The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review
Abstract
:1. Introduction
2. Extraction of Essential Oils
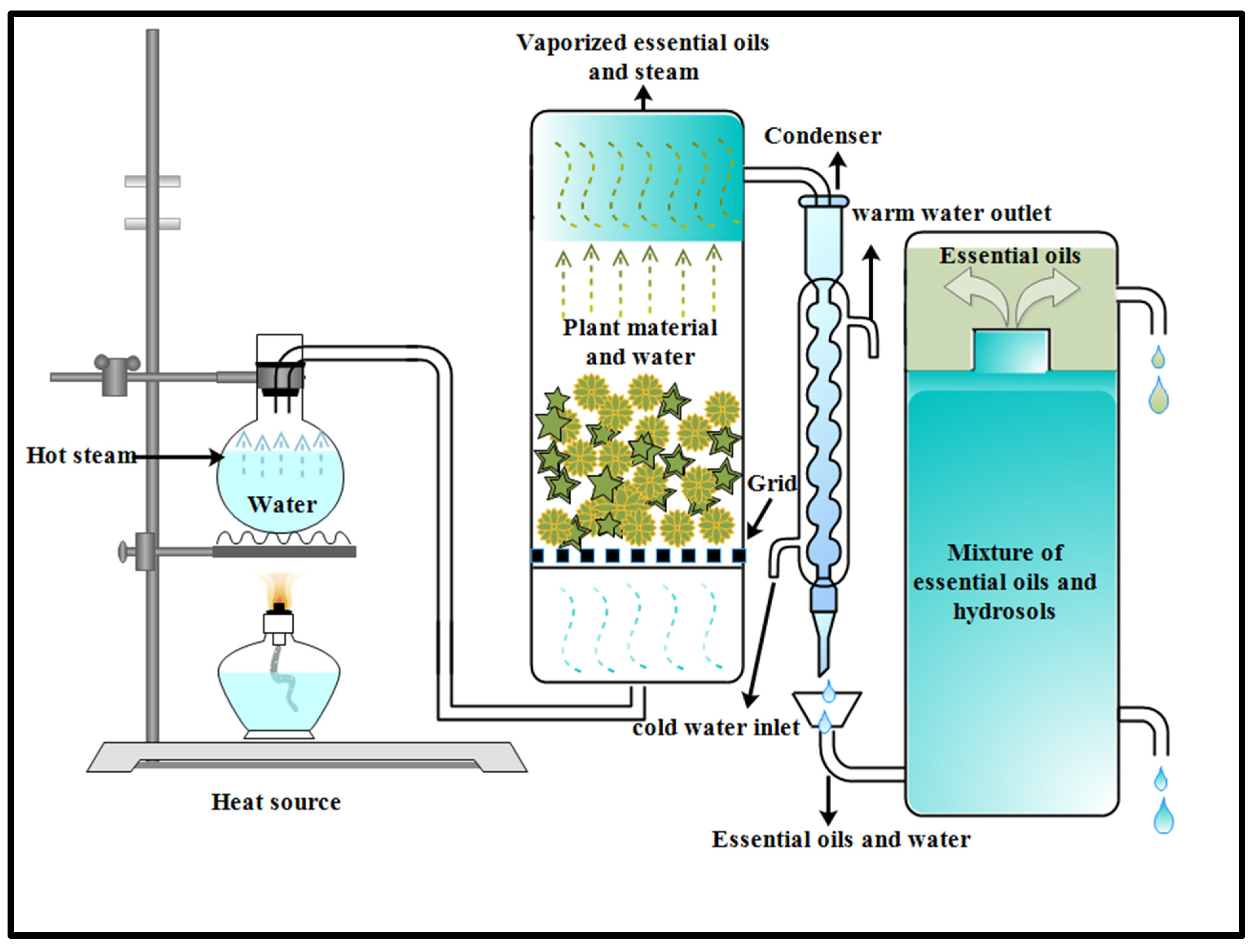
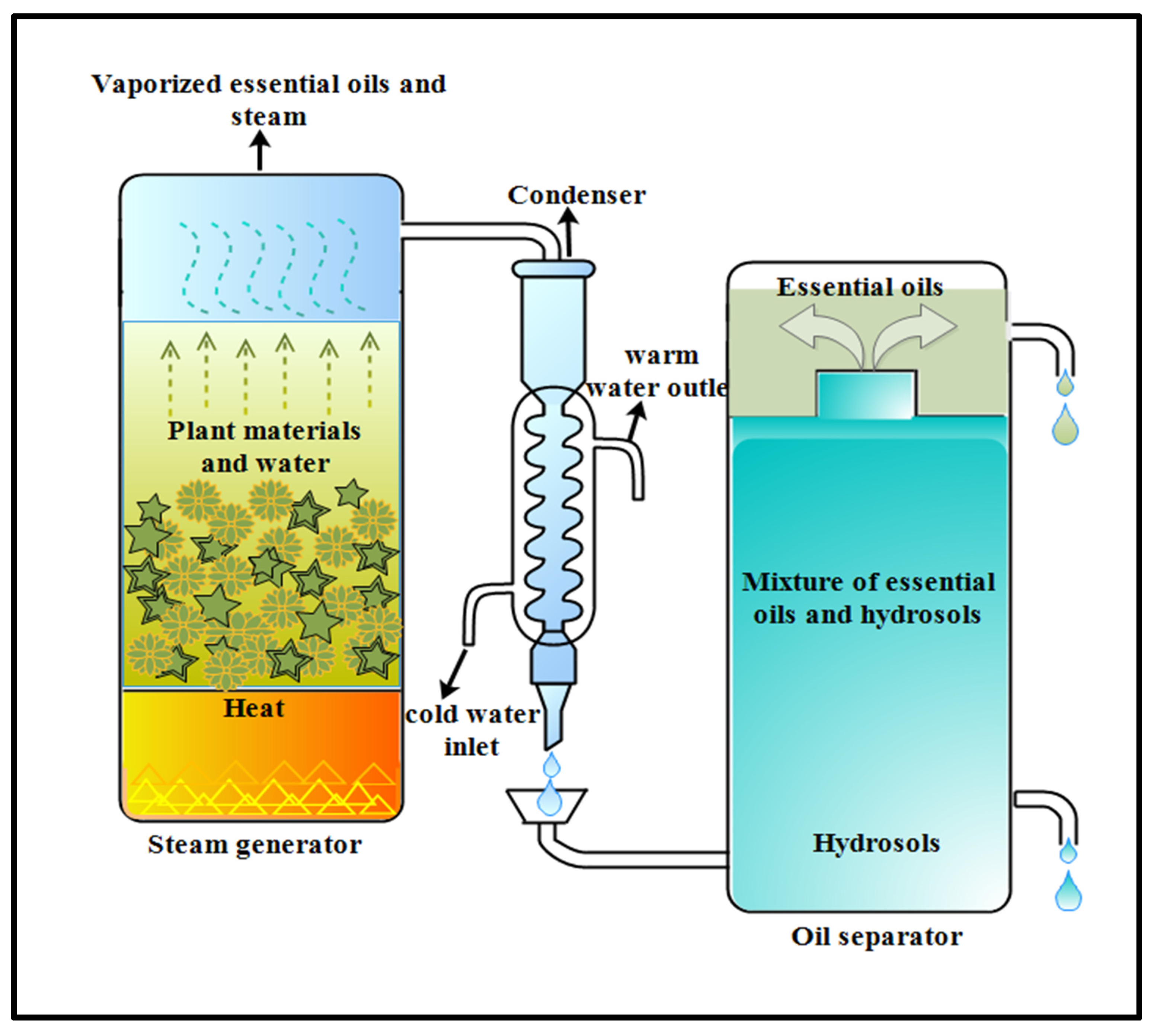
2.1. Distillation
2.1.1. Steam Distillation Method
2.1.2. Hydrodistillation Method
2.1.3. Hydrodiffusion
2.2. Solvent Extraction
2.3. Supercritical Fluid Extraction
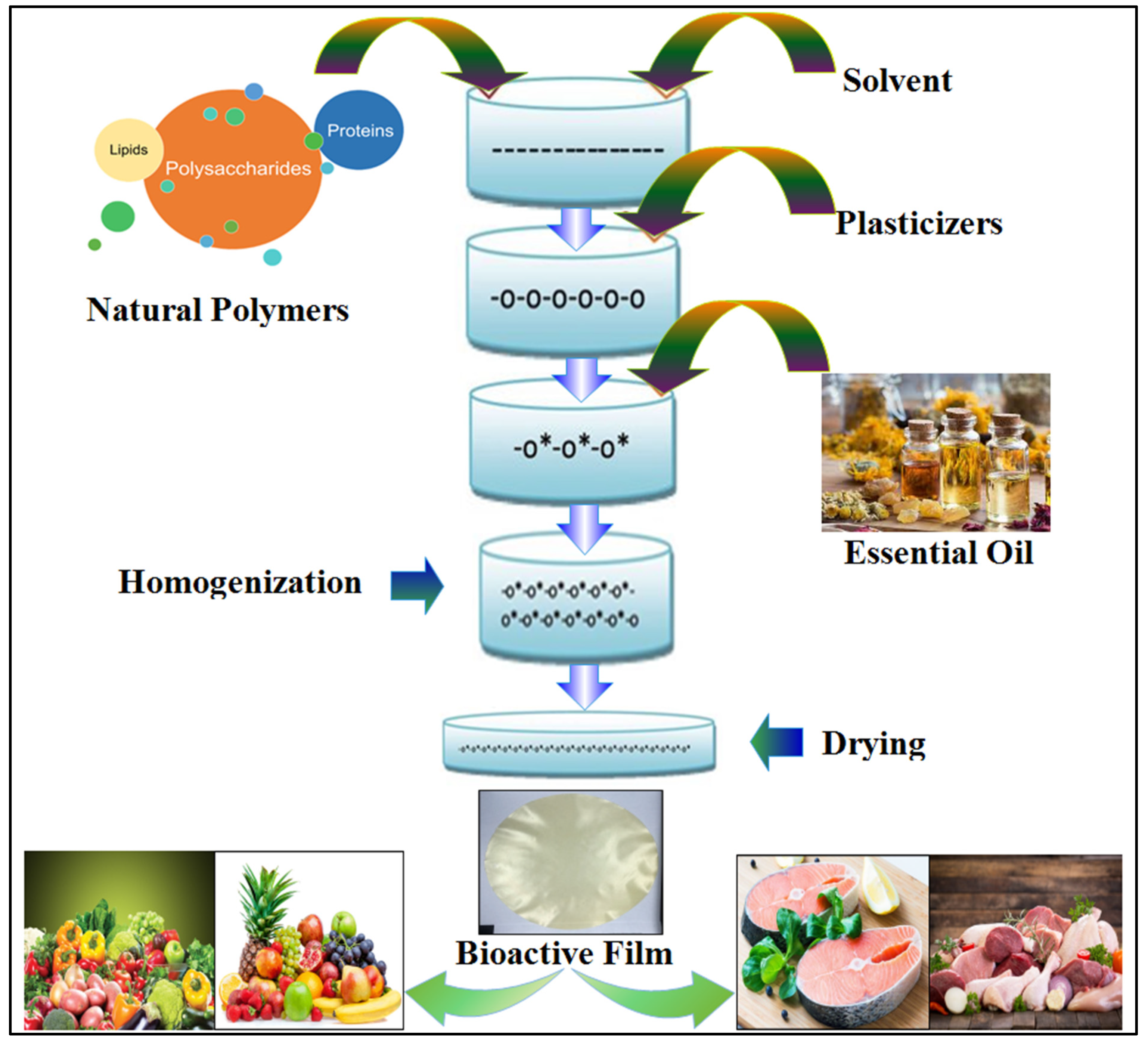
3. Biomaterials
3.1. Polysaccharide-Based Films Loaded with Essential Oils
3.2. Protein-Based Films Loaded with Essential Oils
3.3. Composite Films Loaded with Essential Oils
4. Bioactivity of Essential Oil-Added Film
4.1. Antimicrobial Activity
4.2. Antioxidant Activity
| Essential Oil | Major Active Components | Film Type | EO Levels in the Film | Findings | Reference |
|---|---|---|---|---|---|
| Oregano EO | Phenolic acids and terpenoids | Oxidized esterified tapioca starch film | 0.3, 0.6, 0.9, 1.0, and 1.5% |
| [77] |
| Oregano EO | Phenolic acids and terpenoids | Starch film (Dioscorea zingiberensis starch) | 1, 2, and 3% |
| [103] |
| Oregano EO | Phenolic acids and terpenoids | Poly (lactic acid)/poly (trimenthylene carbonate) | 3, 6, 9, and 12% |
| [115] |
| Licorice EO | Isopropyl palmitate | Zein/pullulan | 30% |
| [116] |
| Rockrose EO | Camphene, bornyl acetate, trans pinocarveol, and α-pinene | Pullulan | 15% |
| [106] |
| Clove EO | Eugenol, α- karyophylene, β-karyophylene, and α-humulene | Edible film | 5% |
| [101] |
| Citrus limonia EO | Limonene, nerol, and 1,8-cineole | Chitosan | 0.4, 0.8, 1.5, and 2% |
| [76] |
| Lemon EO | Phenolic compounds | Gelatin/Chitosan | 0.25, 0.50, 0.75, and 1.0% |
| [117] |
| Orange peel EO | Phenolic compounds | Chitosan and fish skin gelatin | 0.25, 0.5, and 1.0% |
| [97] |
| Neem EO | Sterols, triterpenoids, and active ester derivatives | Active agar (AG) bilayer film | 2.0 g NEO/100 g AG |
| [102] |
| Thyme EO | Sterols, triterpenoids, and active ester derivatives | Chitosan | 1.0% |
| [99] |
| Ginger EO | Eucalyptol (19.36%), (-)-camphene (15.07%), β-bisabolene (11.52%), zingiberene (9.58%), and cineol | Chitosan | 0, 0.1, 0.2, and 0.3 (%) |
| [100] |
| Rosemary EO | Polyphenols such as rosemanol, epirosmanol, and rosmarinic acid | Chitosan/gelatin film | 2% |
| [104] |
| Angelica EO | Polyphenols such as rosemanol, epirosmanol, and rosmarinic acid | Polylactic acid active film | 4% |
| [111] |
| Turmeric EO | Polyphenols such as rosemanol, epirosmanol, and rosmarinic acid | Chitosan film | 1.5 μL/cm2 and 3.0 μL/cm2 |
| [118] |
5. Application of Bioactive Films Incorporated with Essential Oils in Food
5.1. Fruits and Vegetables
5.2. Meat and Its Products
5.3. Fish and Fish Products
5.4. Dairy Products
5.5. Bread and Bakery Products
5.6. Nuts
| Film Composition | Essential Oil | Food Application | Findings | Reference |
|---|---|---|---|---|
| Chitosan/collagen protein | Cinnamon-perilla essential oil | Sea bream fillets |
| [153] |
| Pectin | Oregano essential oil, ginger essential oil | Yellow croaker |
| [154] |
| Chitosan/alginate/gelatin | Sage | Carp fish burger |
| [144] |
| Soy protein | Clove | Bluefin tuna (Thunnus thynnus) fillet |
| [146] |
| Bombacaceae gum | Cinnamon leaf essential oil | Salmon fillets |
| [142] |
| Chitosan | Ginger | Cobia (Rachycentron canadum) fish steak |
| [155] |
| Қ-Carrageenan | Red cabbage extract (Brassica oleraceae) | Rainbow trout fillets |
| [156] |
| Furcellaran/carboxymethyl cellulose | Lingonberry extract | Salmon (Salmo salar) fillets |
| [157] |
| Job’s tears starch | Clove bud essential oil | Pork belly |
| [158] |
| Chitosan | Thyme essential oil | Beef |
| [99] |
| Chitosan | Oregano essential oil | Chicken fillets |
| [159] |
| Chitosan | Apricot (Prunusarmeniaca) kernel essential oil | Spiced beef |
| [160] |
| Dioscorea zingiberensis starch | Oregano essential oil | Chicken |
| [103] |
| Potato starch (St)/apple peel pectin (Pec) | Zataria multiflora essential oil | Quail meat |
| [98] |
| Mung bean protein isolate/pullulan | Marjoram essential oil | Minced beef |
| [131] |
| Whey protein | Rosemary oil | Lamb meat |
| [161] |
| Corn starch | Zataria multiflora | Ground beef patties |
| [162] |
| Starch | Torch ginger essential oil | Chicken meat |
| [132] |
| Whey protein | Rosemary essential oil | Lamb meat |
| [163] |
| Cassava starch | Clove EO | Bananas |
| [124] |
| PLA | Angelica EO | Peaches |
| [123] |
| Carboxymethyl chitosan/pullulan | Galangal essential oil | Mangoes |
| [114] |
| Chitosan | Thyme essential oil | Nectarine fruit |
| [125] |
| Alginate with apple puree | lemongrass | Apples |
| [164] |
| PLA | Ginger essential oil, Angelica essential oil | Peaches |
| [111] |
| Chitosan/Casein | Oregano essential oil | Cherry tomatoes |
| [165] |
| Poly(lactic acid)/poly(ε-caprolactone) | Thymol | Hot peppers |
| [166] |
| Chitosan/starch | Cinnamon leaf essential oil | Tomatoes |
| [127] |
| Methylcellulose | Rosemary extract/Asian spice essential oil | Broccoli |
| [128] |
| Gelatin | Banana leaf EO | Cherry tomatoes |
| [129] |
| Potato starch | Thyme EO | Spinach |
| [130] |
| Whey protein | Garlic/oregano EO | Kasar cheese |
| [90] |
| Sodium alginate | Cinnamon EO | Paneer |
| [148] |
| Zein | Rosmarinus officinalis essential oil | Cheese |
| [167] |
| κ-Carrageenan | Black carob extract | Cheese |
| [168] |
| Gelatin/chitosan | Boldo extract | Sliced Prato cheese |
| [169] |
| Carboxymethyl cellulose (CMC)-polyvinyl alcohol (PVA) | Cinnamon essential oil | Bread |
| [170] |
| Poly (lactic acid)/poly (butylene-succinate-co-adipate) | Thymol | Bread |
| [171] |
| Chitosan/Poly(ε-caprolactone) (PCL) | Grapefruit seed extract (GFSE) | Bread |
| [172] |
| Cashew gum/gelatin | Cymbopogon citratus essential oil | Bread |
| [173] |
| Starch/gum | Grapefruit seed extract | Rice cakes |
| [174] |
| Chitosan | Mango leaf extract | Cashew nuts |
| [175] |
| Chitosan | Green tea extract | Fresh walnut kernels |
| [176] |
| Indian gooseberry puree/methylcellulose | Indian gooseberry extract | Cashew nuts |
| [177] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- da Silva Barbosa, D.C.; Holanda, V.N.; de Assis, C.R.D.; de Oliveira Farias, J.C.R.; Henrique doNascimento, P.; da Silva, W.V.; Navarro, D.M.d.A.F.; da Silva, M.V.; de Menezes Lima, V.L.; dos Santos Correia, M.T. Chemical composition and acetylcholinesterase inhibitory potential, in silico, of Myrciaria floribunda (H. West ex Willd.) O. Berg fruit peel essential oil. Ind. Crops Prod. 2020, 151, 112372. [Google Scholar] [CrossRef]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Chouhan, K.B.S.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. A unique model of gravity assisted solvent free microwave based extraction of essential oil from mentha leaves ensuring biorefinery of leftover waste biomass for extraction of nutraceuticals: Towards cleaner and greener technology. J. Clean. Prod. 2019, 225, 587–598. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.-L.; Huang, R.-H.; Su, Y.; Li, Y.-Q.; Zhang, C.-S. Variation in chemical composition and biological activities of flos chrysanthemi indici essential oil under different extraction methods. Biomolecules 2019, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.; da Cruz, J.N.; Silva, S.G.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; Teixeira, E.; da Silva, N.J.N.; de Aguiar Andrade, E.H.; Neto, A.M.d.J.C. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Darbasi, M.; Askari, G.; Kiani, H.; Khodaiyan, F. Development of chitosan based extended-release antioxidant films by control of fabrication variables. Int. J. Biol. Macromol. 2017, 104, 303–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Khan, S.; Shu, Y.; Liang, T. A Green Film-Forming Investigation of the Edible Film Based on Funoran: Preparation, Characterization, and the Investigation of the Plasticizer Effects. Foods 2022, 11, 2971. [Google Scholar] [CrossRef]
- Khan, S.; Wang, H.; Shu, Y.; Zhang, Z.; Liang, T. Characterization of a novel bioactive film based on Artemisia sphaerocephala Krasch. Gum (ASKG) complexed with β-cyclodextrin/curcumin (β-CD/CUR) inclusion complex and its application in meat preservation. Food Hydrocoll. 2022, 136, 108296. [Google Scholar] [CrossRef]
- Hernández, H.; Fraňková, A.; Sýkora, T.; Klouček, P.; Kouřimská, L.; Kučerová, I.; Banout, J. The effect of oregano essential oil on microbial load and sensory attributes of dried meat. J. Sci. Food Agric. 2017, 97, 82–87. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice-Hernandez, C.; Martins, V.G. Active and sustainable materials from rice starch, fish protein and oregano essential oil for food packaging. Ind. Crops Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Satyal, P.; Setzer, W.N. Chemical compositions of commercial essential oils from Coriandrum sativum fruits and aerial parts. Nat. Prod. Commun. 2020, 15, 1934578X20933067. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Wang, C.; Yan, S.; Lu, P.; Wang, S. Developed chitosan/oregano essential oil biocomposite packaging film enhanced by cellulose nanofibril. Polymers 2020, 12, 1780. [Google Scholar] [CrossRef]
- Ju, J.; Xu, X.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Inhibitory effects of cinnamon and clove essential oils on mold growth on baked foods. Food Chem. 2018, 240, 850–855. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.C.; Nerin, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef]
- Antunes, M.D.; da Silva Dannenberg, G.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.-T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Culmone, A.; Mirabile, G.; Tinebra, I.; Michelozzi, M.; Carrubba, A.; Bellardi, M.G.; Farina, V.; Romanazzi, G.; Torta, L. Hydrolate and EO Application to Reduce Decay of Carica papaya during Storage. Horticulturae 2023, 9, 204. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- del Carmen Razola-Díaz, M.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef]
- Chouhan, K.B.S.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. Critical analysis of microwave hydrodiffusion and gravity as a green tool for extraction of essential oils: Time to replace traditional distillation. Trends Food Sci. Technol. 2019, 92, 12–21. [Google Scholar] [CrossRef]
- Parhi, S.S.; Rangaiah, G.P.; Jana, A.K. Vapor recompressed batch distillation: Optimizing reflux ratio at variable mode. Comput. Chem. Eng. 2019, 124, 184–196. [Google Scholar] [CrossRef]
- Tien, C. Introduction to Adsorption: Basics, Analysis, and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gerbaud, V.; Rodriguez-Donis, I.; Hegely, L.; Lang, P.; Denes, F.; You, X. Review of extractive distillation. Process design, operation, optimization and control. Chem. Eng. Res. Des. 2019, 141, 229–271. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Schubert, M.; Hampel, U. Assessment of separation efficiency modeling and visualization approaches pertaining to flow and mixing patterns on distillation trays. Chem. Eng. Sci. 2018, 185, 182–208. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Meindersma, G.W.; De Haan, A.B. Water extraction of pyrolysis oil: The first step for the recovery of renewable chemicals. Bioresour. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C. Modeling and kinetics study of conventional and assisted batch solvent extraction. Chem. Eng. Res. Des. 2014, 92, 1169–1186. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernandez Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and opportunities for bio-oil refining: A review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Yildirim, A.; Cakir, A.; Mavi, A.; Yalcin, M.; Fauler, G.; Taskesenligil, Y. The variation of antioxidant activities and chemical composition of essential oils of Teucrium orientale L. var. orientale during harvesting stages. Flavour Fragr. J. 2004, 19, 367–372. [Google Scholar] [CrossRef]
- Dorman, H.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Silva, L.; Nelson, D.; Drummond, M.; Dufossé, L.; Glória, M. Comparison of hydrodistillation methods for the deodorization of turmeric. Food Res. Int. 2005, 38, 1087–1096. [Google Scholar] [CrossRef]
- Okoh, O.; Sadimenko, A.; Afolayan, A. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extractionof essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Farahnaky, A.; Javidnia, K.; Majzoobi, M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from Thymus vulgaris L. Innov. Food Sci. Emerg. Technol. 2012, 14, 85–91. [Google Scholar] [CrossRef]
- Wollinger, A.; Perrin, É.; Chahboun, J.; Jeannot, V.; Touraud, D.; Kunz, W. Antioxidant activity of hydro distillation water residues from Rosmarinus officinalis L. leaves determined by DPPH assays. Comptes Rendus Chim. 2016, 19, 754–765. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Papavassilopoulou, E.; Bardouki, H.; Vamvakias, M.; Bimpilas, A.; Oreopoulou, V. Antioxidant recovery from hydrodistillation residues of selected Lamiaceae species by alkaline extraction. J. Appl. Res. Med. Aromat. Plants 2018, 8, 83–89. [Google Scholar] [CrossRef]
- Vian, M.A.; Fernandez, X.; Visinoni, F.; Chemat, F. Microwave hydrodiffusion and gravity, a new technique for extraction of essential oils. J. Chromatogr. A 2008, 1190, 14–17. [Google Scholar] [CrossRef]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Farhat, A.; Fabiano-Tixier, A.-S.; Visinoni, F.; Romdhane, M.; Chemat, F. A surprising method for green extraction of essential oil from dry spices: Microwave dry-diffusion and gravity. J. Chromatogr. A 2010, 1217, 7345–7350. [Google Scholar] [CrossRef] [PubMed]
- Amarni, F.; Kadi, H. Kinetics study of microwave-assisted solvent extraction of oil from olive cake using hexane: Comparison with the conventional extraction. Innov. Food Sci. Emerg. Technol. 2010, 11, 322–327. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Naik, D.V.; Tripathi, D.; Singh, R.; Poddar, M.K.; Konathala, L.S.K.; Sharma, Y.K. Pyrolysis of Jatropha Curcas seed cake followed by optimization of liquid⿿ liquid extraction procedure for the obtained bio-oil. J. Anal. Appl. Pyrolysis 2016, 118, 202–224. [Google Scholar] [CrossRef]
- Stephan, C.; Dicko, M.; Stringari, P.; Coquelet, C. Liquid-liquid equilibria of water+ solutes (acetic acid/acetol/furfural/guaiacol/methanol/phenol/propanal)+ solvents (isopropyl acetate/toluene) ternary systems for pyrolysis oil fractionation. Fluid Phase Equilibria 2018, 468, 49–57. [Google Scholar] [CrossRef]
- Li, X.-J.; Wang, W.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Mu, P.-S.; Fu, Y.-J. Solvent-free microwave extraction of essential oil from Dryopteris fragrans and evaluation of antioxidant activity. Food Chem. 2012, 133, 437–444. [Google Scholar] [CrossRef]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.-P.; Ferroud, C.; Doussot, J. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochemistry 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–259. [Google Scholar]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.; Chemat, F. Rapid extraction of volatile compounds using a new simultaneous microwave distillation: Solvent extraction device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Karrar, E.; Sheth, S.; Wei, W.; Wang, X. Determination of Phenolic Compounds in Gurum (Citrulluslanatus var. Colocynthoide) Seed Oil Obtained by Different Methods Using HPLC. Food Anal. Methods 2020, 13, 1391–1397. [Google Scholar]
- Akhter, R.; Masoodi, F.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Ceni, G.; Silva, M.F.; Valério Jr, C.; Cansian, R.L.; Oliveira, J.V.; Dalla Rosa, C.; Mazutti, M.A. Continuous inactivation of alkaline phosphatase and Escherichia coli in milk using compressed carbon dioxide as inactivating agent. J. CO2 Util. 2016, 13, 24–28. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Karrar, E.; Sheth, S.; Wei, W.; Wang, X. Supercritical CO2 extraction of gurum (Citrulluslanatus var. Colocynthoide) seed oil and its properties comparison with conventional methods. J. Food Process Eng. 2019, 42, e13129. [Google Scholar] [CrossRef]
- Hua, L.; Deng, J.; Wang, Z.; Wang, Y.; Chen, B.; Ma, Y.; Li, X.; Xu, B. Improving the functionality of chitosan-based packaging films by crosslinking with nanoencapsulated clove essential oil. Int. J. Biol. Macromol. 2021, 192, 627–634. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible polymers and secondary bioactive compounds for food packaging applications: Antimicrobial, mechanical, and gas barrier properties. Polymers 2022, 14, 2395. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Bahrami, A.; Mokarram, R.R.; Khiabani, M.S.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W.; Kim, H.-Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zong, X.; Wang, S.; Yin, C.; Gao, X.; Xiong, G.; Xu, X.; Qi, J.; Mei, L. Emulsified blend film based on konjac glucomannan/carrageenan/camellia oil: Physical, structural, and water barrier properties. Carbohydr. Polym. 2021, 251, 117100. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Muppalla, S.R.; Kanatt, S.R.; Chawla, S.; Sharma, A. Carboxymethyl cellulose–polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packag. Shelf Life 2014, 2, 51–58. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Sarıcaoglu, F.T.; Turhan, S. Physicochemical, antioxidant and antimicrobial properties of mechanically deboned chicken meat protein films enriched with various essential oils. Food Packag. Shelf Life 2020, 25, 100527. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; de Deus, I.P.B.; Valadares, A.C.F.; Fernandes, C.C.; Estevam, E.B.B.; Egea, M.B. Chitosan film with citrus limonia essential oil: Physical and morphological properties and antibacterial activity. Colloids Interfaces 2020, 4, 18. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Chen, M.; Wang, X.; Wang, J. Fabrication and characterization of oxidized esterified tapioca starch films encapsulating oregano essential oil with mesoporous nanosilica. Ind. Crops Prod. 2022, 184, 115033. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Petkoska, A.T. Ginger Essential Oil as an Active Addition to Composite Chitosan Films: Development and Characterization. Gels 2022, 8, 327. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A. Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–204. [Google Scholar]
- Pirnia, M.; Shirani, K.; Yazdi, F.T.; Moratazavi, S.A.; Mohebbi, M. Characterization of antioxidant active biopolymer bilayer film based on gelatin-frankincense incorporated with ascorbic acid and Hyssopus officinalis essential oil. Food Chem. X 2022, 14, 100300. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, J.; Ramírez-Toro, C.; Bolívar, G.; Ceballos-González, C. Mechanisms of action of novel ingredients used in edible films to preserve microbial quality and oxidative stability in sausages-A review. Trends Food Sci. Technol. 2019, 89, 100–109. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or ɛ-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef]
- Lau, H.H.; Murney, R.; Yakovlev, N.L.; Novoselova, M.V.; Lim, S.H.; Roy, N.; Singh, H.; Sukhorukov, G.B.; Haigh, B.; Kiryukhin, M.V. Protein-tannic acid multilayer films: A multifunctional material for microencapsulation of food-derived bioactives. J. Colloid Interface Sci. 2017, 505, 332–340. [Google Scholar] [CrossRef]
- Iordache, F.; Gheorghe, I.; Lazar, V.; Curutiu, C.; Ditu, L.M.; Grumezescu, A.M.; Holban, A.M. Nanostructurated materials for prolonged and safe food preservation. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–335. [Google Scholar]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Jaradat, N.; Qadi, M.; Giosafatto, C.V.L.; Dell’Olmo, E.; Gaglione, R.; Arciello, A.; Porta, R. Physicochemical and antimicrobial properties of whey protein-based films functionalized with Palestinian Satureja capitata essential oil. Coatings 2021, 11, 1364. [Google Scholar] [CrossRef]
- da Silva Scudeler, C.G.; de Lima Costa, T.; Cortez-Vega, W.R.; Prentice, C.; Fonseca, G.G. Development and characterization of Nile tilapia (Oreochromis niloticus) protein isolate-based biopolymer films incorporated with essential oils and nanoclay. Food Packag. Shelf Life 2020, 25, 100542. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, X.; Shen, Y.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and characterization of bio-nanocomposites film of chitosan and montmorillonite incorporated with ginger essential oil and its application in chilled beef preservation. Antibiotics 2021, 10, 796. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Luís, Â.; Gallardo, E.; Ramos, A.; Domingues, F. Design and characterization of bioactive bilayer films: Release kinetics of isopropyl palmitate. Antibiotics 2020, 9, 443. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of orange (Citrus sinensis L.) peel essential oil on characteristics of blend films based on chitosan and fish skin gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- Sani, I.K.; Geshlaghi, S.P.; Pirsa, S.; Asdagh, A. Composite film based on potato starch/apple peel pectin/ZrO2 nanoparticles/microencapsulated Zataria multiflora essential oil; investigation of physicochemical properties and use in quail meat packaging. Food Hydrocoll. 2021, 117, 106719. [Google Scholar] [CrossRef]
- Elshamy, S.; Khadizatul, K.; Uemura, K.; Nakajima, M.; Neves, M.A. Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect. J. Food Sci. Technol. 2021, 58, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, R.M.; Al-Hilifi, S.A.; Rashed, M. Fabrication, characterization, and anti-free radical performance of edible packaging-chitosan film synthesized from shrimp shell incorporated with ginger essential oil. J. Food Meas. Charact. 2021, 15, 2951–2962. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.; Holmes, M.; Povey, M. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Yang, C.; Chen, Y.; Yang, Y.; Zhou, C.; Wang, L.; Xia, G.; Yu, X.; Yang, H. Preparation and characterization of oregano essential oil-loaded Dioscorea zingiberensis starch film with antioxidant and antibacterial activity and its application in chicken preservation. Int. J. Biol. Macromol. 2022, 212, 20–30. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-crosslinked gelatin/chitosan-based functional films incorporated with rosemary essential oil and quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Gaspar, A.L.; Gaspar, A.B.; Contini, L.R.; Silva, M.F.; Chagas, E.G.; Bahú, J.O.; Concha, V.O.; Carvalho, R.A.; Severino, P.; Souto, E.B. Lemongrass (Cymbopogon citratus)-incorporated chitosan bioactive films for potential skincare applications. Int. J. Pharm. 2022, 628, 122301. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/essential oils formulations for potential use as wound dressing: Physical and antimicrobial properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Jiang, J.; Watowita, P.; Chen, R.; Shi, Y.; Geng, J.-T.; Takahashi, K.; Li, L.; Osako, K. Multilayer gelatin/myofibrillar films containing clove essential oil: Properties, protein-phenolic interactions, and migration of active compounds. Food Packag. Shelf Life 2022, 32, 100842. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, L.; Dong, Q.; Kang, Y.; Osako, K.; Li, L. Characterization of PLA-P3, 4HB active film incorporated with essential oil: Application in peach preservation. Food Chem. 2020, 313, 126134. [Google Scholar] [CrossRef]
- Ghani, S.; Barzegar, H.; Noshad, M.; Hojjati, M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int. J. Biol. Macromol. 2018, 112, 197–202. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Wang, Y.; Zhao, P.; Wang, K.; Wang, Y.; Wang, X.; Wang, J. Evaluation of film packaging containing mesoporous nanosilica and oregano essential oil for postharvest preservation of mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2023, 198, 112263. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly (lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT 2019, 111, 218–225. [Google Scholar] [CrossRef]
- Tügen, A.; Ocak, B.; Özdestan-Ocak, Ö. Development of gelatin/chitosan film incorporated with lemon essential oil with antioxidant properties. J. Food Meas. Charact. 2020, 14, 3010–3019. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; An, S.; Liu, L.; Hu, Y.; Wan, L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019, 131, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2021, 375, 131810. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced mechanical and antioxidant properties of biodegradable poly (lactic) acid-poly (3-hydroxybutyrate-co-4-hydroxybutyrate) film utilizing α-tocopherol for peach storage. Packag. Technol. Sci. 2021, 34, 187–199. [Google Scholar] [CrossRef]
- de Figueiredo Sousa, H.A.; de Oliveira Filho, J.G.; Egea, M.B.; da Silva, E.R.; Macagnan, D.; Pires, M.; Peixoto, J. Active film incorporated with clove essential oil on storage of banana varieties. Nutr. Food Sci. 2019, 49, 911–924. [Google Scholar] [CrossRef]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Passafiume, R.; Tinebra, I.; Gaglio, R.; Settanni, L.; Sortino, G.; Allegra, A.; Farina, V. Fresh-Cut Mangoes: How to Increase Shelf Life by Using Neem Oil Edible Coating. Coatings 2022, 12, 664. [Google Scholar] [CrossRef]
- He, X.; Li, M.; Gong, X.; Niu, B.; Li, W. Biodegradable and antimicrobial CSC films containing cinnamon essential oil for preservation applications. Food Packag. Shelf Life 2021, 29, 100697. [Google Scholar] [CrossRef]
- Takala, P.N.; Vu, K.D.; Salmieri, S.; Khan, R.A.; Lacroix, M. Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 C. LWT Food Sci. Technol. 2013, 53, 499–506. [Google Scholar] [CrossRef]
- Kamari, A.; Halim, A.; Yusoff, S.; Ishak, S. Gelatin film incorporated with banana leaf essential oil for food preservation. J. Phys. Conf. Ser. 2018, 1097, 012047. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Omar-Aziz, M.; Gharaghani, M.; Khodaiyan, F.; Hosseini, S.S.; Kennedy, J.F. Effect of mung bean protein isolate/pullulan films containing marjoram (Origanum majorana L.) essential oil on chemical and microbial properties of minced beef meat. Int. J. Biol. Macromol. 2022, 201, 318–329. [Google Scholar] [CrossRef]
- Marzlan, A.A.; Muhialdin, B.J.; Abedin, N.H.Z.; Manshoor, N.; Ranjith, F.H.; Anzian, A.; Hussin, A.S.M. Incorporating torch ginger (Etlingera elatior Jack) inflorescence essential oil onto starch-based edible film towards sustainable active packaging for chicken meat. Ind. Crops Prod. 2022, 184, 115058. [Google Scholar] [CrossRef]
- Souza, V.G.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf life assessment of fresh poultry meat packaged in novel bionanocomposite of chitosan/montmorillonite incorporated with ginger essential oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Karimnezhad, F.; Razavilar, V.; Anvar, A.; Eskandari, S. Study the antimicrobial effects of chitosan-based edible film containing the Trachyspermum ammi essential oil on shelf-life of chicken meat. Microbiol. Res. 2017, 8, 7226. [Google Scholar] [CrossRef]
- dos Santos Caetano, K.; Almeida Lopes, N.; Haas Costa, T.; Brandelli, A.; Rodrigues, E.; Hickmann Flôres, S.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Supardan, M.D.; Annisa, Y.; Arpi, N.; Satriana, S.; Mustapha, W. Cassava starch edible film incorporated with lemongrass oil: Characteristics and application. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 216–220. [Google Scholar] [CrossRef]
- Khan, S.; Shu, Y.; Li, C.; Liang, T.; Zhang, Z. The influence of forsythia essential oil and ZnO nanoparticles on the physicochemical properties of ASKG-based film and its effect on the preservation of meat quality. Food Biosci. 2023, 56, 103239. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.C.; Horita, C.N.; Sant Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Cao, T.L.; Song, K.B. Development of bioactive Bombacaceae gum films containing cinnamon leaf essential oil and their application in packaging of fresh salmon fillets. LWT 2020, 131, 109647. [Google Scholar] [CrossRef]
- Martins, P.C.; Bagatini, D.C.; Martins, V.G. Oregano essential oil addition in rice starch films and its effects on the chilled fish storage. J. Food Sci. Technol. 2021, 58, 1562–1573. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT 2020, 117, 108633. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Awonyemi, I.O. Citrus peel extract starch-based bioplastic: Effect of extract concentration on packed fish and bioplastic properties. J. Polym. Environ. 2021, 29, 1706–1716. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite-clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements-a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Raju, A.; Sasikala, M.S. Natural antimicrobial edible film for preservation of paneer. Biosci. Biotechnol. Res. Asia 2016, 13, 1083–1088. [Google Scholar] [CrossRef]
- De Pilli, T. Development of a vegetable oil and egg proteins edible film to replace preservatives and primary packaging of sweet baked goods. Food Control 2020, 114, 107273. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Barba, F.J. Essential oils as natural preservatives for bakery products: Understanding the mechanisms of action, recent findings, and applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 310–321. [Google Scholar] [CrossRef]
- Nader, J.; Afif, C.; Louka, N. Impact of a novel partial defatting technology on oxidative stability and sensory properties of peanut kernels. Food Chem. 2021, 334, 127581. [Google Scholar] [CrossRef] [PubMed]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zheng, P.; Tian, F.; Zhang, Z.; Sun, Z.; Cai, L. Development of edible composite film based on chitosan nanoparticles and their application in packaging of fresh red sea bream fillets. Food Control 2022, 132, 108545. [Google Scholar] [CrossRef]
- Lan, W.q.; Lang, A.; Chen, M.L.; Xie, J. Combined effects of pectin–plant essential oil coating with vacuum packaging on the quality of large yellow croaker (Pseudosciaena crocea) during iced storage. J. Food Saf. 2022, 42, e12960. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Ravishankar, C.N. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 C. Food Control 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Rostamzad, H.; Kamali Sabeti, N.; Babakhani, A. Production and evaluation of smart biodegradable film based on carrageenan for fish fillet packaging. J. Fish. 2019, 72, 85–95. [Google Scholar]
- Tkaczewska, J.; Jamróz, E.; Guzik, P.; Kopeć, M. Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films. Polymers 2022, 14, 1717. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Song, K.B. Characterization of Job’s tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT 2019, 111, 711–718. [Google Scholar] [CrossRef]
- Karimnezhad, F.; Razavilar, V.; Anvar, A.; Dashtgol, S.; Pilehvar Zavareh, A. Combined effect of chitosan-based edible film containing oregano essential oil on the shelf-life extension of fresh chicken meat. J. Nutr. Food Secur. 2019, 4, 236–242. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Chen, X.; Liu, Y.; Wang, J.; Wang, X.; Wang, C.; Song, H. Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 2020, 162, 838–844. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Aguiar Campolina, G.; das Graças Cardoso, M.; Rodrigues-Silva-Caetano, A.; Lee Nelson, D.; Mendes Ramos, E. Essential Oil and Plant Extracts as Preservatives and Natural Antioxidants Applied to Meat and Meat Products: A Review. Food Technol. Biotechnol. 2023, 61, 212–225. [Google Scholar] [CrossRef]
- Sani, M.A.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Chafer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Roshandel-Hesari, N.; Mokaber-Esfahani, M.; Taleghani, A.; Akbari, R. Investigation of physicochemical properties, antimicrobial and antioxidant activity of edible films based on chitosan/casein containing Origanum vulgare L. essential oil and its effect on quality maintenance of cherry tomato. Food Chem. 2022, 396, 133650. [Google Scholar] [CrossRef]
- Qin, Y.; Zhuang, Y.; Wu, Y.; Li, L. Quality evaluation of hot peppers stored in biodegradable poly (lactic acid)-based active packaging. Sci. Hortic. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Pérez, M.J.; Moreno, M.A.; Martínez-Abad, A.; Cattaneo, F.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Interest of black carob extract for the development of active biopolymer films for cheese preservation. Food Hydrocoll. 2021, 113, 106436. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Gelatin-chitosan edible film activated with Boldo extract for improving microbiological and antioxidant stability of sliced Prato cheese. Int. J. Food Sci. Technol. 2019, 54, 1617–1624. [Google Scholar] [CrossRef]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and antimicrobial properties of carbohydrate-based films enriched with cinnamon essential oil by Pickering emulsion method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly (lactic acid)/poly (butylene-succinate-co-adipate)(PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Wang, K.; Lim, P.N.; Tong, S.Y.; San Thian, E. Development of grapefruit seed extract-loaded poly (ε-caprolactone)/chitosan films for antimicrobial food packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Gonzaga, M.L.; Bastos, M.S.; Magalhães, H.C.; Benevides, S.D.; Furtado, R.F.; Zambelli, R.A.; Garruti, D.S. Packaging with cashew gum/gelatin/essential oil for bread: Release potential of the citral. Food Packag. Shelf Life 2020, 23, 100431. [Google Scholar] [CrossRef]
- Lee, E.-s.; Song, H.-g.; Choi, I.; Lee, J.-S.; Han, J. Effects of mung bean starch/guar gum-based edible emulsion coatings on the staling and safety of rice cakes. Carbohydr. Polym. 2020, 247, 116696. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Suppakul, P.; Boonlert, R.; Buaphet, W.; Sonkaew, P.; Luckanatinvong, V. Efficacy of superior antioxidant Indian gooseberry extract-incorporated edible Indian gooseberry puree/methylcellulose composite films on enhancing the shelf life of roasted cashew nut. Food Control 2016, 69, 51–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Abdo, A.A.A.; Shu, Y.; Zhang, Z.; Liang, T. The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods 2023, 12, 4169. https://doi.org/10.3390/foods12224169
Khan S, Abdo AAA, Shu Y, Zhang Z, Liang T. The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods. 2023; 12(22):4169. https://doi.org/10.3390/foods12224169
Chicago/Turabian StyleKhan, Sohail, Abdullah A. A. Abdo, Ying Shu, Zhisheng Zhang, and Tieqiang Liang. 2023. "The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review" Foods 12, no. 22: 4169. https://doi.org/10.3390/foods12224169
APA StyleKhan, S., Abdo, A. A. A., Shu, Y., Zhang, Z., & Liang, T. (2023). The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods, 12(22), 4169. https://doi.org/10.3390/foods12224169







