jiménez-Falcao, S.; Sanz, M.L.; Ramos, L. An untargeted evaluation of the volatile and semi-volatile compounds migrating into food simulants from polypropylene food containers by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Talanta 2019, 195, 800–806. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Li, H.; Li, D.; Zheng, J.; Lin, Q.; Nerín, C.; Zhong, H.; Dong, B. The characterization and influence factors of semi-volatile compounds from mechanically recycled polyethylene terephthalate (rPET) by combining GC×GC-TOFMS and chemometrics. J. Hazard. Mater. 2022, 439, 129583. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Wu, X.; Wu, S.; Su, Q.-z.; Dong, B.; Li, D.; Ma, T.; Zhong, H.; Wang, X.; et al. Characterization of volatile organic compounds in food contact paperboards and elucidation of their potential origins from the perspective of the raw materials. Food Packag. Shelf Life 2023, 37, 101062. [Google Scholar] [CrossRef]
- Hao, T.-Y.; Xu, X.; Lin, Q.-B.; Wu, S.-L.; Wu, X.-F.; Hu, J.-L.; Zhong, H.-N.; Dong, B.; Chen, Z.-F.; Ye, Z.-K.; et al. Rapid discrimination of recycled and virgin poly(ethylene terephthalate) based on non-targeted screening of semi-volatile organic compounds using a novel method of DSI/GC×GC-Q-TOF-MS coupled with various chemometrics. Food Packag. Shelf Life 2022, 34, 100978. [Google Scholar] [CrossRef]
- Dong, B.; Wu, X.; Wu, S.; Li, H.; Su, Q.-Z.; Li, D.; Lin, Q.; Chen, S.; Zheng, J.; Zhu, L.; et al. Occurrence of volatile contaminants in recycled poly(ethylene terephthalate) by HS-SPME-GC×GC-QTOF-MS combined with chemometrics for authenticity assessment of geographical recycling regions. J. Hazard. Mater. 2023, 445, 130407. [Google Scholar] [CrossRef]
| Type | Extraction Method | Advantage | Disadvantage | Typical Example | Reference | |
|---|---|---|---|---|---|---|
| Extraction technology of volatile and semi-volatile compounds | HS | Simplify your pre-treatment and avoid organic solvents, the most simple and commonly used, suitable for GC and ion migration spectrometry | The extraction effect is limited due to the low air pressure and slow mass transfer | Utilizing HS-gas chromatography mass spectrometry (GC-MS) and multivariate statistical analysis, Chen et al. were able to differentiate between primary and recycled polyethylene (PE). Their findings revealed 47 volatile organic compounds (VOCs) within four categories, including aliphatic hydrocarbons and additives. Through the use of orthogonal partial least squares discriminant analysis and non-parametric tests, they successfully identified 16 VOC markers. | [18,19,20] | |
| Purge and capture technique | Has high sensitivity, good enrichment effect, and high upper boiling point, which is conducive to the detection of trace analytes | Easy to form foam, easy to overload the instrument, time-consuming, may introduce impurities, universal adsorbent selection is difficult | Ibarra et al. used purge and capture technology combined with GC-MS to analyze VOCs and semi-volatile organic compounds (SVOCs) in organic solvent extracts from 12 plastic packaging materials, detecting approximately 100 different compounds. | [21,22,23] | ||
| Headspace solid-phase microextraction technology (HS-SPME) | It can extract VOCs and SVOCs easily and without solvents, while also handling sample collection, concentration, injection, and analysis | Extracting weak volatile components is challenging due to interference from experimental conditions | Parigoridi et al. studied methods to separate plasticizer mixtures from recycled paperboard for food packaging and developed a technique to detect low concentrations of comparable chemicals. | [24,25,26] | ||
| Multi-headspace solid-phase microextraction technique (MHS-SPME) | Trace analysis is more sensitive, matrix effects are gone, accurate quantification results and multiple analytes can be quantified without external calibration. | Headspace saturation needs to be avoided | A study by Salazar et al. analyzed VOCs in polylactic acid particles and identified aldehydes, ethanol, and acetone using MHS-SPME. They also found three specific compounds: acetaldehyde, 2-methyl-2-propanol, and 2,3-pentanedione. | [27,28] | ||
| Extraction technology of non-volatile compounds | Solid–liquid extraction combined with field-assisted extraction technology | Microwave-assisted extraction (MAE) | Ensure efficient energy transfer, minimize solvent use, shorten extraction time. | The reaction cycle is lengthy and the operational process is complex | A study conducted by Moreno-Gordaliza et al. analyzed the potential migration of silver ions and microplastics in antibacterial food plastic containers during regular use and microwave heating. The results revealed that in certain scenarios, the migration levels surpassed the acceptable limits of risk. | [29,30] |
| Ultrasound-assisted extraction (UAE) | Efficient and simple extraction with low instrument threshold for good results | Sample damage from ultrasonic attenuation makes control and handling difficult. | A review by Peters et al. looked at ways to detect non-intentionally added materials (NIAs) in paper-based FCMs. Methods included UAE for extracting NIAs. | [17,31] | ||
| Accelerated solvent extraction (ASE) | Efficient extraction with minimal solvent and low impact on the body | High equipment requirements | Dorival-Garcia et al. used ASE optimization and LC-HRMS to identify over 100 additives and degradation products in multilayer polymer systems of disposable plastic bags. | [32,33,34] | ||
| The QuEChERS method | FCM impurity purification requirements can be met with this fast, simple, economical, effective, stable, and safe option | The QuEChERS method needs improvement to detect complex FCMs | Diamantidou et al. developed an ultra-high-pressure liquid chromatography(UPLC)-quadruply-time-of-flight (qTOF) mass spectrometry (MS) method for the analysis of NIA migration in FCMs, olive oil, and food simulants in different saturated polyester (PET) bottles. | [35,36,37,38,39] | ||
| Enrichment technology | Solid-phase extraction (SPE) | Flexible and diverse, high sensitivity, good reproducibility, can be set sampling, extraction, concentration, sampling in one | The cost is high, the method development is difficult, and it is not suitable for solid samples | Liu et al. established a pipette tip SPE combined with HPLC and photodiode array detector for the detection of atomic absorbents in polyamide (PA) FCMs for migration detection of six atomic absorbents in PA kitchenware. | [40,41,42,43,44] | |
| Liquid–liquid extraction (LLE) | Simple and fast operation, high selectivity, no special equipment required | Extracting water-soluble compounds from water is tough due to the high organic solvent content and long operation time. | Tsochatzis et al. has developed and refined the salt-out LLE technique to analyze caprolactam and 2,4-di-tert-butylphenol (2,4-DTBP) in water and food simulant samples. The method achieved high accuracy with recovery rates of 87% and 95%, respectively, and a relative standard deviation (RSD) below 12%. | [45,46,47] | ||
| Solid-phase microextraction (SPME) | Easy to operate, efficient and sensitive, can selectively enrich compounds, small sample size | May be affected by interference, selectivity and sensitivity are affected by the sample and solid-phase material | Li et al. utilized oxygenated carbon nanotube cage materials, which were created by oxidizing zeolite imidazole-frame-67, as SPME packages for extracting aromatic amines from FCMs. And they developed a new detection method with gas chromatography-tandem mass spectrometry (GC-MS/MS). | [48,49,50,51,52,53,54,55] | ||
| Liquid-phase microextraction (LPME) | Simple operation, high enrichment efficiency, small extractant dosage, easy to combine with chromatographic system | Extraction solvent, temperature, salt, pH, and stirring affect it easily. | Li et al. designed eight novel low-viscosity hydrophobic eutectic solvents as extractants for eddy-assisted LLME technology to extract and preen rich phthalates from water samples, and determined phthalates content in plastic FCMs using GC-MS. | [56,57,58,59,60] | ||
| Number | Sample | The Migrants | Food Simulants | Research Content | Reference |
|---|---|---|---|---|---|
| 1 | Spike paper | Photoinitiators such as benzophenone (BP), 2-hydroxybenzophenone (2-HBP) | Tenax®, Porapak®, and Tylose® | The migration behavior of Porapak® was similar to that of Tenax®, but Tylose® was slightly lower in the order of 2HBP > BP > 4-hydroxybenzophenone (4-HBP) among all simulants, with the migration amount of 4-HBP significantly lower. | [66] |
| 2 | Multilayer PA packaging | ε-caprolactam | Water, 3% acetic acid solution, olive oil | At high temperature and atmospheric pressure, ε-caprolactam migrates more to different simulants than at high pressure, but remains below the permitted specific migration value of 15 mg/kg. | [67] |
| 3 | Primary carton packaging | Mineral oil aromatic hydrocarbons (MOAHs) | Modified polyphenoxyethylene | The migration patterns of model compounds are influenced by their volatility and food substrate. The behavior of the most volatile and heaviest compounds is distinctive. | [68] |
| 4 | Plastic FCMs | Antioxidant | 95% ethanol, water, and 4% acetic acid | Irganox 1010, Irganox 1076, and antioxidant LTDP had the highest detection frequency and concentration | [69] |
| 5 | Paper FCMs | Mineral oil hydrocarbons | Tenax® | The maximum temperature of Tenax® in paper-based FCMs migration testing should not be higher than 40 °C. | [70] |
| 6 | Water | Primary aromatic amines (PAAs) | Water; 3% acetic acid; 10%, 20%, and 50% ethanol | PAAs is most unstable in 3% acetic acid and more stable in 3 mmol/L HCl solution. In ethanol-containing food simulants, most PAAs are stable. Reducing the temperature can improve its stability, and shortening the storage time can improve its recovery rate. | [71] |
| 7 | Paper FCMs | Perfluorocarboxylic acid/sulfonic acid (PFCAs/PFSAs), polyfluoroalkyl phosphate (PAPs), and fluoropolyols (FTOHs) | 20%, 50% ethanol | Migration of PFCAs and FTOHs to 50% ethanol is higher than migration to real food, while FTOHs do not migrate to 20% ethanol. Children’s estimated dietary exposure to polyfluoroalkyl substances (PFASs) is exceeding the safe threshold and poses a health risk. | [72] |
| 8 | Paper and cardboard materials | Photoinitiators, phthalates and plasticizers | 50%, 95% ethanol, and Tenax | Tenax was an adequate simulation model for the migration to rice and cereals, but underestimated the migration to infant milk powder, 95% ethanol was a superior simulant for this particular food | [73] |
| 9 | Paper and board materials | Per- and polyfluorinated compounds | 50% ethanol, 95% ethanol, and Tenax® | Tenax®-based techniques underestimate the migration of PFASs to food stuffs, particularly for short-carbon-chain PFASs and milk powder. | [74] |
| 10 | Contaminated food contact articles | Brominated flame retardants (BFRs) | Water, 3% acetic acid, 10% ethanol and 50% ethanol | HBCD not detected. Phenolic BFRs (tributyl phosphate and tetrabromobisphenol A) migrated in food simulants from nondetected to 73 µg/kg, and in foods from 1 to 23 µg/kg. Phenolic BFRs migrated more into 50% ethanol than aqueous simulants and foods. | [75] |
| 11 | Bio-based food packaging material | Brominated flame retardants | 96% ethanol | Real samples had low chemical migration in most cases, except for one case. Low percentage suggests low health concern. | [76] |
| Number | Sample | Screened Compound | Instrumental Method | Detection Limit/ Quantification Limit | Detected Quantity | Recovery | Reference |
|---|---|---|---|---|---|---|---|
| 1 | FCMs | 11 PFAS | UPLC- IMS-QTOF | 0.07~3.42/0.20~11.4 μg/kg | 3.2~22.3 μg/kg | 119 ± 22% | [81] |
| 2 | FCM extract | 64 small-molecule compounds | UPLC-QTOF-MS | — | 0.0001~14.2470 mg/d | — | [93] |
| 3 | Liquid food simulator, PA/PE FCM multilayer film | 13 PA monomers and oligomers | QTOF-MS | 0.6~4.8/1.7~14.5 μg/L | 18.1~212.2 ng /mL | 78.3%~108.7% | [94] |
| 4 | Plastic FCMs for microwave and conventional oven heating | 74 kinds of migration compounds | LC-Orbitrap-MS | — | — | — | [95] |
| 5 | Juice milk bag, 18 kinds of packaging samples | 10 kinds of UV ink photoinitiator and 8 kinds of PAAs | LC-Orbitrap-HRMS | —/0.5~5µg/kg | 0.004~658 ng/g | 72%~120% | [96] |
| 6 | Envelope | 22 kinds of plasticizers | UPLC-MS | 0.04~10/1.0~50 μg/kg | — | 75.6~124.5% | [98] |
| 7 | FCM UV varnish | 54 kinds of NIAs | UPLC-ion mobility-QTOF-MS | 0.01~0.1/— mg/kg | 0.01~3.83 mg/kg | — | [99] |
| Number | Sample | Screened Compound | Instrumental Method | Detection Limit/ Quantification Limit | Detected Quantity | Recovery | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Plastic FCMs for microwave and conventional oven heating | 74 kinds of migratory compounds | GC-Orbitrap-MS | — | — | — | [95] |
| 2 | Polypropylene-based FCMs | 27 kinds of VOCs, SVOCs | APGC-QTOF-MS | — | — | — | [103] |
| 3 | FCMs | 27 best markers of MOAH | Ambient solid analysis probe (ASAP)/APGC-QTOF-MS | 0.01~0.06/0.1~0.3 μg/g | 8.62~25.12 mg/kg | — | [104] |
| 4 | Paper FCMs | 35 kinds of migratory compounds | GC-Orbitrap-MS | — | 189~600 μg/kg | — | [105] |
| 5 | Polylactic acid and PET blend | 15 kinds of VOCs | APGC-QTOF-MS | 226~2800/310~8400 µg/kg | — | 40.0%~91.3% | [106] |
| 6 | Water | 12 neutral PFAS substances | HS-SPME-GC-atmospheric pressure-photoionization-HRMS | 0.02~0.24/0.08~50 ng/L | — | — | [107] |
| 7 | Polypropylene FCMs | 45 kinds of VOCs, SVOCs | GC-electron impact (EI)-QMS/GC-EI-TOF-MS | — | 5.4%~98.9% | — | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, H.; Huang, H.; Zhang, B.; Ye, Z.; Yu, X.; Shentu, X. Recent Advances in Non-Targeted Screening of Compounds in Plastic-Based/Paper-Based Food Contact Materials. Foods 2023, 12, 4135. https://doi.org/10.3390/foods12224135
Chen Y, Li H, Huang H, Zhang B, Ye Z, Yu X, Shentu X. Recent Advances in Non-Targeted Screening of Compounds in Plastic-Based/Paper-Based Food Contact Materials. Foods. 2023; 12(22):4135. https://doi.org/10.3390/foods12224135
Chicago/Turabian StyleChen, Ya, Hongyan Li, Haizhi Huang, Biao Zhang, Zihong Ye, Xiaoping Yu, and Xuping Shentu. 2023. "Recent Advances in Non-Targeted Screening of Compounds in Plastic-Based/Paper-Based Food Contact Materials" Foods 12, no. 22: 4135. https://doi.org/10.3390/foods12224135





