Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Used in This Study
2.2. Preparation and Purification of Spore Suspension
2.3. Preparation of B. licheniformis Germinants
2.4. Selection of B. licheniformis Germinants
2.4.1. Measurement of OD600
2.4.2. Thermal Inactivation and Germination Rate of Spores
2.4.3. Determination of DPA Release Rate
2.5. Selection of Cold Plasma Conditions
2.6. Inactivation Effect and Mechanism of Cold Plasma on B. licheniformis
2.6.1. Thermal Inactivation of B. licheniformis
2.6.2. B. licheniformis Growth Curve Determination
2.6.3. DPA Release Rate of B. licheniformis Spores
2.6.4. Survival Status of Spores of B. licheniformis in Hypertonic Medium
2.6.5. Confocal Laser Scanning Microscope (CLSM) Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Selection of Germinants
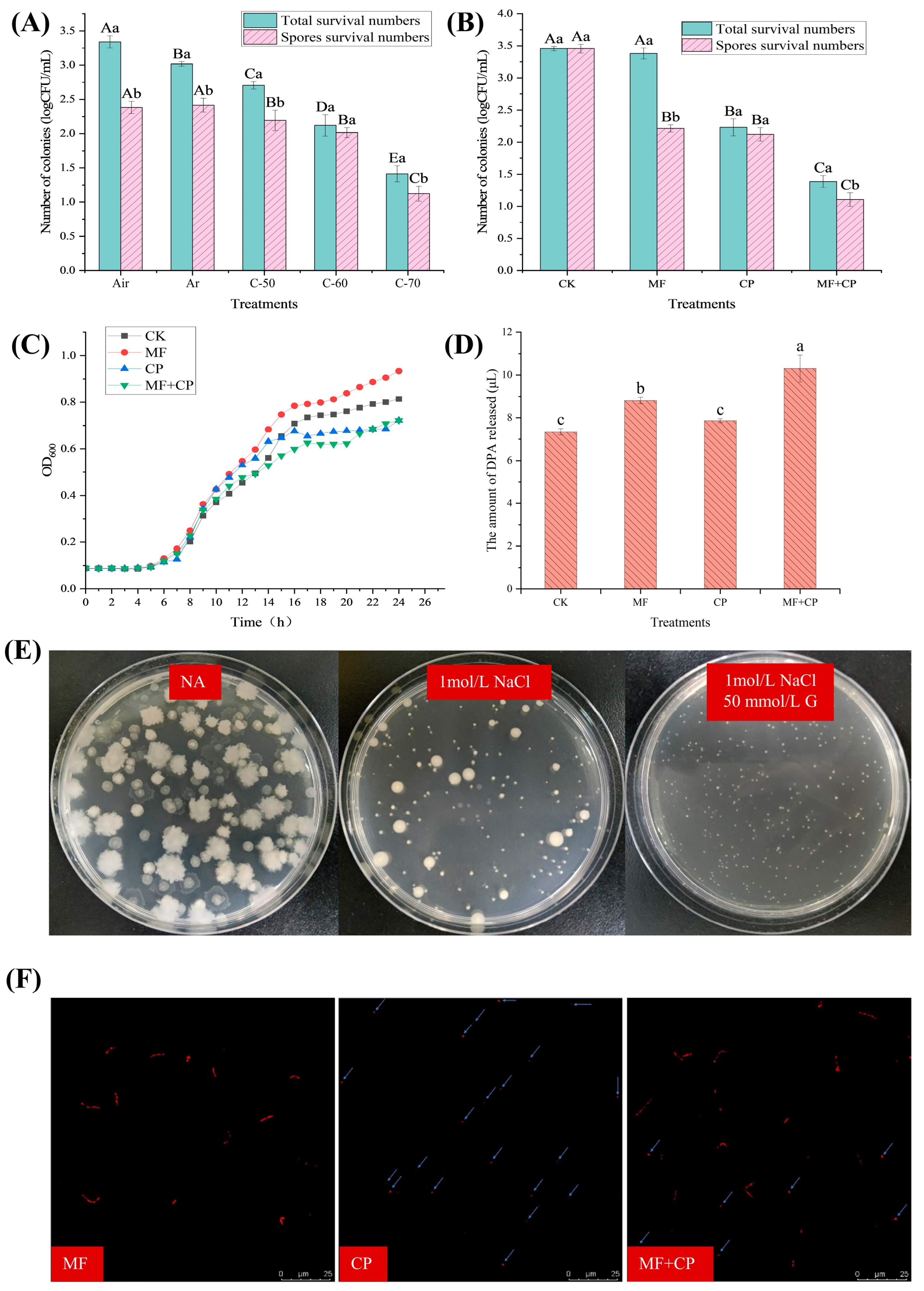
3.2. Selection of Cold Plasma Conditions
3.3. Inactivation Effect of Germinants Combined with Cold Plasma on B. licheniformis
3.4. Mechanism of Cold Plasma Inactivation of B. licheniformis Spores
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richmond, B.; Fields, M.L. Distribution of Thermophilic Aerobic Sporeforming Bacteria in Food Ingredients. Appl. Microbiol. 1966, 14, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Petrova, L. Diferentsirane na kulturi ot rod Bacillus, izolirani ot mesni polukonservi. Vet. Med. Nauk. 1975, 12, 82–88. [Google Scholar]
- Iacona, V.A.; Simonetta, A.C.; Renzulli, P.M. Bacteria of genus Bacillus in chicken carcasses and hamburgers. Rev. Argent. Microbiol. 1995, 27, 21–27. [Google Scholar] [PubMed]
- Yeak, K.Y.C.; Perko, M.; Staring, G.; Fernandez-Ciruelos, B.M.; Wells, J.M.; Abee, T.; Wells-Bennik, M.H.J. Lichenysin Production by Bacillus licheniformis Food Isolates and Toxicity to Human Cells. Front. Microbiol. 2022, 13, 831033. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Barbosa, J.; Maciel, E.; da Costa, E.; Alves, E.; Domingues, P.; Mendo, S.; Domingues, M.R.M. Lipidomic Signature of Bacillus licheniformis 189 during the Different Growth Phases Unravelled by High-Resolution Liquid Chromatography-Mass Spectrometry. Arch. Biochem. Biophys. 2019, 663, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Almalki, T.A.; Anand, S. Recovery Potential of Cavitation-Induced Injured Cells of Common Spore-Forming Bacteria in Skim Milk Exposed to Ultrasonication. JDS Commun. 2021, 2, 305–308. [Google Scholar] [CrossRef]
- Modugno, C.; Kmiha, S.; Simoni, H.; Aouadhi, C.; Canizares, E.D.; Lang, E.; Andre, S.; Mejri, S.; Maaroufi, A.; Perrier-Cornet, J.-M. High Pressure Sensitization of Heat-Resistant and Pathogenic Foodborne Spores to Nisin. Food Microbiol. 2019, 84, 103244. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Huang, T.; Zhang, Y.; Ali Khan, I.; Huang, M. The Formation, Germination, and Cold Plasma Inactivation of Bacterial Spore. Food Chem. Adv. 2022, 1, 100056. [Google Scholar] [CrossRef]
- Delbruck, A.I.; Zhang, Y.; Heydenreich, R.; Mathys, A. Bacillus Spore Germination at Moderate High Pressure: A Review on Underlying Mechanisms, Influencing Factors, and Its Comparison with Nutrient Germination. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4159–4181. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, T.; Gui, M.; Wang, Y.; Zhao, L.; Wu, X.; Rao, L.; Liao, X. The Underlying Mechanism of Bacterial Spore Germination: An Update Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2728–2746. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Ismail, B.B.; Muhammad, A.I.; Li, G.; Liu, D. Bacillus Spore Germination: Mechanisms, Identification, and Antibacterial Strategies. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sinai, L.; Rosenberg, A.; Smith, Y.; Segev, E.; Ben-Yehuda, S. The Molecular Timeline of a Reviving Bacterial Spore. Mol. Cell 2015, 57, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Amon, J.D.; Artzi, L.; Ramirez-Guadiana, F.H.; Brock, K.P.; Cofsky, J.C.; Marks, D.S.; Kruse, A.C.; Rudner, D.Z. Bacterial Spore Germination Receptors Are Nutrient-Gated Ion Channels. Science 2023, 380, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Wang, S.; Li, Y.-Q. Germination of Spores of the Orders Bacillales and Clostridiales. In Annual Review of Microbiology; Gottesman, S., Ed.; Annual Reviews; Palo Alto: Santa Clara, CA, USA, 2017; Volume 71, pp. 459–477. ISBN 978-0-8243-1171-1. [Google Scholar]
- Rao, L.; Zhou, B.; Serruya, R.; Moussaieff, A.; Sinai, L.; Ben-Yehuda, S. Glutamate Catabolism during Sporulation Determines the Success of the Future Spore Germination. Iscience 2022, 25, 105242. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhu, P.; Zhang, Y.; Ju, N.; Si, X.; Pang, X.; Lv, J.; Zhang, S. Preparation and Quality Assessment of Processed Cream Cheese by High Hydrostatic Pressure Combined Thermal Processing and Spore-Induced Germination. J. Food Eng. 2023, 341, 111319. [Google Scholar] [CrossRef]
- Song, M.; Lei, Y.; Ali, A.; Xu, Y.; Sheng, K.; Huang, T.; Huang, J.; Huang, M. Inhibitory Effect of Licorice Extract on the Germination and Outgrowth of Paraclostridium bifermentans Spores. Front. Microbiol. 2022, 13, 1076144. [Google Scholar] [CrossRef]
- Rajan, S.; Pandrangi, S.; Balasubramaniam, V.M.; Yousef, A.E. Inactivation of Bacillus stearothermophilus Spores in Egg Patties by Pressure-Assisted Thermal Processing. LWT Food Sci. Technol. 2006, 39, 844–851. [Google Scholar] [CrossRef]
- Lopes, R.P.; Mota, M.J.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Application of High Pressure with Homogenization, Temperature, Carbon Dioxide, and Cold Plasma for the Inactivation of Bacterial Spores: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 532–555. [Google Scholar] [CrossRef]
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of Supercritical Fluid Carbon Dioxide in Improving Food Shelf-Life and Safety by Inactivating Spores: A Review. J. Food Sci. Technol. 2022, 59, 417–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Huang, S.; Dong, X.; Huang, J.; Huang, M. In-Package Cold Plasma Treatment of Braised Chicken: Voltage Effect. Food Sci. Hum. Wellness 2022, 11, 845–853. [Google Scholar] [CrossRef]
- Liao, X.; Muhannnnad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial Spore Inactivation Induced by Cold Plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Doona, C.J.; Setlow, P.; Li, Y. Use of Raman Spectroscopy and Phase-Contrast Microscopy to Characterize Cold Atmospheric Plasma Inactivation of Individual Bacterial Spores. Appl. Environ. Microbiol. 2016, 82, 5775–5784. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhorst-van de Veen, H.; Xie, H.; Esveld, E.; Abee, T.; Mastwijk, H.; Groot, M.N. Inactivation of Chemical and Heat-Resistant Spores of Bacillus and Geobacillus by Nitrogen Cold Atmospheric Plasma Evokes Distinct Changes in Morphology and Integrity of Spores. Food Microbiol. 2015, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, J.; Hu, H.; Deveau, I.F.; Foley, S.L.; Chen, H. Optimization of Sporulation and Purification Methods for Sporicidal Efficacy Assessment on Bacillus Spores. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac014. [Google Scholar] [CrossRef]
- Francis, M.B.; Sorg, J.A. Dipicolinic Acid Release by Germinating Clostridium Difficile Spores Occurs through a Mechanosensing Mechanism. Msphere 2016, 1, e00306-16. [Google Scholar] [CrossRef]
- Yi, X.; Bond, C.; Sarker, M.R.; Setlow, P. Efficient Inhibition of Germination of Coat-Deficient Bacterial Spores by Multivalent Metal Cations, Including Terbium (Tb3+). Appl. Environ. Microbiol. 2011, 77, 5536–5539. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Li, M.; Zhao, L.; Ren, H.; Yan, L.; Zhao, G.; Zhu, C. Rapid Determination of Spore Germinability of Clostridium perfringens Based on Microscopic Hyperspectral Imaging Technology and Chemometrics. J. Food Eng. 2020, 280, 109896. [Google Scholar] [CrossRef]
- Lin, T.; Bian, H.; Sun, Z.; Wang, X.; Liu, F.; Wang, D. Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid. Foods 2022, 11, 3771. [Google Scholar] [CrossRef]
- Baloh, M.; Sorg, J.A. Clostridioides Difficile Spore Germination: Initiation to DPA Release. Curr. Opin. Microbiol. 2022, 65, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Loshon, C.A.; Wahome, P.G.; Maciejewski, M.W.; Setlow, P. Levels of Glycine Betaine in Growing Cells and Spores of Bacillus Species and Lack of Effect of Glycine Betaine on Dormant Spore Resistance. J. Bacteriol. 2006, 188, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, P.; Ong, L.; Gras, S.; Pellegrino, L. A Fluorescence in Situ Staining Method for Investigating Spores and Vegetative Cells of Clostridia by Confocal Laser Scanning Microscopy and Structured Illuminated Microscopy. Micron 2018, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
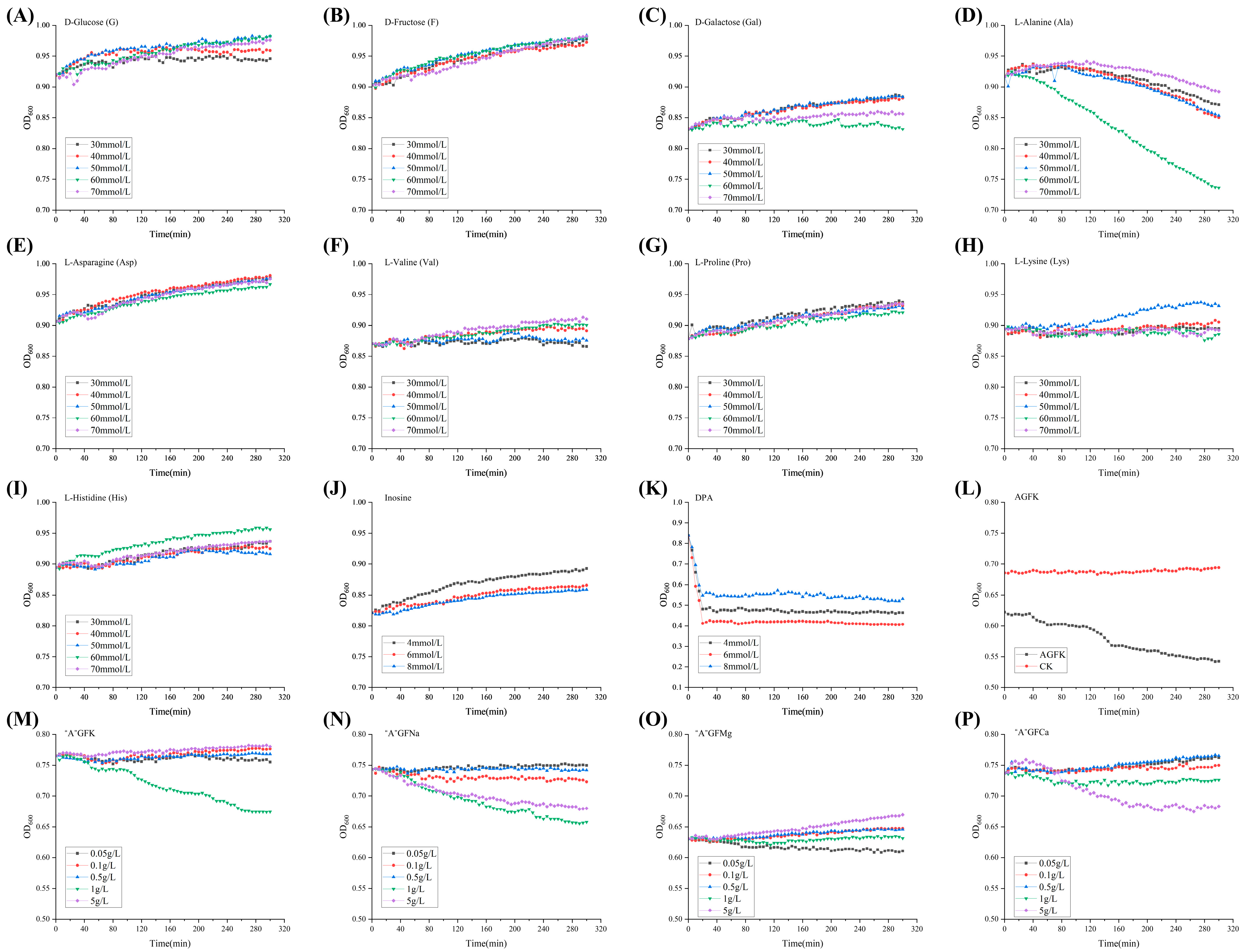

| Germinants | Formulation Concentration | |
|---|---|---|
| D-glucose (G) | 30 mmol/L 40 mmol/L 50 mmol/L 60 mmol/L 70 mmol/L | |
| D-fructose (F) | ||
| D-galactose (Gal) | ||
| L-Alanine (Ala) | ||
| L-Asparagine (Asp) | ||
| L-Valine (Val) | ||
| L-Proline (Pro) | ||
| L-Lysine (Lys) | ||
| L-Histidine (His) | ||
| Inosine | 4 mmol/L 6 mmol/L 8 mmol/L | |
| DPA 1 | ||
| AGFK | Asp (10 mmol/L) G (10 mmol/L) F (10 mmol/L) KCl (50 mmol/L) | |
| Combined germinants “A”GFK/Na/Mg/Ca | Ala (60 mmol/L) G (10 mmol/L) F (10 mmol/L) KCl/NaCl/MgCl2/CaCl2 | 0.05 g/L |
| 0.1 g/L | ||
| 0.5 g/L | ||
| 1 g/L | ||
| 5 g/L | ||
| Treatment | Number of Spores Remaining on Different Media (log CFU/mL) | ||
|---|---|---|---|
| NA 5 | NA + 1 mol/L NaCl | NA + 1 mol/L NaCl + 50 mmol/L Glucose | |
| CK 1 | 3.46 ± 0.03 Aa | 3.39 ± 0.07 Aa | 3.22 ± 0.07 Aa |
| MF 2 | 3.38 ± 0.09 Aa | 2.44 ± 0.1 Bc | 2.78 ± 0.1 Bb |
| CP 3 | 2.23 ± 0.13 Ba | 1.12 ± 0.1 Cb | 1.22 ± 0.12 Cb |
| MF + CP 4 | 1.39 ± 0.1 Ca | 0.98 ± 0.06 Dc | 1.27 ± 0.13 Cb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Sheng, K.; Zhang, Y.; Song, M.; Ali, A.; Huang, T.; Huang, M. Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores. Foods 2023, 12, 4319. https://doi.org/10.3390/foods12234319
Huang J, Sheng K, Zhang Y, Song M, Ali A, Huang T, Huang M. Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores. Foods. 2023; 12(23):4319. https://doi.org/10.3390/foods12234319
Chicago/Turabian StyleHuang, Jichao, Kairan Sheng, Yali Zhang, Mengmeng Song, Ahtisham Ali, Tianran Huang, and Ming Huang. 2023. "Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores" Foods 12, no. 23: 4319. https://doi.org/10.3390/foods12234319




