Association of Dietary Vitamin C Consumption with Serum Klotho Concentrations
Abstract
1. Introduction
2. Materials and Methods
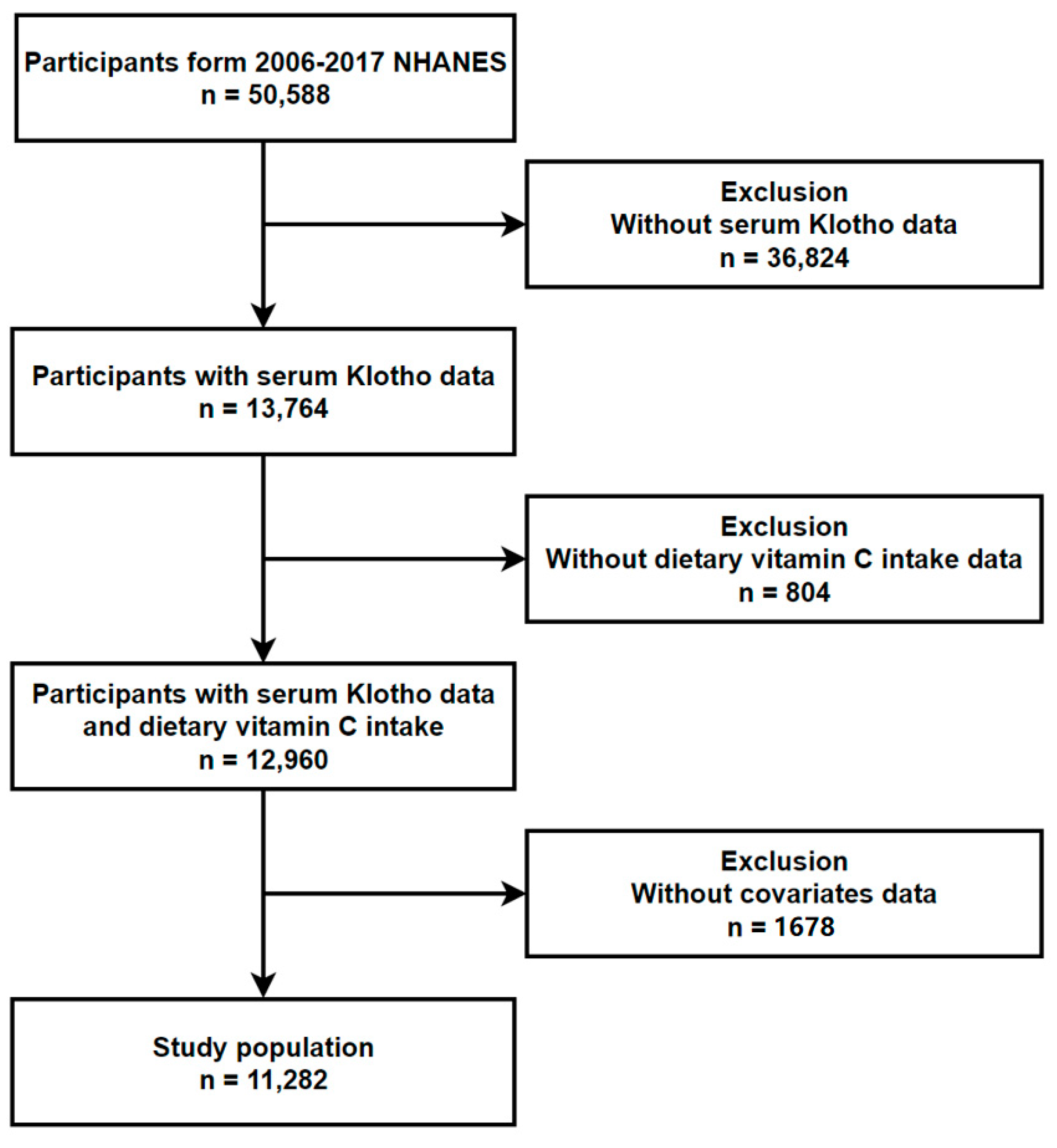
2.1. Study Population
2.2. Determination of Serum Klotho Concentrations
2.3. Assessment of Vitamin C Consumption
2.4. Covariate Adjustment
2.5. Methods of Statistical Analysis
3. Results
3.1. Basic Characteristics of All Participants
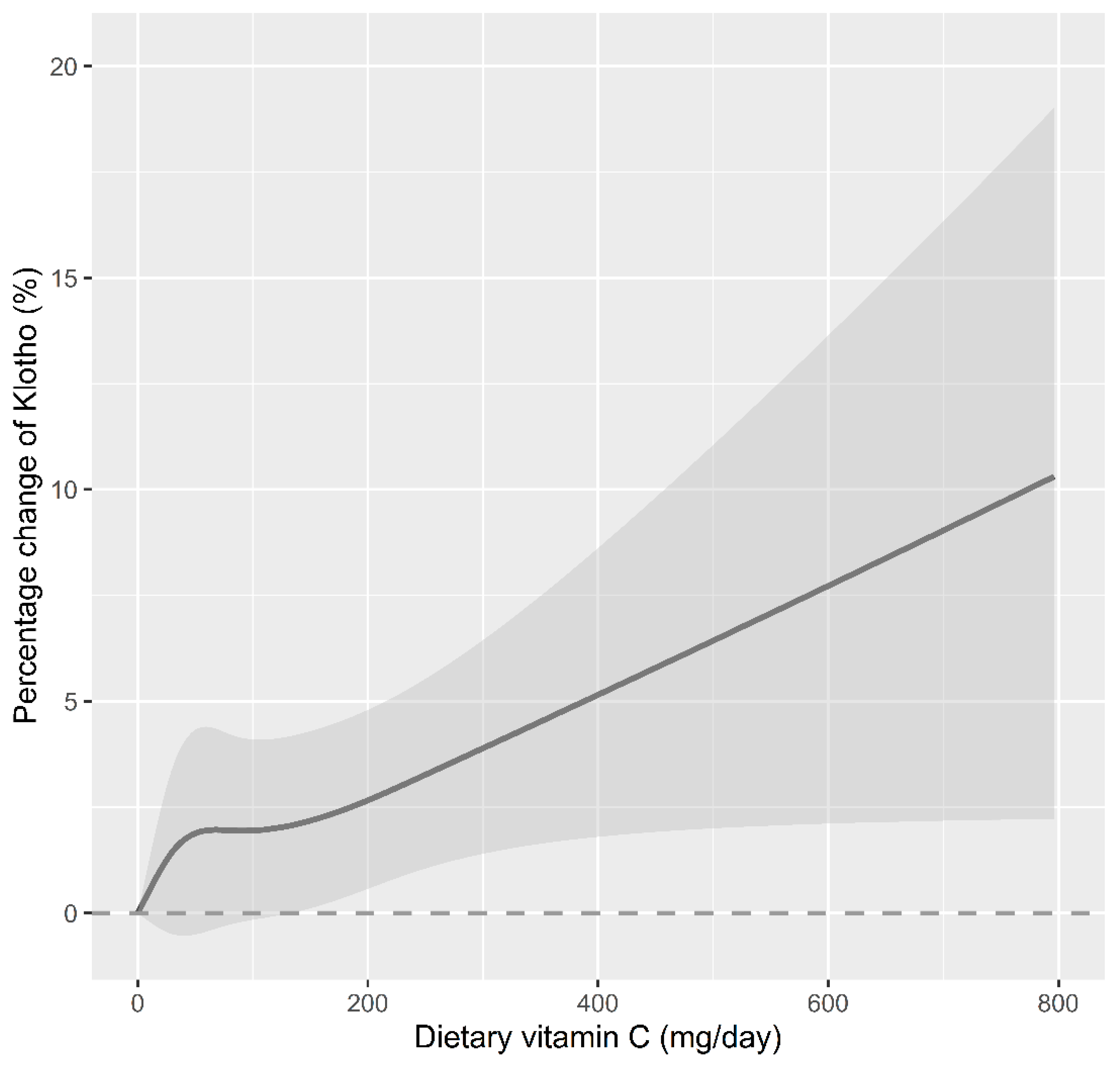
3.2. Association between Serum Klotho Concentrations and Dietary Vitamin C Consumption
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Castner, S.A.; Gupta, S.; Wang, D.; Moreno, A.J.; Park, C.; Chen, C.; Poon, Y.; Groen, A.; Greenberg, K.; David, N.; et al. Longevity factor klotho enhances cognition in aged nonhuman primates. Nat. Aging 2023, 3, 931–937. [Google Scholar] [CrossRef]
- Koyama, D.; Sato, Y.; Aizawa, M.; Maki, T.; Kurosawa, M.; Kuro-o, M.; Furukawa, Y. Soluble αKlotho as a candidate for the biomarker of aging. Biochem. Biophys. Res. Commun. 2015, 467, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, E.; Donate-Correa, J.; Ferri, C.; López-Castillo, Á.; Delgado-Molinos, A.; Hernández-Carballo, C.; Pérez-Delgado, N.; Rodríguez-Ramos, S.; Cerro-López, P.; Tagua, V.G.; et al. Association between serum levels of Klotho and inflammatory cytokines in cardiovascular disease: A case-control study. Aging 2020, 12, 1952–1964. [Google Scholar] [CrossRef]
- Sanz, B.; Arrieta, H.; Rezola-Pardo, C.; Fernández-Atutxa, A.; Garin-Balerdi, J.; Arizaga, N.; Rodriguez-Larrad, A.; Irazusta, J. Low serum klotho concentration is associated with worse cognition, psychological components of frailty, dependence, and falls in nursing home residents. Sci. Rep. 2021, 11, 9098. [Google Scholar] [CrossRef]
- Lim, S.W.; Jin, L.; Luo, K.; Jin, J.; Shin, Y.J.; Hong, S.Y.; Yang, C.W. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 2017, 8, e2972. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Bao, Y.; Zhang, T.; Ainiwaer, D.; Xiong, X.; Wang, G.; Sun, Z. The serum soluble Klotho alleviates cardiac aging and regulates M2a/M2c macrophage polarization via inhibiting TLR4/Myd88/NF-κB pathway. Tissue Cell 2022, 76, 101812. [Google Scholar] [CrossRef]
- Shin, E.-J.; Chung, Y.H.; Le, H.-L.T.; Jeong, J.H.; Dang, D.-K.; Nam, Y.; Wie, M.B.; Nah, S.-Y.; Nabeshima, Y.-I.; Nabeshima, T.; et al. Melatonin attenuates memory impairment induced by Klotho gene deficiency via interactive signaling between MT2 receptor, ERK, and Nrf2-related antioxidant potential. Int. J. Neuropsychopharmacol. 2014, 18, pyu105. [Google Scholar] [CrossRef]
- Ho, T.-J.; Goswami, D.; Kuo, W.-W.; Kuo, C.-H.; Yen, S.C.; Lin, P.-Y.; Lin, S.-Z.; Hsieh, D.J.-Y.; Shibu, M.A.; Huang, C.-Y. Artemisia argyi exhibits anti-aging effects through decreasing the senescence in aging stem cells. Aging 2022, 14, 6187–6201. [Google Scholar] [CrossRef]
- Xuan, N.T.; Trang, P.T.T.; Van Phong, N.; Toan, N.L.; Trung, D.M.; Bac, N.D.; Nguyen, V.L.; Hoang, N.H.; Van Hai, N. Klotho sensitive regulation of dendritic cell functions by vitamin E. Biol. Res. 2016, 49, 45. [Google Scholar] [CrossRef] [PubMed]
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Study on the Anti-cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wu, X.; Wen, J.; Fei, Y.; Wu, J.; Li, X.; Zhang, Q.; Dong, Y.; Xu, T.; Fan, Y.; et al. Nicotinamide retains Klotho expression and ameliorates rhabdomyolysis-induced acute kidney injury. Nutrition 2021, 91–92, 111376. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Zhang, B.L.; Zhang, X.M.; Tong, J.D.; Gu, Y.H.; Guo, L.L.; Jin, H.M. EGCG Attenuates Renal Damage via Reversing Klotho Hypermethylation in Diabetic db/db Mice and HK-2 Cells. Oxid. Med. Cell Longev. 2020, 2020, 6092715. [Google Scholar] [CrossRef]
- Rizzo, B.; Maltese, G.; Paraskevi, M.P.; Hrelia, S.; Mann, G.; Siow, R. Induction of antioxidant genes by sulforaphane and klotho in human aortic smooth muscle cells. Free Radic. Biol. Med. 2014, 75 (Suppl. 1), S14–S15. [Google Scholar] [CrossRef]
- He, H.; Chen, X.; Miao, D.; Zhang, H.; Wang, Y.; He, X.; Chen, X.; Dai, N. Composite Dietary Antioxidant Index and Plasma Levels of Soluble Klotho: Insights from NHANES. Oxid. Med. Cell Longev. 2023, 2023, 3524611. [Google Scholar] [CrossRef]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef]
- Jaturakan, O.; Buranakarl, C.; Dissayabutra, T.; Chaiyabutr, N.; Kijtawornrat, A.; Rungsipipat, A. Changes of Klotho protein and Klotho mRNA expression in a hydroxy-L-proline induced hyperoxaluric rat model. J. Vet. Med. Sci. 2017, 79, 1861–1869. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Yin, X.; Zhao, Y.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological ageing and the risks of all-cause and cause-specific mortality among people with diabetes: A prospective cohort study. J. Epidemiol. Community Health 2022, 76, 771–778. [Google Scholar] [CrossRef]
- Cai, Y.; Zhong, Y.-d.; Zhang, H.; Lu, P.-L.; Liang, Y.-Y.; Hu, B.; Wu, H. Association between dietary vitamin C and telomere length: A cross-sectional study. Front. Nutr. 2023, 10, 1025936. [Google Scholar] [CrossRef]
- Kale, A.; Sankrityayan, H.; Anders, H.-J.; Gaikwad, A.B. Klotho in kidney diseases: A crosstalk between the renin-angiotensin system and endoplasmic reticulum stress. Nephrol. Dial. Transpl. Transplant. 2023, 38, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.D.; Xie, J.; An, S.-W.; Huang, C.-L. New Insights into the Mechanism of Action of Soluble Klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Espuch-Oliver, A.; Vázquez-Lorente, H.; Jurado-Fasoli, L.; de Haro-Muñoz, T.; Díaz-Alberola, I.; López-Velez, M.D.S.; de Haro-Romero, T.; Castillo, M.J.; Amaro-Gahete, F.J. References Values of Soluble α-Klotho Serum Levels Using an Enzyme-Linked Immunosorbent Assay in Healthy Adults Aged 18–85 Years. J. Clin. Med. 2022, 11, 2415. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Min, J.-Y.; Min, K.-B. Anti-aging protein klotho was associated with vitamin B12 concentration in adults. Medicine 2022, 101, e30710. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Hillesund, E.R.; Øverby, N.C.; Vik, F.N.; Medin, A.C. Individual nutrients and serum klotho levels in adults aged 40–79 years. Food Sci. Nutr. 2023, 11, 3279–3286. [Google Scholar] [CrossRef]
- Tan, S.-J.; Smith, E.R.; Hewitson, T.D.; Holt, S.G.; Toussaint, N.D. Diurnal variation and short-term pre-analytical stability of serum soluble α-klotho in healthy volunteers: A pilot study. Ann. Clin. Biochem. 2015, 52, 506–509. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Shardell, M.; Semba, R.D.; Kalyani, R.R.; Bandinelli, S.; Prather, A.A.; Chia, C.W.; Ferrucci, L. Plasma Klotho and Frailty in Older Adults: Findings From the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1052–1057. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Arenander, J.; Broestl, L.; Garay, B.I.; Wang, D.; Dubal, D.B. Longevity factor klotho and chronic psychological stress. Transl. Psychiatry 2015, 5, e585. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-E.; Chen, Y.-J.; Chen, W.-L. Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging. Nutrients 2022, 14, 3910. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, M.; Cumming, R.G.; Lazarus, R. Cohort study of vitamin C intake and cognitive impairment. Am. J. Epidemiol. 1998, 148, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef]
- Lin, B.; Xu, D.; Wu, S.; Qi, S.; Xu, Y.; Liu, X.; Zhang, X.; Chen, C. Antioxidant Effects of Sophora davidi (Franch.) Skeels on d-Galactose-Induced Aging Model in Mice via Activating the SIRT1/p53 Pathway. Front. Pharmacol. 2021, 12, 754554. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhao, J.; Chen, Y.; Ren, C.; Chen, Y. Antioxidant effects of compound walnut oil capsule in mice aging model induced by D-galactose. Food Nutr. Res. 2018, 62, 29219. [Google Scholar] [CrossRef]
- Bella, Y.F.; Oliveira, C.R.; Mateus-Silva, J.R.; Brandao-Rangel, M.A.R.; Silva-Reis, A.; Santos, J.d.M.B.; Albertini, R.; Lopes-Martins, R.A.B.; de Oliveira, L.V.F.; Vieira, R.P. A phytotherapic blend immunity-6™ inhibits myeloid leukemic cells 2 activation involving purinergic signaling. Biomed. Pharmacother. 2023, 159, 114263. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Buga, A.M.; Docea, A.O.; Sarandi, E.; Mitrut, R.; Renieri, E.; Spandidos, D.A.; Rogoveanu, I.; Cercelaru, L.; Niculescu, M.; et al. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int. J. Mol. Med. 2021, 48, 199. [Google Scholar] [CrossRef]
- Wei, F.; Qu, C.; Song, T.; Ding, G.; Fan, Z.; Liu, D.; Liu, Y.; Zhang, C.; Shi, S.; Wang, S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J. Cell Physiol. 2012, 227, 3216–3224. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Langlois, K.; Cooper, M.; Colapinto, C.K. Vitamin C status of Canadian adults: Findings from the 2012/2013 Canadian Health Measures Survey. Health Rep. 2016, 27, 3–10. [Google Scholar] [PubMed]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Martín-Calvo, N.; Martínez-González, M.Á. Vitamin C Intake is Inversely Associated with Cardiovascular Mortality in a Cohort of Spanish Graduates: The SUN Project. Nutrients 2017, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, J.; Jiang, H.; Kong, J. Klotho Levels are Decreased and Associated with Enhanced Oxidative Stress and Inflammation in the Aqueous Humor in Patients with Exudative Age-related Macular Degeneration. Ocul. Immunol. Inflamm. 2022, 30, 630–637. [Google Scholar] [CrossRef]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef]
- Valdecantos, M.P.; Pérez-Matute, P.; Quintero, P.; Martínez, J.A. Vitamin C, resveratrol and lipoic acid actions on isolated rat liver mitochondria: All antioxidants but different. Redox Rep. 2010, 15, 207–216. [Google Scholar] [CrossRef]
- Izquierdo, M.C.; Perez-Gomez, M.V.; Sanchez-Niño, M.D.; Sanz, A.B.; Ruiz-Andres, O.; Poveda, J.; Moreno, J.A.; Egido, J.; Ortiz, A. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transpl. Transplant. 2012, 27 (Suppl. 4), iv6–iv10. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Korac, A.; Srdic-Galic, B.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Vucetic, M.; Markelic, M.; Velickovic, K.; Golic, I.; et al. Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism 2014, 63, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Zengin, K.; Taskin, M.; Aydin, S.; Simsek, G.; Dariyerli, N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes. Surg. 2004, 14, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Flocea, E.-I.; Lazado, C.C.; Simionov, I.-A.; Nicoara, M.; Ciobica, A.; Faggio, C.; Jijie, R. Vitamin C Mitigates Oxidative Stress and Behavioral Impairments Induced by Deltamethrin and Lead Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 12714. [Google Scholar] [CrossRef]
- McGregor, G.P.; Biesalski, H.K. Rationale and impact of vitamin C in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 697–703. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Orces, C.H. The Association of Obesity and the Antiaging Humoral Factor Klotho in Middle-Aged and Older Adults. Sci. World J. 2022, 2022, 7274858. [Google Scholar] [CrossRef]
- Rose, F.J.; Webster, J.; Barry, J.B.; Phillips, L.K.; Richards, A.A.; Whitehead, J.P. Synergistic effects of ascorbic acid and thiazolidinedione on secretion of high molecular weight adiponectin from human adipocytes. Diabetes Obes. Metab. 2010, 12, 1084–1089. [Google Scholar] [CrossRef]
- Olmedilla, B.; Granado, F.; Southon, S.; Wright, A.J.; Blanco, I.; Gil-Martinez, E.; Berg, H.; Corridan, B.; Roussel, A.M.; Chopra, M.; et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001, 85, 227–238. [Google Scholar] [CrossRef]
- Itoh, R.; Yamada, K.; Oka, J.; Echizen, H.; Murakami, K. Sex as a factor in levels of serum ascorbic acid in a healthy elderly population. Int. J. Vitam. Nutr. Res. 1989, 59, 365–372. [Google Scholar]
| Characteristic | All | The Quintile of Dietary Vitamin C Intake (mg/day) | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p Value | ||
| Number of participants | 11,282 | 2250 | 2262 | 2254 | 2259 | 2257 | |
| Dietary vitamin C intake, mean (SD) | 81.00 (90.78) | 8.24 (5.06) | 26.40 (5.62) | 52.58 (9.73) | 97.31 (16.67) | 220.31 (109.64) | <0.001 |
| Serum Klotho concentration, pg/mL, median (25th–75th) | 800.55 (653.95, 990.00) | 787.50 (643.50, 973.72) | 797.85 (644.58, 989.98) | 802.30 (660.22, 994.20) | 794.20 (647.10, 981.90) | 821.70 (671.00, 1003.90) | 0.001 |
| Age, years, mean (SD) | 57.78 (10.81) | 56.52 (10.77) | 56.70 (10.59) | 58.24 (10.95) | 58.70 (10.90) | 57.75 (10.74) | <0.001 |
| Sex, n (%) | <0.001 | ||||||
| male | 5544 (49.10) | 1101 (48.9) | 1063 (47.0) | 1055 (46.8) | 1083 (47.9) | 1242 (55.0) | |
| female | 5738 (50.90) | 1149 (51.1) | 1199 (53.0) | 1199 (53.2) | 1176 (52.1) | 1015 (45.0) | |
| BMI, kg/m2, mean (SD) | 29.89 (6.74) | 30.35 (7.30) | 30.01 (6.62) | 29.95 (6.74) | 29.66 (6.51) | 29.46 (6.47) | <0.001 |
| Race/Ethnicity, n (%) | <0.001 | ||||||
| Non-Hispanic White | 5203 (46.10) | 1084 (48.2) | 1107 (48.9) | 1052 (46.7) | 1051 (46.5) | 909 (40.3) | |
| Non-Hispanic Black | 2234 (19.80) | 502 (22.3) | 423 (18.7) | 394 (17.5) | 425 (18.8) | 490 (21.7) | |
| Other Hispanic | 1199 (10.60) | 224 (10.0) | 225 (9.9) | 249 (11.0) | 228 (10.1) | 273 (12.1) | |
| Mexican American or Other | 2646 (23.5) | 440 (19.6) | 507 (22.4) | 559 (24.8) | 555 (24.6) | 585 (25.9) | |
| Educational attainment, n (%) | <0.001 | ||||||
| <High school | 2950 (26.10) | 745 (33.1) | 607 (26.8) | 583 (25.9) | 527 (23.3) | 488 (21.6) | |
| High school | 2519 (22.30) | 556 (24.7) | 582 (25.7) | 480 (21.3) | 476 (21.1) | 425 (18.8) | |
| College or above | 5813 (51.50) | 949 (42.2) | 1073 (47.4) | 1191 (52.8) | 1256 (55.6) | 1344 (59.5) | |
| PIR, mean (SD) | 2.65 (1.65) | 2.26 (1.54) | 2.56 (1.62) | 2.76 (1.66) | 2.85 (1.67) | 2.84 (1.69) | <0.001 |
| Serum cotinine, ng/mL, median (25–75th) | 0.04 (0.01, 2.54) | 0.13 (0.02, 187.75) | 0.05 (0.01, 64.12) | 0.03 (0.01, 0.36) | 0.03 (0.01, 0.23) | 0.03 (0.01, 0.20) | <0.001 |
| Alcohol consumption, n (%) | 0.138 | ||||||
| ≥12 drinks/year | 3199 (28.40) | 625 (27.8) | 655 (29.0) | 667 (29.6) | 655 (29.0) | 597 (26.5) | |
| <12 drinks/year | 8083 (71.60) | 1625 (72.2) | 1607 (71.0) | 1587 (70.4) | 1604 (71.0) | 1660 (73.5) | |
| Diabetes, n (%) | <0.001 | ||||||
| No | 8569 (76.00) | 1709 (76.0) | 1652 (73.0) | 1667 (74.0) | 1740 (77.0) | 1801 (79.8) | |
| Yes | 2713 (24.00) | 541 (24.0) | 610 (27.0) | 587 (26.0) | 519 (23.0) | 456 (20.2) | |
| Hypertension, n (%) | 0.230 | ||||||
| No | 5133 (45.50) | 989 (44.0) | 1010 (44.7) | 1027 (45.6) | 1044 (46.2) | 1063 (47.1) | |
| Yes | 6149 (54.50) | 1261 (56.0) | 1252 (55.3) | 1227 (54.4) | 1215 (53.8) | 1194 (52.9) | |
| eGFR, mL/min/1.73 m2, mean (SD) | 84.00 (19.64) | 83.94 (20.69) | 83.62 (20.10) | 84.26 (19.11) | 83.20 (19.77) | 84.96 (18.45) | 0.034 |
| Dietary energy intake, kcal/day, mean (SD) | 2023.66 (914.05) | 1747.34 (848.38) | 1941.31 (836.79) | 2045.95 (906.66) | 2072.94 (901.60) | 2310.06 (977.78) | <0.001 |
| Dietary Vitamin C Consumption (mg/day) | Percent Changes (%) and 95% CI | |||||
|---|---|---|---|---|---|---|
| Model 1 | p Value | Model 2 | p Value | Model 3 | p Value | |
| Per SD increases | 1.33 (0.56, 2.12) | 0.001 | 1.54 (0.78, 2.31) | <0.001 | 1.17 (0.37, 1.99) | 0.006 |
| Quintile 1 | Ref. | Ref. | Ref. | |||
| Quintile 2 | 0.69 (−1.75, 3.19) | 0.585 | 0.97 (−1.43, 3.44) | 0.434 | 0.77 (−1.60, 3.20) | 0.530 |
| Quintile 3 | 0.68 (−1.71, 3.14) | 0.581 | 1.18 (−1.22, 3.63) | 0.342 | 0.51 (−1.68, 2.74) | 0.653 |
| Quintile 4 | 0.48 (−1.54, 2.54) | 0.644 | 1.09 (−0.88, 3.10) | 2.833 | 0.37 (−1.69, 2.48) | 0.726 |
| Quintile 5 | 4.08 (1.51, 6.72) | 0.002 | 4.89 (2.38, 7.47) | <0.001 | 3.66 (1.05, 6.32) | 0.007 |
| p for trend | <0.001 | <0.001 | 0.011 | |||
| Participants | Dietary Vitamin C Consumption (mg/day) | Percent Changes (%) and 95% CI | p Value | * p for Interaction |
|---|---|---|---|---|
| Age subgroup | 0.457 | |||
| Age < 60 years | Per SD increases | 0.69 (−0.23, 1.62) | 0.147 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | 2.03 (−1.15, 5.31) | 0.219 | ||
| Quintile 3 | 0.14 (−3.09, 3.49) | 0.933 | ||
| Quintile 4 | −0.10 (−3.11, 3.00) | 0.948 | ||
| Quintile 5 | 3.51 (0.10, 7.03) | 0.048 | ||
| p for trend | 0.087 | |||
| Age ≥ 60 years | Per SD increases | 1.90 (0.37, 3.43) | 0.017 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | −1.53 (−5.20, 2.28) | 0.43 | ||
| Quintile 3 | 0.94 (−2.82, 4.84) | 0.63 | ||
| Quintile 4 | 0.87 (−2.68, 4.54) | 0.64 | ||
| Quintile 5 | 3.38 (−1.08, 8.05) | 0.14 | ||
| p for trend | 0.0720 | |||
| BMI subgroup | 0.009 | |||
| BMI < 25 kg/m2 | Per SD increases | 2.38 (1.17, 3.61) | <0.001 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | 4.10 (−1.85, 10.22) | 0.189 | ||
| Quintile 3 | −0.93 (−6.39,4.86) | 0.749 | ||
| Quintile 4 | 4.12 (−0.81, 9.30) | 0.108 | ||
| Quintile 5 | 8.27 (2.98, 13.84) | 0.003 | ||
| p for trend | 0.002 | |||
| BMI ≥ 25 kg/m2 | Per SD increases | 0.62 (−0.38, 1.63) | 0.231 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | −0.13 (−2.86, 2.69) | 0.939 | ||
| Quintile 3 | 0.98 (−1.70, 3.73) | 0.481 | ||
| Quintile 4 | −0.93 (−3.62, 1.73) | 0.490 | ||
| Quintile 5 | 2.13 (−1.05, 5.40) | 0.196 | ||
| p for trend | 0.239 | |||
| Sex subgroup | 0.146 | |||
| Male | Per SD increases | 1.80 (0.73, 2.89) | 0.002 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | 1.85 (−1.31, 5.10) | 0.259 | ||
| Quintile 3 | 1.85 (−1.47, 5.28) | 0.283 | ||
| Quintile 4 | 0.34 (−2.84, 3.62) | 0.838 | ||
| Quintile 5 | 5.68 (1.97, 9.51) | 0.004 | ||
| p for trend | 0.009 | |||
| Female | Per SD increases | 0.42 (−0.73, 1.59) | 0.480 | |
| Quintile 1 | Ref. | |||
| Quintile 2 | −0.09 (−3.52, 3.47) | 0.962 | ||
| Quintile 3 | −0.83 (−3.73, 2.17) | 0.587 | ||
| Quintile 4 | 0.37 (−2.75, 3.60) | 0.817 | ||
| Quintile 5 | 1.66 (−1.82, 5.26) | 0.358 | ||
| p for trend | 0.260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, M.; Xiang, L.; Liu, S.; Luo, G.; Lin, Q.; Xiao, L. Association of Dietary Vitamin C Consumption with Serum Klotho Concentrations. Foods 2023, 12, 4230. https://doi.org/10.3390/foods12234230
Wang Y, Wu M, Xiang L, Liu S, Luo G, Lin Q, Xiao L. Association of Dietary Vitamin C Consumption with Serum Klotho Concentrations. Foods. 2023; 12(23):4230. https://doi.org/10.3390/foods12234230
Chicago/Turabian StyleWang, Yan, Mingyang Wu, Lu Xiang, Si Liu, Gang Luo, Qian Lin, and Lin Xiao. 2023. "Association of Dietary Vitamin C Consumption with Serum Klotho Concentrations" Foods 12, no. 23: 4230. https://doi.org/10.3390/foods12234230
APA StyleWang, Y., Wu, M., Xiang, L., Liu, S., Luo, G., Lin, Q., & Xiao, L. (2023). Association of Dietary Vitamin C Consumption with Serum Klotho Concentrations. Foods, 12(23), 4230. https://doi.org/10.3390/foods12234230








