The Progressive Utilization of Ponkan Peel Residue for Regulating Human Gut Microbiota through Sequential Extraction and Modification of Its Dietary Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Agents
2.2. Preparation of MSDF and MIDF from Ponkan Peel Residue
2.3. Analysis of Chemical Compositions of Ponkan Peel Residue and Its Modified Dietary Fibers
2.4. In Vitro Fermentation of Modified Dietary Fibers
2.4.1. Preparation of Fecal Inocula
2.4.2. Preparation of Fermentation Media
2.4.3. In Vitro Fermentation Process
2.4.4. Determination of pH and SCFA Contents in Fermentation Broths
2.4.5. Analysis of Gut Microbiota
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Compositions of Ponkan Peel Residue and Its Modified Dietary Fibers
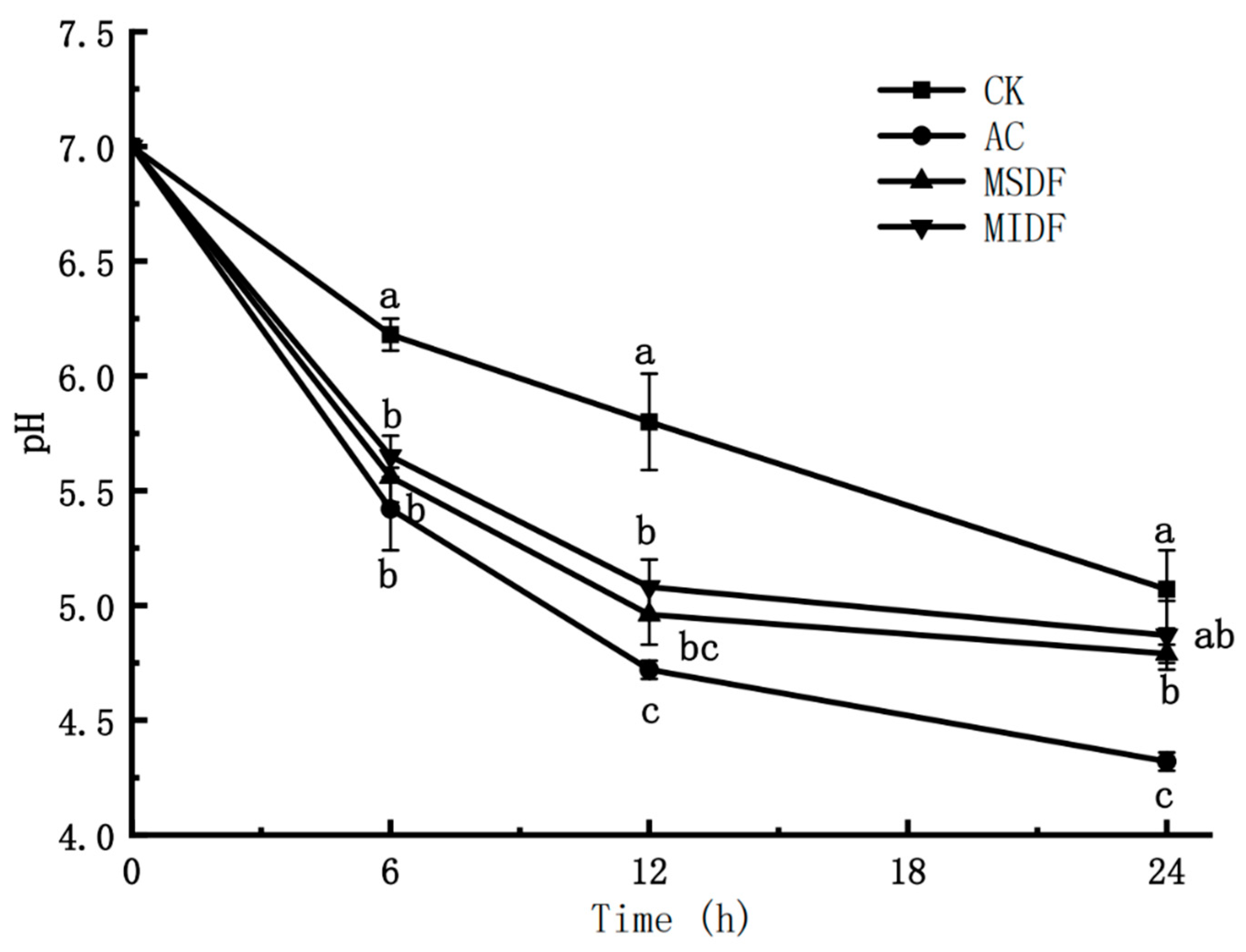
3.2. Effect of Modified Dietary Fibers on pH of In Vitro Fermentation Broths
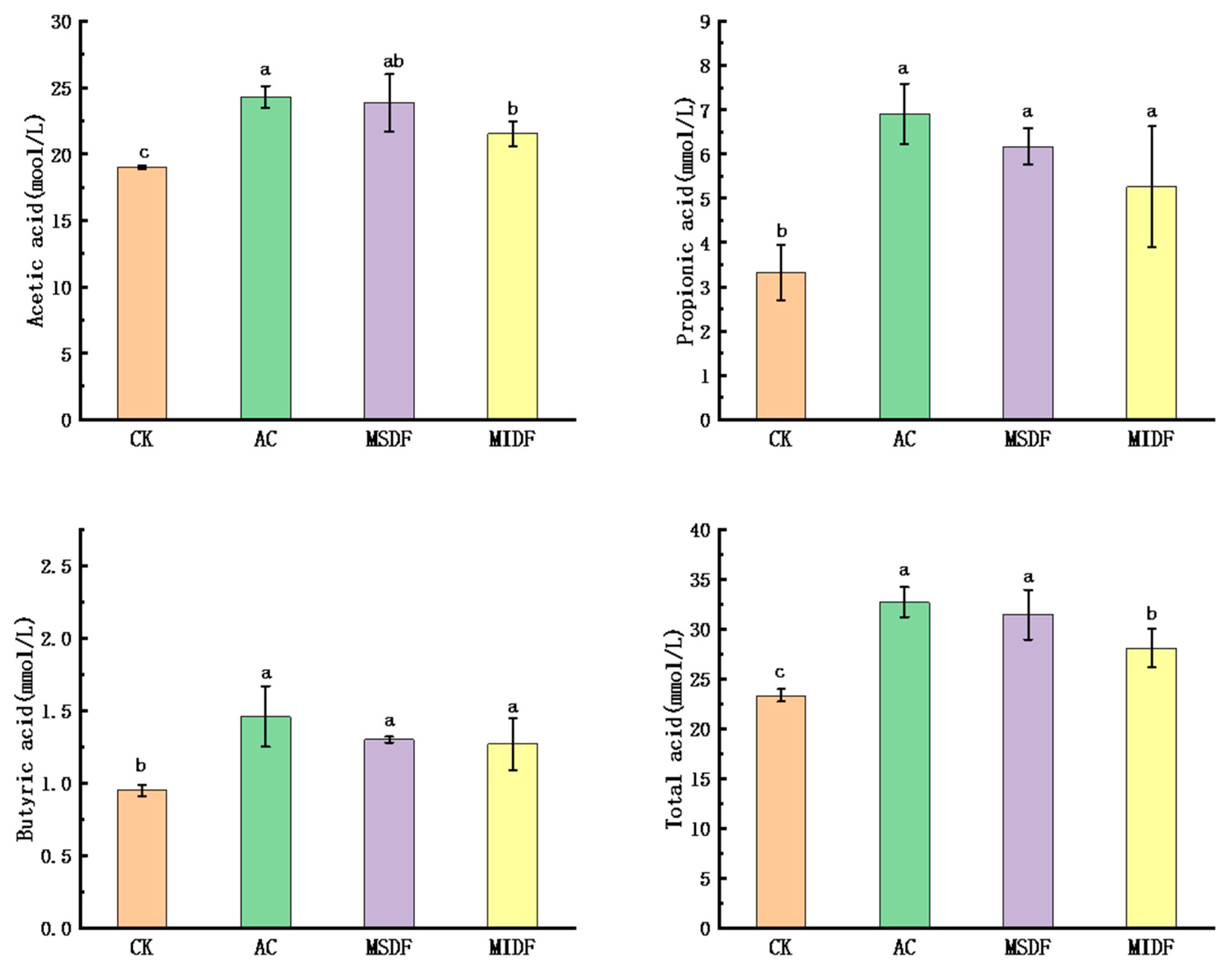
3.3. Effect of Modified Dietary Fibers on the SCFA Contents of In Vitro Fermentation Broths
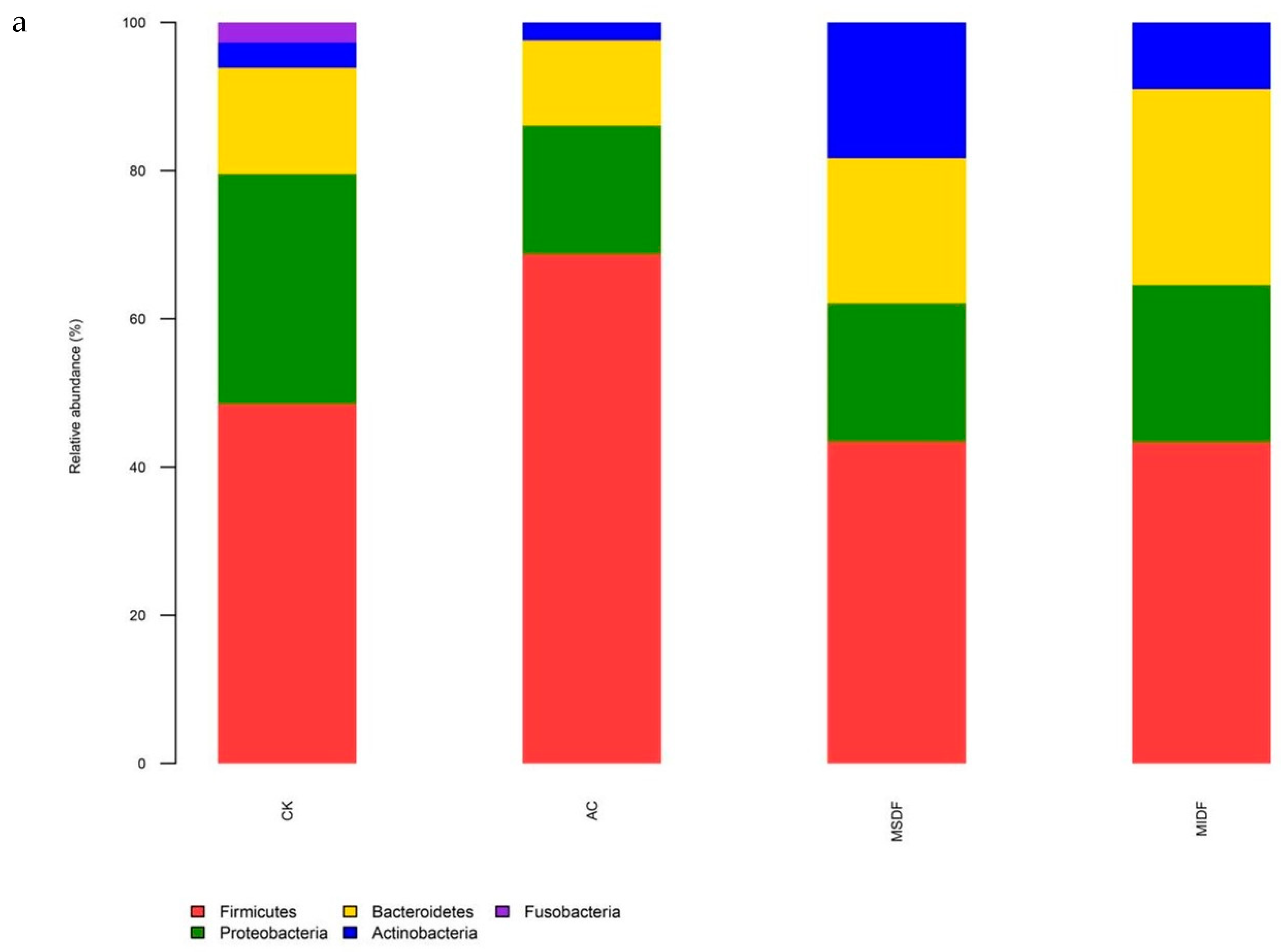
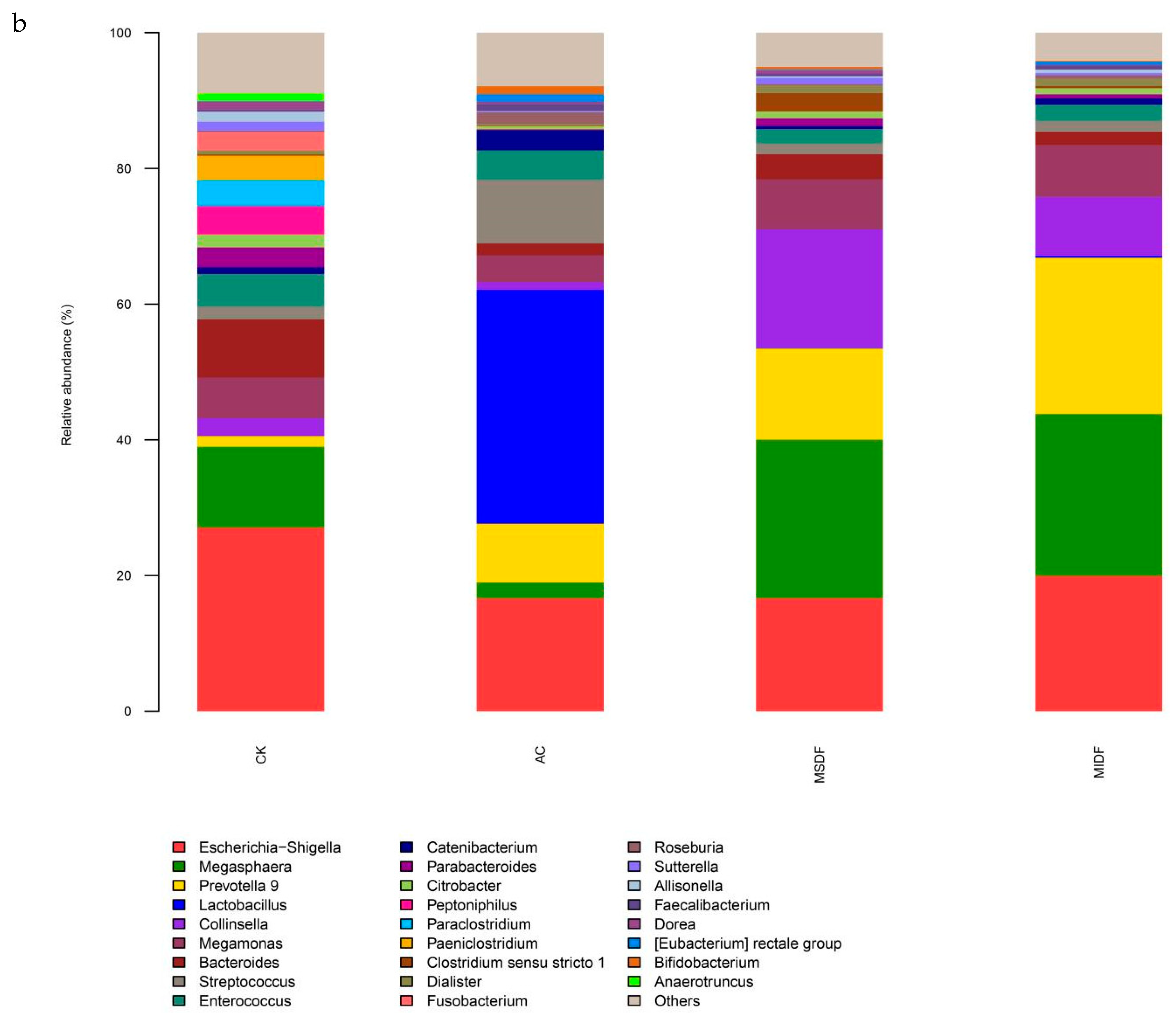
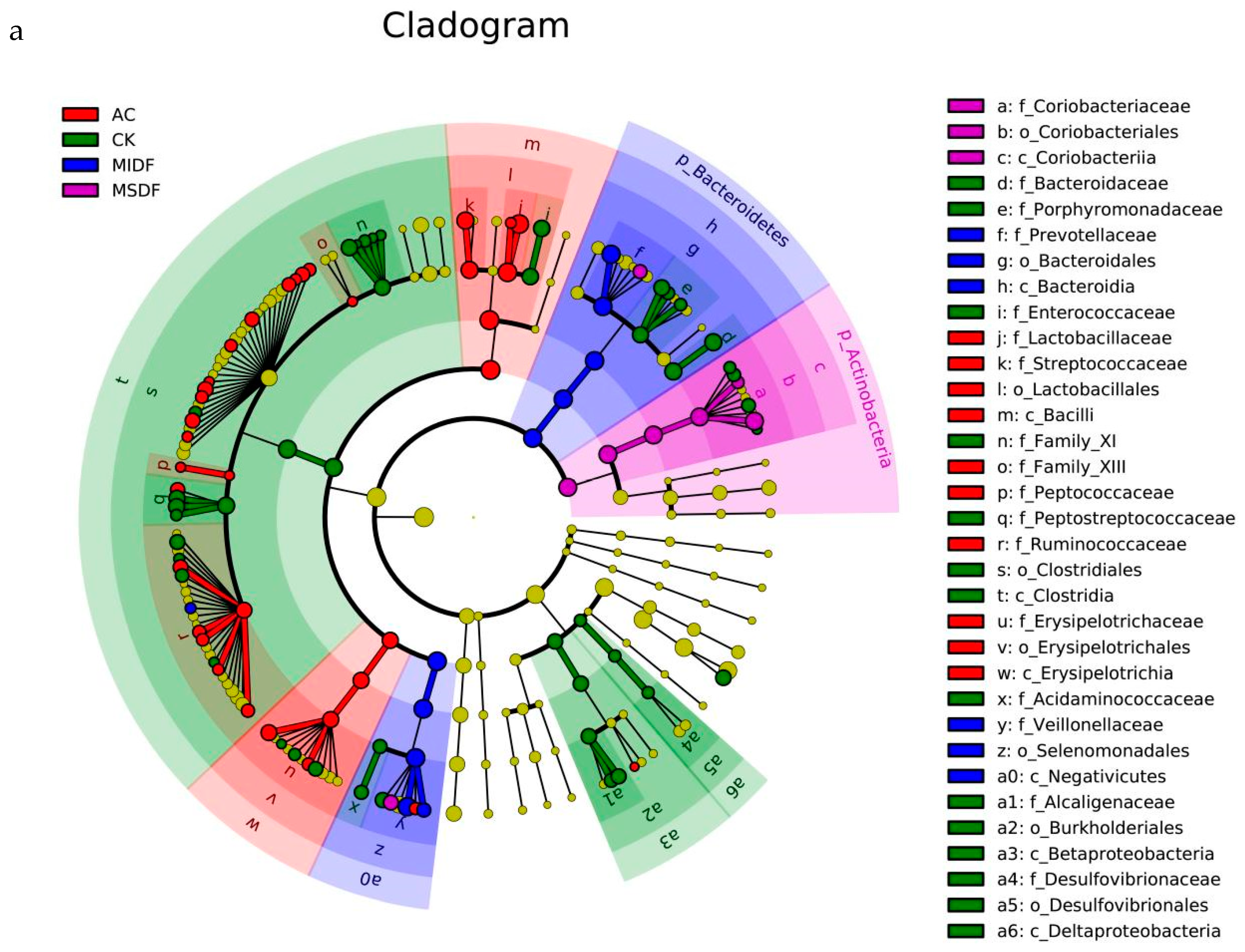
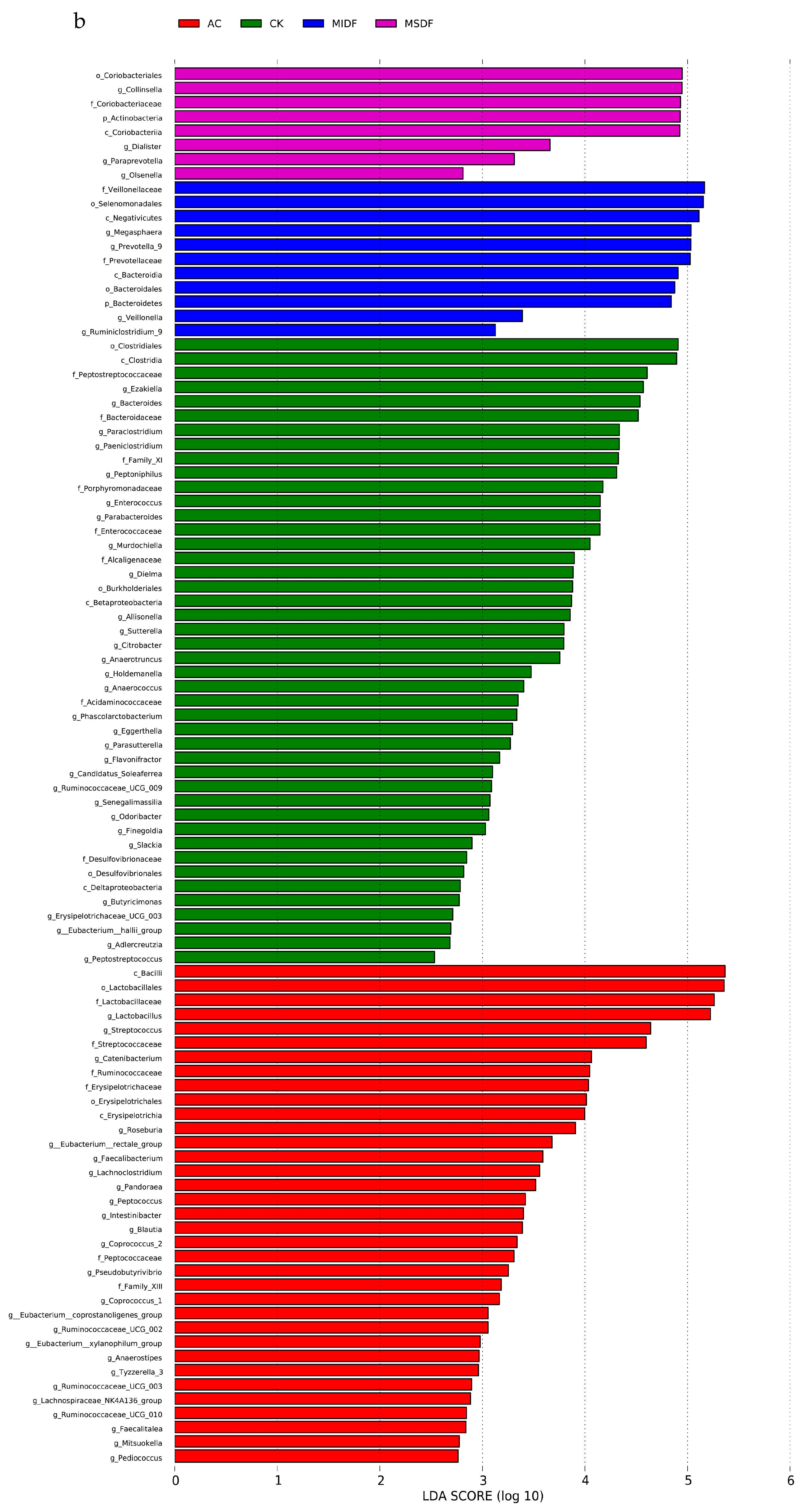
3.4. Effect of Modified Dietary Fibers on Gut Microbiota of In Vitro Fermentation Broths
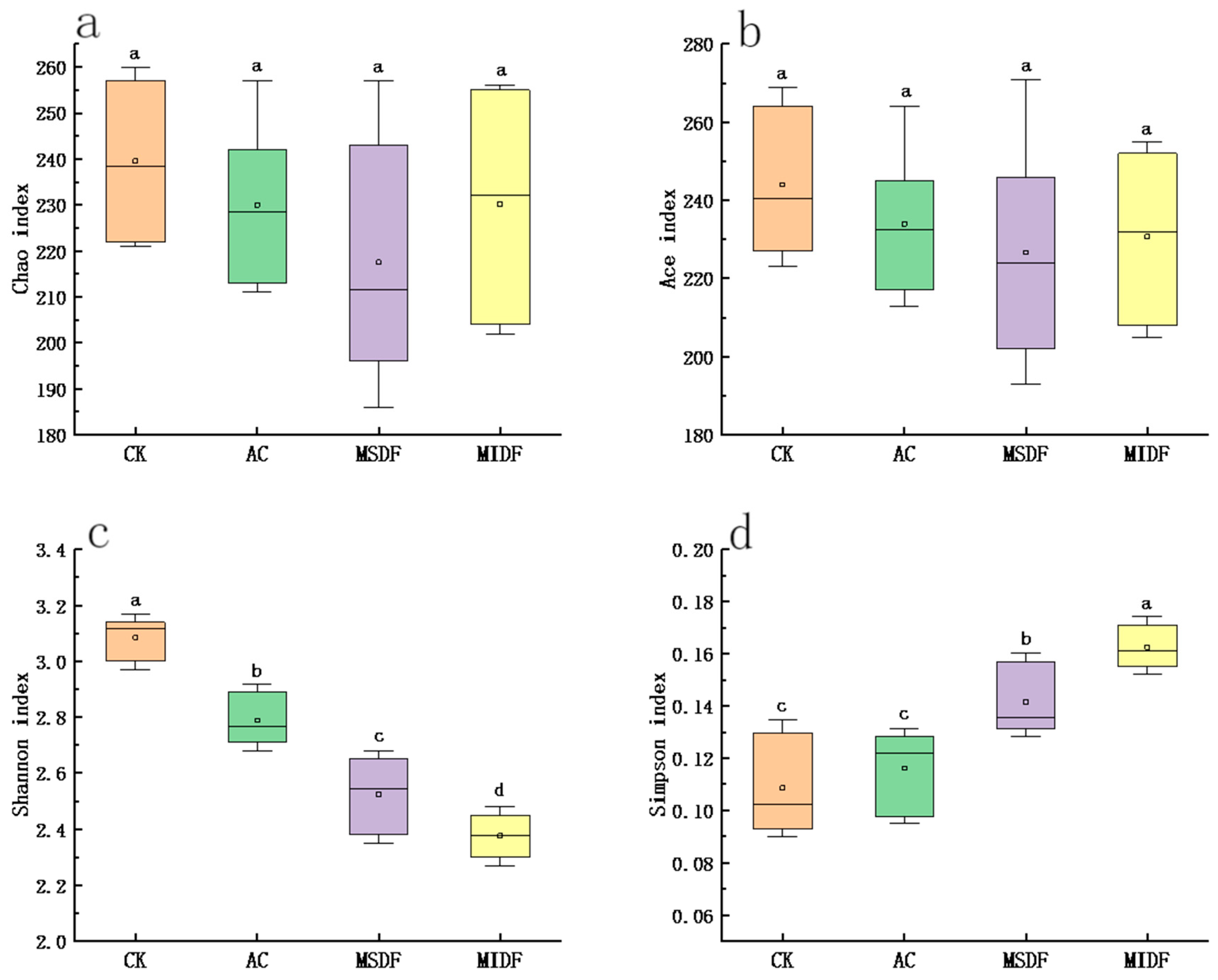
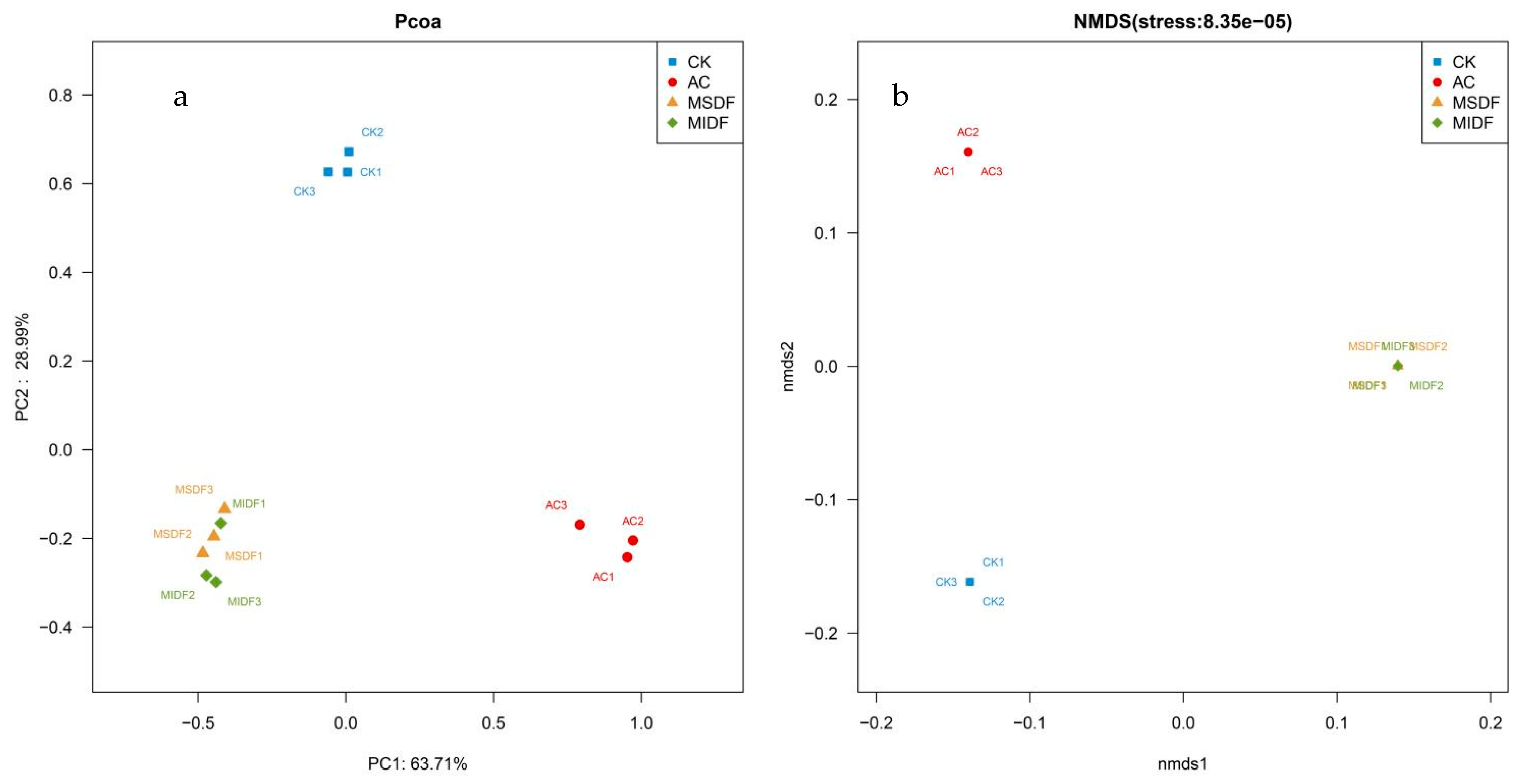
3.4.1. Alpha Diversity and β Diversity
3.4.2. Composition of Gut Microbiota
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Da Silva, J.C.G.; Pereira, J.L.C.; Andersen, S.L.F.; de Fatima Peralta Muniz Moreira, R.; José, H.J. Torrefaction of ponkan peel waste in tubular fixed-bed reactor: In-depth bioenergetic evaluation of torrefaction products. Energy 2020, 210, 118569. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Aboul Naser, A.; Younis, E.; El-Feky, A.; Elbatanony, M.; Hamed, M. Management of Citrus sinensis peels for protection and treatment against gastric ulcer induced by ethanol in rats. Biomarkers 2020, 25, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Campanella, O.H.; Hamaker, B.R.; Welti-Chanes, J. Extrusion effect on in vitro fecal fermentation of fruit peels used as dietary fiber sources. LWT 2022, 153, 112569. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Bussolo de Souza, C.; Jonathan, M.; Isay Saad, S.M.; Schols, H.A.; Venema, K. Degradation of fibers from fruit by-products allows selective modulation of the gut bacteria in an in vitro model of the proximal colon. J. Funct. Foods 2019, 57, 275–285. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Zhang, X.; Kazem, A.E.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. Microwave treatment enhances human gut microbiota fermentability of isolated insoluble dietary fibers. Food Res. Int. 2021, 143, 110293. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocolloid. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Koc, F.; Sugrue, I.; Murphy, K.; Renzetti, S.; Noort, M.; Ross, R.P.; Stanton, C. The microbiome modulating potential of superheated steam (SHS) treatment of dietary fibers. Innov. Food Sci. Emerg. 2022, 80, 103082. [Google Scholar] [CrossRef]
- Luo, S.; Hou, Y.; Xie, L.; Zhang, H.; Liu, C.; Chen, T. Effects of microwave on the potential microbiota modulating effects of agro-industrial by-product fibers among different individuals. LWT 2023, 178, 114621. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Ma, Z.; Liu, Y.; Mu, J.; Wang, J.; Stipkovits, L.; Wu, G.; Sun, J.; Hui, X. Enzymatic-modified dietary fiber fraction extracted from potato residue regulates the gut microbiotas and production of short-chain fatty acids of C57BL/6 mice. J. Funct. Foods 2021, 84, 104606. [Google Scholar] [CrossRef]
- Liu, X.-M.; Liu, Y.; Shan, C.-H.; Yang, X.-Q.; Zhang, Q.; Xu, N.; Xu, L.-Y.; Song, W. Effects of five extraction methods on total content, composition, and stability of flavonoids in jujube. Food Chem. X 2022, 14, 100287. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Z.; Wang, W.; Mu, J.; Liu, Y.; Wang, J.; Stipkovits, L.; Hui, X.; Wu, G.; Sun, J. The effects of enzymatic modification on the functional ingredient—Dietary fiber extracted from potato residue. LWT 2022, 153, 112511. [Google Scholar] [CrossRef]
- GB 5009.3; National Health and Family Planning Commission. Determination of Water in Foods. National Standard of Food Safety: Beijing, China, 2016.
- GB 5009.4; National Health and Family Planning Commission. Determination of Ash in Foods. National Standard of Food Safety: Beijing, China, 2016.
- GB 5009.5; National Health and Family Planning Commission. Determination of Protein in Foods. National Standard of Food Safety: Beijing, China, 2016.
- GB 5009.6; National Health and Family Planning Commission. Determination of Fat in Foods. National Standard of Food Safety: Beijing, China, 2016.
- GB 5009.88; National Health and Family Planning Commission. Determination of Fiber in Foods. National Standard of Food Safety: Beijing, China, 2014.
- Schwab, C.; Ruscheweyh, H.-J.; Bunesova, V.; Pham, V.T.; Beerenwinkel, N.; Lacroix, C. Trophic interactions of infant bifidobacteria and Eubacterium hallii during L-fucose and fucosyllactose degradation. Front. Microbiol. 2017, 8, 95. [Google Scholar] [CrossRef]
- Wu, W.; Li, Q.; Chen, H.; Fang, X.; Niu, B.; Liu, R.; Mu, H.; Gao, H. In vitro fermentation characteristics of the dietary fiber in bamboo (Phyllostachys edulis) shoots and its regulatory effects on the intestinal microbiota and metabolites. Food Chem. 2023, 404, 134707. [Google Scholar] [CrossRef]
- Qiao, H.; Shao, H.; Zheng, X.; Liu, J.; Liu, J.; Huang, J.; Zhang, C.; Liu, Z.; Wang, J.; Guan, W. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef]
- Cao, Z.; Guo, Y.; Liu, Z.; Zhang, H.; Zhou, H.; Shang, H. Ultrasonic enzyme-assisted extraction of comfrey (Symphytum officinale L.) polysaccharides and their digestion and fermentation behaviors in vitro. Process Biochem. 2022, 112, 98–111. [Google Scholar] [CrossRef]
- Fang, F.; He, Y.; Zhao, J.; Zhang, Y.; Chen, C.; He, H.; Wu, Q.; Hu, M.; Nie, S.; Xie, M.; et al. Effects of boiling and steaming process on dietary fiber components and in vitro fermentation characteristics of 9 kinds of whole grains. Food Res. Int. 2023, 164, 112328. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Bradu, P.; Murali, R.; Kannampuzha, S.; Loganathan, T.; Doss C, G.P.; Prakash, B.P.A.; Renu, K.; Dey, A.; et al. The crosstalk of the human microbiome in breast and colon cancer: A metabolomics analysis. Crit. Rev. Oncol. Hemat. 2022, 176, 103757. [Google Scholar] [CrossRef]
- Moreira Júnior, R.E.; de Carvalho, L.M.; dos Reis, D.C.; Cassali, G.D.; Faria, A.M.C.; Maioli, T.U.; Brunialti-Godard, A.L. Diet-induced obesity leads to alterations in behavior and gut microbiota composition in mice. J. Nutr. Biochem. 2021, 92, 108622. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, E.J.C.; Iljazovic, A.; Amend, L.; Lesker, T.R.; Renault, T.; Thiemann, S.; Hao, L.; Roy, U.; Gronow, A.; Charpentier, E.; et al. Distinct polysaccharide utilization determines interspecies competition between intestinal Prevotella spp. Cell Host Micro. 2020, 28, 838–852.e836. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Xie, M.; Gao, H. In vitro fecal fermentation characteristics of bamboo shoot (Phyllostachys edulis) polysaccharide. Food Chem. X 2021, 11, 100129. [Google Scholar] [CrossRef] [PubMed]
| Basic Composition (%) | PPR | MDF |
|---|---|---|
| Moisture | 2.27 ± 0.19 a | 1.10 ± 0.10 b |
| Ash | 8.41 ± 0.38 a | 5.57 ± 0.22 b |
| Protein | 6.89 ± 0.14 a | 1.14 ± 0.09 b |
| Crude fat | 2.20 ± 0.21 a | 0.51 ± 0.09 b |
| TDF | 66.89 ± 2.60 b | 89.59 ± 0.79 a |
| IDF | 58.38 ± 2.07 b | 60.87 ± 0.85 b |
| SDF | 8.51 ± 0.65 b | 28.72 ± 0.83 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Zheng, M.; Lu, H.; Lu, S. The Progressive Utilization of Ponkan Peel Residue for Regulating Human Gut Microbiota through Sequential Extraction and Modification of Its Dietary Fibers. Foods 2023, 12, 4148. https://doi.org/10.3390/foods12224148
Gao P, Zheng M, Lu H, Lu S. The Progressive Utilization of Ponkan Peel Residue for Regulating Human Gut Microbiota through Sequential Extraction and Modification of Its Dietary Fibers. Foods. 2023; 12(22):4148. https://doi.org/10.3390/foods12224148
Chicago/Turabian StyleGao, Pu, Meiyu Zheng, Hanyu Lu, and Shengmin Lu. 2023. "The Progressive Utilization of Ponkan Peel Residue for Regulating Human Gut Microbiota through Sequential Extraction and Modification of Its Dietary Fibers" Foods 12, no. 22: 4148. https://doi.org/10.3390/foods12224148
APA StyleGao, P., Zheng, M., Lu, H., & Lu, S. (2023). The Progressive Utilization of Ponkan Peel Residue for Regulating Human Gut Microbiota through Sequential Extraction and Modification of Its Dietary Fibers. Foods, 12(22), 4148. https://doi.org/10.3390/foods12224148





