Investigation of the Suitability of a Combination of Ethyl-Να-dodecanyl-L-arginat_HCl (LAE) and Starter Culture Bacteria for the Reduction of Bacteria from Fresh Meat of Different Animal Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Treatment and Sample Processing
2.3. Physical Parameters
2.4. Chemical Parameters
2.5. Microbiological Parameters
2.6. Statistical Analysis
3. Results
3.1. Microbiological Results
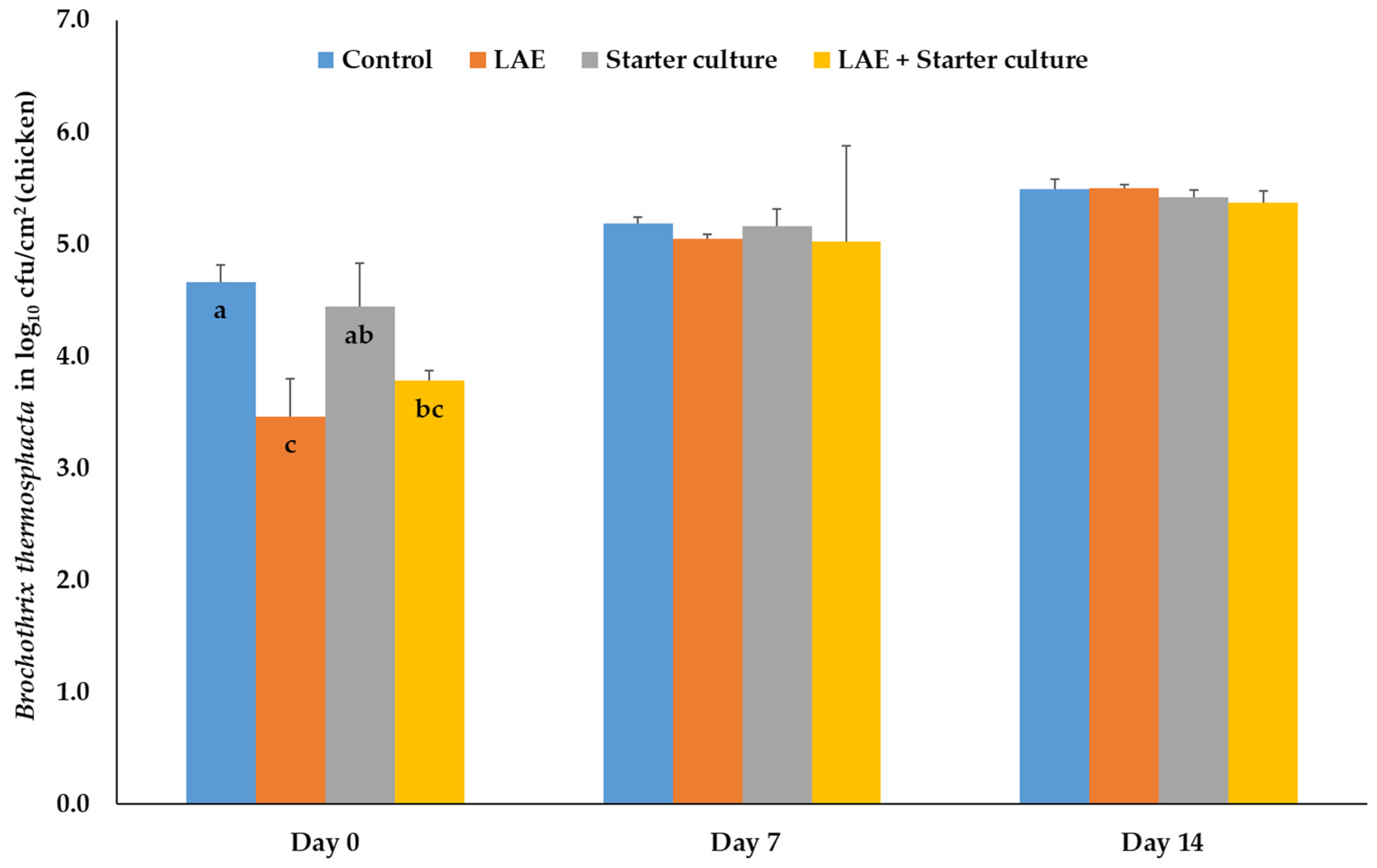
3.1.1. Brochothrix thermosphacta
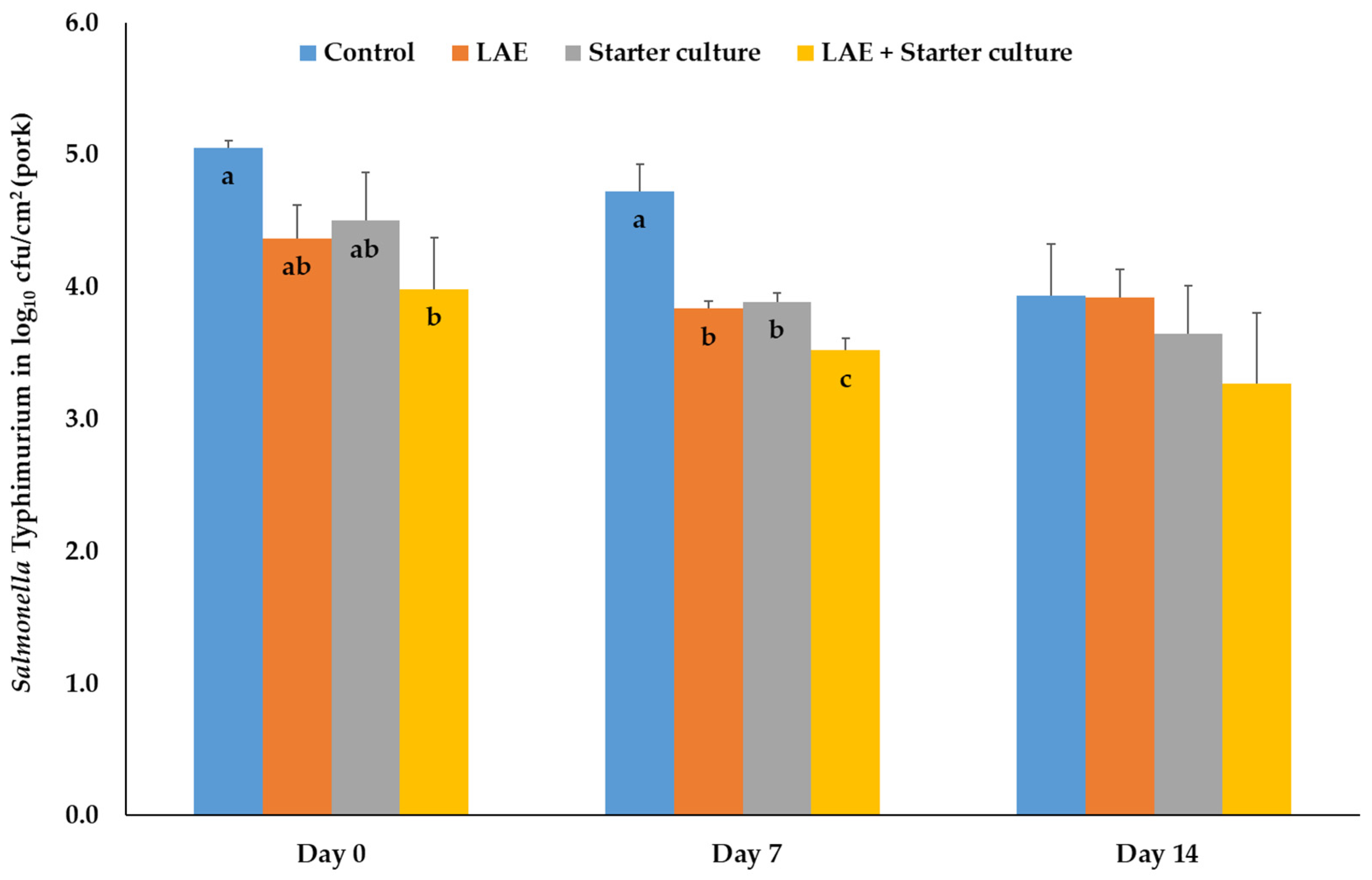
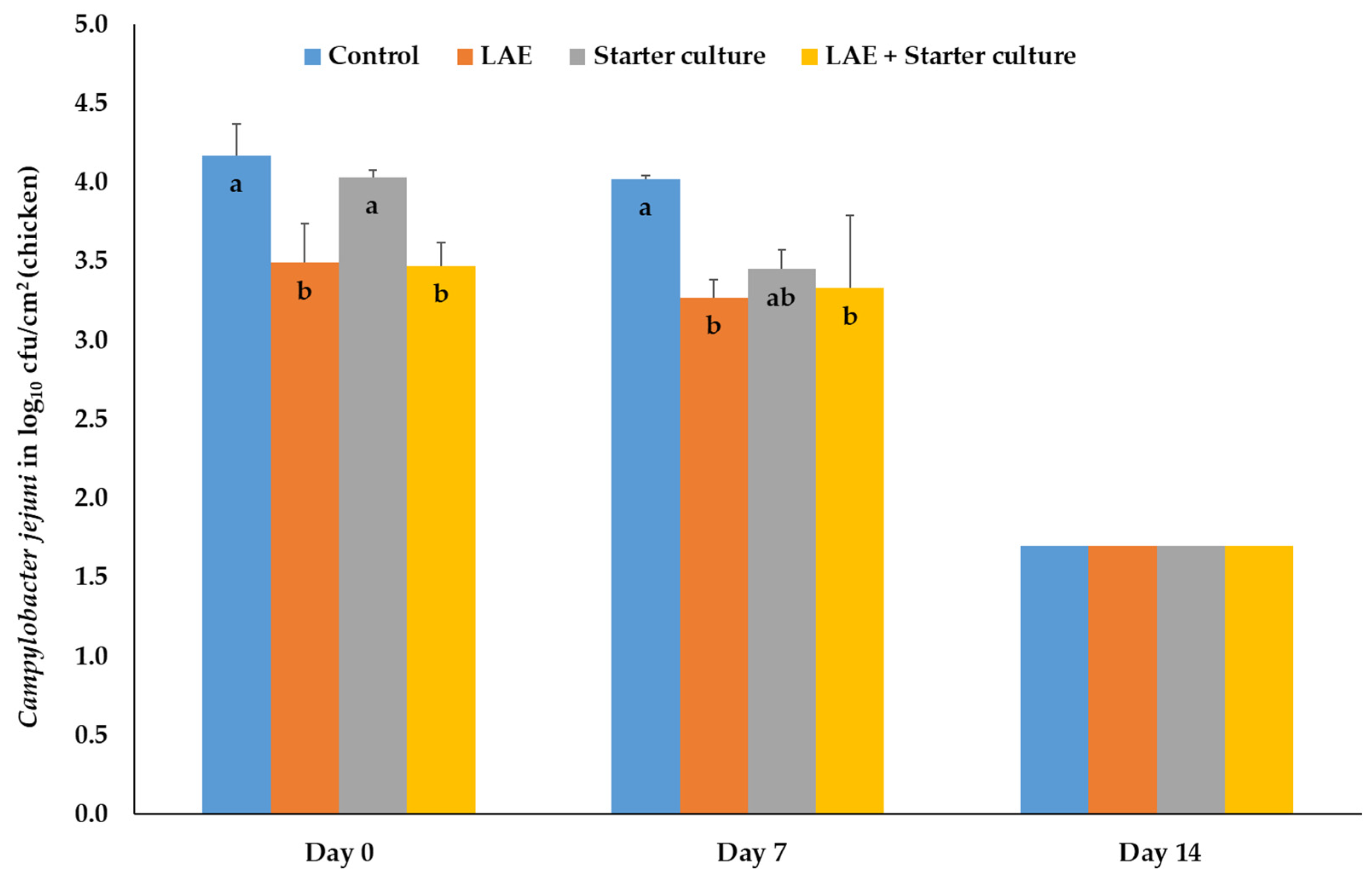
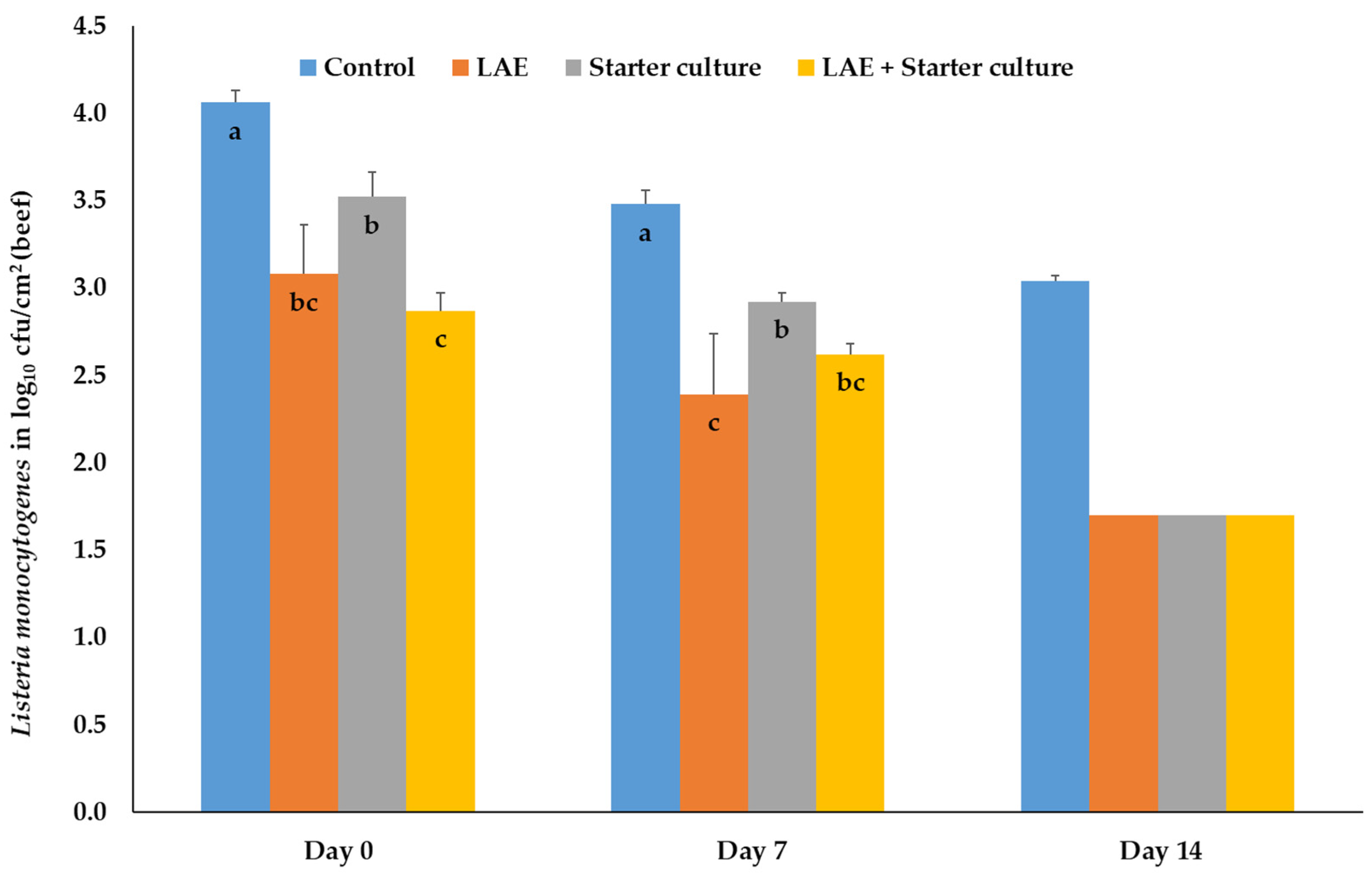
3.1.2. Salmonella Typhimurium, Campylobacter jejuni, Listeria monocytogenes
3.2. Physicochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauta, M.J.; van der Wal, F.J.; Putirulan, F.F.; Post, J.; van de Kassteele, J.; Bolder, N.M. Evaluation of the “testing and scheduling” strategy for control of Campylobacter in broiler meat in The Netherlands. Int. J. Food Microbiol. 2009, 134, 216–222. [Google Scholar] [CrossRef]
- Andritsos, N.D.; Mataragas, M. Characterization and Antibiotic Resistance of Listeria monocytogenes Strains Isolated from Greek Myzithra Soft Whey Cheese and Related Food Processing Surfaces over Two-and-a-Half Years of Safety Monitoring in a Cheese Processing Facility. Foods 2023, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Picouet, P.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02004R0853-20230215&qid=1698061936624 (accessed on 1 October 2023).
- Lazaro, C.A.; Conte-Junior, C.A.; Monteiro, M.L.G.; Canto, A.C.V.S.; Costa-Lima, B.R.C.; Mano, S.B.; Franco, R.M. Effects of ultraviolet light on biogenic amines and other quality indicators of chicken meat during refrigerated storage. Poult. Sci. 2014, 93, 2304–2313. [Google Scholar] [CrossRef]
- Reichel, J.; Kehrenberg, C.; Krischek, C. Inactivation of Yersinia enterocolitica and Brochothrix thermosphacta on pork by UV-C irradiation. Meat Sci. 2019, 158, 107909. [Google Scholar] [CrossRef] [PubMed]
- Meredith, H.; Walsh, D.; McDowell, D.A.; Bolton, D.J. An investigation of the immediate and storage effects of chemical treatments on Campylobacter and sensory characteristics of poultry meat. Int. J. Food Microbiol. 2013, 166, 309–315. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Vimini, B.; Schwarz, J.G.; Nichols, P.; Parveen, S. Application of Antimicrobial Agents via Commercial Spray Cabinet To Inactivate Salmonella on Skinless Chicken Meat. J. Food Prot. 2016, 79, 569–573. [Google Scholar] [CrossRef]
- Bechstein, D.V.; Popp, J.; Sudhaus-Joern, N.; Krischek, C. Effect of ethyl-lauroyl-arginate hypochloride in combination with high hydrostatic pressure processing on the microbial load and physico-chemical characteristics of minced and portioned chicken breast meat. Poult. Sci. 2019, 98, 966–976. [Google Scholar] [CrossRef]
- Bertram, R.; Kehrenberg, C.; Seinige, D.; Krischek, C. Peracetic acid reduces Campylobacter spp. numbers and total viable counts on broiler breast muscle and drumstick skins during modified atmosphere package storage. Poult. Sci. 2019, 98, 5064–5073. [Google Scholar] [CrossRef]
- Xu, M.M.; Kaur, M.; Pillidge, C.J.; Torley, P.J. Microbial biopreservatives for controlling the spoilage of beef and lamb meat: Their application and effects on meat quality. Crit. Rev. Food Sci. Nutr. 2022, 62, 4571–4592. [Google Scholar] [CrossRef]
- Xu, M.M.; Kaur, M.; Pillidge, C.J.; Torley, P.J. Effect of protective cultures on spoilage bacteria and the quality of vacuum-packaged lamb meat. Food Biosci. 2022, 50, 102148. [Google Scholar] [CrossRef]
- Yang, H.X.; Luo, X.; Zhu, L.X.; Liang, R.R.; Mao, Y.W.; Yang, X.Y.; Niu, L.B.; Zhang, Y.M.; Dong, P.C. The biological effect of a beef-derived Latilactobacillus sakei on beef steaks during chilled storage. Food Sci. Nutr. 2023, 11, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Manso, S.; Nerin, C.; Gómez-Lus, R. Antimicrobial activity of Lauroyl Arginate Ethyl (LAE), against selected food-borne bacteria. Food Control 2013, 32, 404–408. [Google Scholar] [CrossRef]
- Becerril, R.; Precone, M.; Nerin, C. Antibiofilm activity of LAE (ethyl lauroyl arginate) against food-borne fungi and its application in polystyrene surface coating. Food Microbiol. 2023, 113, 104284. [Google Scholar] [CrossRef]
- Ma, Y.F.; Ma, Y.Q.; Chi, L.; Wang, S.D.; Zhang, D.H.; Xiang, Q.S. Ethyl lauroyl arginate: An update on the antimicrobial potential and application in the food systems: A review. Front. Microbiol. 2023, 14, 1125808. [Google Scholar] [CrossRef] [PubMed]
- Oladunjoye, A.; Soni, K.A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L.; Mikel, B.; Bailey, R.H.; Mahmoud, B.S.M.; Sharma, C.S. Synergistic activity between lauric arginate and carvacrol in reducing Salmonella in ground turkey. Poult. Sci. 2013, 92, 1357–1365. [Google Scholar] [CrossRef]
- Sharma, C.S.; Ates, A.; Joseph, P.; Soni, K.A.; Schilling, M.W.; Kiess, A. Evaluation of antimicrobial effects of lauric arginate on reduction of Salmonella spp. in ground chicken. Int. J. Food Sci. Technol. 2013, 48, 1410–1415. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Nannapaneni, R.; Kiess, A.; Mahmoud, B.; Sharma, C.S. Antimicrobial efficacy of lauric arginate against Campylobacter jejuni and spoilage organisms on chicken breast fillets. Poult. Sci. 2014, 93, 2636–2640. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef]
- Fisher, K.D.; Bratcher, C.L.; Jin, T.Z.; Bilgili, S.F.; Owsley, W.F.; Wang, L.X. Evaluation of a novel antimicrobial solution and its potential for control Escherichia coli O157:H7, non-O157:H7 shiga toxin-producing E. coli, Salmonella spp., and Listeria monocytogenes on beef. Food Control 2016, 64, 196–201. [Google Scholar] [CrossRef]
- Yang, S.; Sadekuzzaman, M.; Ha, S.D. Treatment with lauric arginate ethyl ester and commercial bacteriophage, alone or in combination, inhibits Listeria monocytogenes in chicken breast tissue. Food Control 2017, 78, 57–63. [Google Scholar] [CrossRef]
- Moreno, O.; Atares, L.; Chiralt, A.; Cruz-Romero, M.C.; Kerry, J. Starch-gelatin antimicrobial packaging materials to extend the shelf life of chicken breast fillets. LWT-Food Sci. Technol. 2018, 97, 483–490. [Google Scholar] [CrossRef]
- Hudson, J.C.; Tolen, T.N.; Kirsch, K.R.; Acuff, G.; Taylor, T.M.; Lucia, L.M.; Castillo, A. Comparison of Antimicrobial Treatments Applied via Conventional or Handheld Electrostatic Spray To Reduce Shiga Toxin-Producing Escherichia coli on Chilled Beef Outside Rounds. J. Food Prot. 2019, 82, 862–868. [Google Scholar] [CrossRef]
- Punchihewage-Don, A.J.; Parveen, S.; Schwarz, J.; Hamill, L.; Nindo, C.; Hall, P.; Vimini, B. Efficacy and Quality Attributes of Antimicrobial Agent Application via a Commercial Electrostatic Spray Cabinet To Inactivate Salmonella on Chicken Thigh Meat. J. Food Prot. 2021, 84, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, E.; Bernardi, C.; Stella, S. Ethyl Lauroyl Arginate (LAE): Antimicrobial Activity of LAE-Coated Film for the Packaging of Raw Beef and Pork. J. Food Qual. 2021, 2021, 6643717. [Google Scholar] [CrossRef]
- Djenane, D.; Martinez, L.; Blanco, D.; Yanguela, J.; Beltran, J.A.; Roncales, P. Effect of lactic acid bacteria on extention of shelf life and growth of Listeria monocytogenes in beef steaks stored in CO2-rich atmosphere. Braz. J. Microbiol. 2005, 36, 405–412. [Google Scholar] [CrossRef]
- Castellano, P.; Vignolo, G. Inhibition of Listeria innocua and Brochothrix thermosphacta in vacuum-packaged meat by addition of bacteriocinogenic Lactobacillus curvatus CRL705 and its bacteriocins. Lett. Appl. Microbiol. 2006, 43, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Gonzalez, C.; Carduza, F.; Vignolo, G. Protective action of Lactobacillus curvatus CRL705 on vacuum-packaged raw beef. Effect on sensory and structural characteristics. Meat Sci. 2010, 85, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.P.; Misiou, O.D.; Kakagianni, M.N. Climate change threatens the microbiological stability of non-refrigerated foods. Food Res. Int. 2022, 162, 111990. [Google Scholar] [CrossRef]
- Kernberger-Fischer, I.; Kehrenberg, C.; Klein, G.; Schaudien, D.; Krischek, C. Influence of modified atmosphere and vacuum packaging with and without nanosilver-coated films on different quality parameters of pork. J. Food Sci. Technol.-Mysore 2017, 54, 3251–3259. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki revisited: Equations for spectrophotometric determination of myoglobin redox forms in aqueous meat extracts. J. Food Sci. 2004, 69, C717–C720. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; Kittikun, A.; Cutter, C.N. Effect of lauric arginate, nisin Z, and a combination against several food-related bacteria. Int. J. Food Microbiol. 2014, 188, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Valles, N.; Ruiz-Torrubia, F.; Mitchell, S.G.; Nerin, C.; Silva, F. Developing ethyl lauroyl arginate antimicrobial films to combat Listeria monocytogenes in cured ham. Food Control 2022, 141, 109164. [Google Scholar] [CrossRef]
- Loeffler, M.; Schwab, V.; Terjung, N.; Weiss, J.; McClements, D.J. Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben. Foods 2020, 9, 270. [Google Scholar] [CrossRef]
- Rodriguez, E.; Seguer, J.; Rocabayera, X.; Manresa, A. Cellular effects of monohydrochloride of L-arginine, N-alpha-lauroyl ethylester (LAE) on exposure to Salmonella Typhimurium and Staphylococcus aureus. J. Appl. Microbiol. 2004, 96, 903–912. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Kim, H.S.; Mizan, M.F.R.; Ha, S.D. Evaluation of a novel antimicrobial (lauric arginate ester) substance against biofilm of Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella spp. Int. J. Food Sci. Technol. 2017, 52, 2058–2067. [Google Scholar] [CrossRef]
- Zhao, D.B.; Wang, S.D.; Hu, Y.S.; Liu, X.; Tao, J.; Sagratini, G.; Xiang, Q.S. Insight into the antibacterial activity of lauric arginate against Escherichia coli O157:H7: Membrane disruption and oxidative stress. LWT-Food Sci. Technol. 2022, 162, 113449. [Google Scholar] [CrossRef]
- Cauchie, E.; Delhalle, L.; Bare, G.; Tahiri, A.; Taminiau, B.; Korsak, N.; Burteau, S.; Fall, P.A.; Farnir, F.; Daube, G. Modeling the Growth and Interaction Between Brochothrix thermosphacta, Pseudomonas spp., and Leuconostoc gelidum in Minced Pork Samples. Front. Microbiol. 2020, 11, 639. [Google Scholar] [CrossRef]
- Koller, V.; Seinige, D.; Saathoff, J.; Kehrenberg, C.; Krischek, C. Impact of a Combination of UV-C Irradiation and Peracetic Acid Spray Treatment on Brochothrix thermosphacta and Yersinia enterocolitica Contaminated Pork. Foods 2021, 10, 204. [Google Scholar] [CrossRef]
- Esmer, O.K.; Irkin, R.; Degirmencioglu, N.; Degirmencioglu, A. The effects of modified atmosphere gas composition on microbiological criteria, color and oxidation values of minced beef meat. Meat Sci. 2011, 88, 221–226. [Google Scholar] [CrossRef]
- Kamenik, J.; Salakova, A.; Pavlik, Z.; Borilova, G.; Hulankova, R.; Steinhauserova, I. Vacuum skin packaging and its effect on selected properties of beef and pork meat. Eur. Food Res. Technol. 2014, 239, 395–402. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lucera, A.; Sinigaglia, M.; Corbo, M.R. Microbiological characteristics of poultry patties in relation to packaging atmospheres. Int. J. Food Sci. Technol. 2009, 44, 2620–2628. [Google Scholar] [CrossRef]
- Nowak, A.; Rygala, A.; Oltuszak-Walczak, E.; Walczak, P. The prevalence and some metabolic traits of Brochothrix thermosphacta in meat and meat products packaged in different ways. J. Sci. Food Agric. 2011, 92, 1304–1310. [Google Scholar] [CrossRef]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the Genome of Brochothrix thermosphacta, a Problematic Meat Spoilage Bacterium. Appl. Environ. Microbiol. 2017, 83, e02786-16. [Google Scholar] [CrossRef]
- Blickstad, E. The effect of water activity on growth and end-product formation of two Lactobacillus spp. and Brochothrix thermosphacta ATCC-11509T. Appl. Microbiol. Biotechnol. 1984, 19, 13–17. [Google Scholar] [CrossRef]
- Goncalves, L.D.D.; Piccoli, R.H.; Peres, A.D.; Saude, A.V. Primary and secondary modeling of Brochothrix thermosphacta growth under different temperature and ph values. Food Sci. Technol. 2018, 38, 37–43. [Google Scholar] [CrossRef]
- Werner, C.; Janisch, S.; Kuembet, U.; Wicke, M. Comparative study of the quality of broiler and turkey meat. Br. Poult. Sci. 2009, 50, 318–324. [Google Scholar] [CrossRef] [PubMed]
- French, P.; O’Riordan, E.G.; Monahan, F.J.; Caffrey, P.J.; Mooney, M.T.; Troy, D.J.; Moloney, A.P. The eating duality of meat of steers fed grass and/or concentrates. Meat Sci. 2001, 57, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Natter, R.; Schellander, K.; Wicke, M. Mitochondrial respiratory activity in porcine longissimus muscle fibers of different pig genetics in relation to their meat quality. Meat Sci. 2010, 85, 127–133. [Google Scholar] [CrossRef]
- Sibut, V.; Le Bihan-Duval, E.; Tesseraud, S.; Godet, E.; Bordeau, T.; Cailleau-Audouin, E.; Chartrin, P.; Duclos, M.J.; Berri, C. Adenosine monophosphate-activated protein kinase involved in variations of muscle glycogen and breast meat quality between lean and fat chickens. J. Anim. Sci. 2008, 86, 2888–2896. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Yen, C.N.; Elgin, J.M.; Beline, M.; Silva, S.D.E.; Wicks, J.C.; England, E.M.; Dalloul, R.A.; Persia, M.E.; Omara, I.I.; et al. Phosphofructokinase and mitochondria partially explain the high ultimate pH of broiler pectoralis major muscle. Poult. Sci. 2018, 97, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.J.; Zhang, L.; Li, J.L.; Xing, T.; Gao, F. Intracellular Calcium Overload and Activation of CaMKK/AMPK Signaling Are Related to the Acceleration of Muscle Glycolysis of Broiler Chickens Subjected to Acute Stress. J. Agric. Food Chem. 2023, 71, 4091–4100. [Google Scholar] [CrossRef] [PubMed]
- Caine, W.R.; Schaefer, A.L.; Aalhus, J.L.; Dugan, M.E.R. Behaviour, growth performance and pork quality of pigs differing in porcine stress syndrome genotype receiving dietary magnesium aspartate hydrochloride. Can. J. Anim. Sci. 2000, 80, 175–182. [Google Scholar] [CrossRef]
- Matarneh, S.K.; England, E.M.; Scheffler, T.L.; Oliver, E.M.; Gerrard, D.E. Net lactate accumulation and low buffering capacity explain low ultimate pH in the longissimus lumborum of AMPK gamma 3(R200Q) mutant pigs. Meat Sci. 2015, 110, 189–195. [Google Scholar] [CrossRef]
- England, E.M.; Matarneh, S.K.; Oliver, E.M.; Apaoblaza, A.; Scheffler, T.L.; Shi, H.; Gerrard, D.E. Excess glycogen does not resolve high ultimate pH of oxidative muscle. Meat Sci. 2016, 114, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wicks, J.C.; Yen, C.N.; Scheffler, T.L.; Richert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Ractopamine changes in pork quality are not mediated by changes in muscle glycogen or lactate accumulation postmortem. Meat Sci. 2021, 174, 108418. [Google Scholar] [CrossRef]
- Neath, K.E.; Del Barrio, A.N.; Lapitan, R.M.; Herrera, J.R.V.; Cruz, L.C.; Fujihara, T.; Muroya, S.; Chikuni, K.; Hirabayashi, M.; Kanai, Y. Difference in tenderness and pH decline between water buffalo meat and beef during postmortem aging. Meat Sci. 2007, 75, 499–505. [Google Scholar] [CrossRef]
- Apaoblaza, A.; Gerrard, S.D.; Matarneh, S.K.; Wicks, J.C.; Kirkpatrick, L.; England, E.M.; Scheffler, T.L.; Duckett, S.K.; Shi, H.; Silva, S.L.; et al. Muscle from grass- and grain-fed cattle differs energetically. Meat Sci. 2020, 161, 107996. [Google Scholar] [CrossRef] [PubMed]
- Immonen, K.; Ruusunen, M.; Puolanne, E. Some effects of residual glycogen concentration on the physical and sensory quality of normal pH beef. Meat Sci. 2000, 55, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Evaluation of USDA approved antimicrobials on the reduction of Salmonella and Campylobacter in ground chicken frames and their effect on meat quality. Poult. Sci. 2017, 96, 2385–2392. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J. Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum-packaged beef. Int. J. Food Microbiol. 2004, 90, 273–282. [Google Scholar] [CrossRef]
- Katikou, P.; Ambrosiadis, I.; Georgantelis, D.; Koidis, P.; Georgakis, S.A. Effect of Lactobacillus-protective cultures with bacteriocin-like inhibitory substances’ producing ability on microbiological, chemical and sensory changes during storage of refrigerated vacuum-packaged sliced beef. J. Appl. Microbiol. 2005, 99, 1303–1313. [Google Scholar] [CrossRef]
- Jones, R.J.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Inhibition by Lactobacillus sakei of other species in the flora of vacuum packaged raw meats during prolonged storage. Food Microbiol. 2009, 26, 876–881. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhu, L.X.; Dong, P.C.; Liang, R.R.; Mao, Y.W.; Qiu, S.B.; Luo, X. Bio-protective potential of lactic acid bacteria: Effect of Lactobacillus sakei and Lactobacillus curvatus on changes of the microbial community in vacuum-packaged chilled beef. Asian-Australas. J. Anim. Sci. 2018, 31, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef]
- Saraoui, T.; Leroi, F.; Chevalier, F.; Cappelier, J.M.; Passerini, D.; Pilet, M.F. Bioprotective Effect of Lactococcus piscium CNCM I-4031 Against Listeria monocytogenes Growth and Virulence. Front. Microbiol. 2018, 9, 1564. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Simsek, Ö.; Çon, A.H.; Tulumoglu, S. Isolating lactic starter cultures with antimicrobial activity for sourdough processes. Food Control 2006, 17, 263–270. [Google Scholar] [CrossRef]
- Laszkiewicz, B.; Szymanski, P.; Kolozyn-Krajewska, D. The effect of selected lactic acid bacterial strains on the technological and microbiological quality of mechanically separated poultry meat cured with a reduced amount of sodium nitrite. Poult. Sci. 2021, 100, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.R.; Ingham, S.C. Evaluation of Potential for Inhibition of Growth of Escherichia coli O157:H7 and Multidrug-Resistant Salmonella Serovars in Raw Beef by Addition of a Presumptive Lactobacillus sakei Ground Beef Isolate. J. Food Prot. 2009, 72, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Olaoye, O.A.; Onilude, A.A.; Idowu, O.A. Microbiological Profile of Goat Meat Inoculated with Lactic Acid Bacteria Cultures and Stored at 30A degrees C for 7 days. Food Bioprocess Technol. 2011, 4, 312–319. [Google Scholar] [CrossRef]
- Chaillou, S.; Christieans, S.; Rivollier, M.; Lucquin, I.; Champomier-Verges, M.C.; Zagorec, M. Quantification and efficiency of Lactobacillus sakei strain mixtures used as protective cultures in ground beef. Meat Sci. 2014, 97, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Nikodinoska, I.; Baffoni, L.; Di Gioia, D.; Manso, B.; García-Sánchez, L.; Melero, B.; Rovira, J. Protective cultures against foodborne pathogens in a nitrite reduced fermented meat product. LWT-Food Sci. Technol. 2019, 101, 293–299. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Tian, F.W.; Jin, X.; Chen, H.Q.; Liu, X.M.; Zhang, Q.X.; Zhao, J.X.; Chen, Y.Q.; Zhang, H.; et al. Screening of adhesive lactobacilli with antagonistic activity against Campylobacter jejuni. Food Control 2014, 44, 49–57. [Google Scholar] [CrossRef]
- Parks, A.R.H.; Brashears, M.M.; Woerner, W.D.; Martin, J.N.; Thompson, L.D.; Brooks, J.C. Spoilage characteristics of ground beef with added lactic acid bacteria and rosemary oleoresin packaged in a modified-atmosphere package and displayed at abusive temperatures. J. Anim. Sci. 2012, 90, 2054–2060. [Google Scholar] [CrossRef][Green Version]
- Parks, A.R.H.; Brashears, M.M.; Martin, J.N.; Woerner, W.D.; Thompson, L.D.; Brooks, J.C. Shelf life and stability traits of traditionally and modified atmosphere packaged ground beef patties treated with lactic acid bacteria, rosemary oleoresin, or both prior to retail display. Meat Sci. 2012, 90, 20–27. [Google Scholar] [CrossRef]
- Li, P.J.; Luo, H.T.; Kong, B.H.; Liu, Q.; Chen, C.G. Formation of red myoglobin derivatives and inhibition of spoilage bacteria in raw meat batters by lactic acid bacteria and Staphylococcus xylosus. LWT-Food Sci. Technol. 2016, 68, 251–257. [Google Scholar] [CrossRef]


| Treatment | Day of Storage | Lightness L* | Redness a* | Yellowness b* | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Pork | |||||||
| Control | 0 | 57.0 | 1.4 | 8.2 | 2.0 | 8.0 | 1.6 |
| LAE | 0 | 56.4 | 1.9 | 9.0 | 2.9 | 8.6 | 1.4 |
| Starter | 0 | 54.4 | 4.3 | 8.1 | 1.8 | 7.3 | 0.6 |
| LAE + Starter | 0 | 55.3 | 4.3 | 8.4 | 2.1 | 7.8 | 0.6 |
| Control | 14 | 58.8 | 2.3 | 7.9 | 2.0 | 9.8 | 1.6 |
| LAE | 14 | 59.2 | 1.0 | 7.7 | 2.1 | 10.2 | 2.4 |
| Starter | 14 | 59.8 | 1.7 | 8.2 | 1.5 | 10.2 | 1.3 |
| LAE + Starter | 14 | 60.2 | 2.0 | 7.1 | 1.7 | 10.0 | 1.2 |
| Chicken meat | |||||||
| Control | 0 | 57.0 | 5.4 | 2.4 | 0.3 | 6.9 | 2.3 |
| LAE | 0 | 58.0 | 2.3 | 2.0 | 1.0 | 7.9 | 0.5 |
| Starter | 0 | 57.3 | 3.9 | 2.6 | 0.4 | 7.4 | 2.1 |
| LAE + Starter | 0 | 57.5 | 4.5 | 2.2 | 0.2 | 7.6 | 1.5 |
| Control | 14 | 64.4 | 1.2 | 0.9 | 0.4 | 8.8 | 0.7 |
| LAE | 14 | 63.8 | 2.5 | 0.9 | 0.3 | 8.1 | 2.1 |
| Starter | 14 | 65.1 | 1.6 | 0.7 | 0.4 | 9.1 | 1.1 |
| LAE + Starter | 14 | 64.4 | 2.6 | 0.7 | 0.1 | 8.9 | 1.4 |
| Beef | |||||||
| Control | 0 | 38.8 | 5.0 | 22.0 | 0.6 | 11.8 | 1.1 |
| LAE | 0 | 40.1 | 3.2 | 22.3 | 2.1 | 12.1 | 0.3 |
| Starter | 0 | 42.5 | 4.5 | 23.2 | 0.6 | 12.9 | 0.8 |
| LAE + Starter | 0 | 40.3 | 2.1 | 22.3 | 0.8 | 12.4 | 0.5 |
| Control | 14 | 40.5 | 3.1 | 8.9 | 1.1 | 11.4 | 0.5 |
| LAE | 14 | 39.1 | 1.7 | 10.5 | 3.2 | 10.7 | 0.3 |
| Starter | 14 | 43.7 | 4.9 | 9.9 | 5.3 | 11.5 | 0.4 |
| LAE + Starter | 14 | 40.7 | 3.7 | 11.0 | 7.6 | 11.4 | 0.5 |
| Treatment | Day of Storage | OxyMb (%) | MetMb (%) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Pork | |||||
| Control | 0 | 33.1 | 3.0 | 49.5 | 2.0 |
| LAE | 0 | 31.9 | 2.2 | 50.5 | 1.5 |
| Starter | 0 | 35.5 | 2.8 | 47.4 | 2.3 |
| LAE + Starter | 0 | 32.9 | 1.8 | 49.8 | 1.7 |
| Control | 14 | 31.2 | 5.7 | 49.9 | 5.5 |
| LAE | 14 | 30.6 | 2.4 | 50.3 | 2.9 |
| Starter | 14 | 29.7 | 2.4 | 50.9 | 2.0 |
| LAE + Starter | 14 | 28.2 | 2.2 | 52.5 | 2.8 |
| Chicken meat | |||||
| Control | 0 | 19.5 | 1.8 | 59.4 | 1.8 |
| LAE | 0 | 17.9 | 3.6 | 60.9 | 2.8 |
| Starter | 0 | 17.4 | 1.8 | 61.2 | 1.4 |
| LAE + Starter | 0 | 19.7 | 2.4 | 59.5 | 1.9 |
| Control | 14 | 12.4 | 2.0 | 65.2 | 1.5 |
| LAE | 14 | 13.0 | 1.7 | 64.6 | 2.0 |
| Starter | 14 | 13.3 | 1.1 | 64.3 | 1.2 |
| LAE + Starter | 14 | 13.7 | 1.8 | 63.9 | 2.3 |
| Beef | |||||
| Control | 0 | 68.5 | 3.4 | 25.3 | 3.2 |
| LAE | 0 | 66.3 | 6.2 | 27.5 | 6.0 |
| Starter | 0 | 62.9 | 1.3 | 31.7 | 1.6 |
| LAE + Starter | 0 | 61.7 | 5.7 | 33.0 | 4.9 |
| Control | 14 | 13.4 | 4.3 | 81.1 | 5.7 |
| LAE | 14 | 15.8 | 9.0 | 79.2 | 9.0 |
| Starter | 14 | 17.1 | 17.6 | 77.9 | 17.0 |
| LAE + Starter | 14 | 18.4 | 21.1 | 76.4 | 20.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drevin, M.; Plötz, M.; Krischek, C. Investigation of the Suitability of a Combination of Ethyl-Να-dodecanyl-L-arginat_HCl (LAE) and Starter Culture Bacteria for the Reduction of Bacteria from Fresh Meat of Different Animal Species. Foods 2023, 12, 4138. https://doi.org/10.3390/foods12224138
Drevin M, Plötz M, Krischek C. Investigation of the Suitability of a Combination of Ethyl-Να-dodecanyl-L-arginat_HCl (LAE) and Starter Culture Bacteria for the Reduction of Bacteria from Fresh Meat of Different Animal Species. Foods. 2023; 12(22):4138. https://doi.org/10.3390/foods12224138
Chicago/Turabian StyleDrevin, Maike, Madeleine Plötz, and Carsten Krischek. 2023. "Investigation of the Suitability of a Combination of Ethyl-Να-dodecanyl-L-arginat_HCl (LAE) and Starter Culture Bacteria for the Reduction of Bacteria from Fresh Meat of Different Animal Species" Foods 12, no. 22: 4138. https://doi.org/10.3390/foods12224138
APA StyleDrevin, M., Plötz, M., & Krischek, C. (2023). Investigation of the Suitability of a Combination of Ethyl-Να-dodecanyl-L-arginat_HCl (LAE) and Starter Culture Bacteria for the Reduction of Bacteria from Fresh Meat of Different Animal Species. Foods, 12(22), 4138. https://doi.org/10.3390/foods12224138




