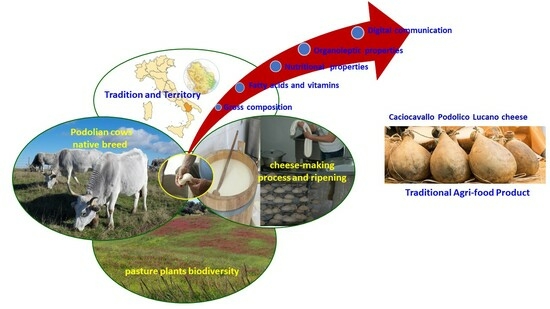
Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication
Abstract
:1. Introduction
2. Materials and Methods
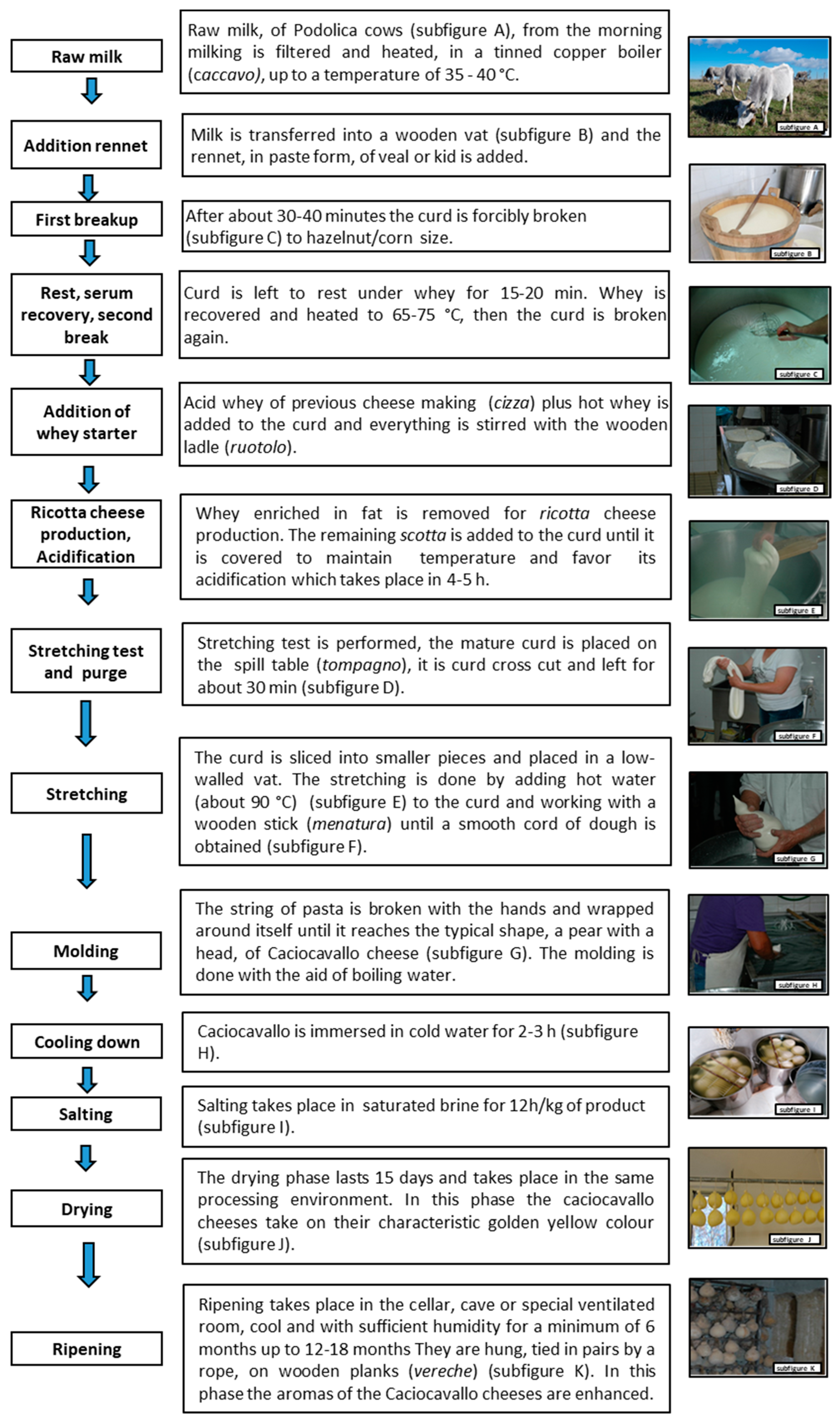
2.1. Survey Design and Cheese Sample Collection
2.2. Gross Composition
2.3. Fatty Acid Profile
2.4. Total Retinol, α-Tocopherol, and Cholesterol
2.5. Total Polyphenol Content and Antioxidant Capacity
2.6. Nutritional Indexes
2.7. Artificial Sensory Analyses
2.8. Digital Communication and Trends
2.9. Statistical Analyses
3. Results and Discussion
3.1. Gross Composition of CPL Cheese
3.2. Fatty Acid Profile of CPL Cheese
3.3. Fat-Soluble Vitamins and Cholesterol in CPL Cheese
3.4. Polyphenol Content and Total Antioxidant Capacity of CPL Cheese
3.5. Nutritional Indexes of CPL Cheese
3.6. Artificial Sensory Profile of CPL Cheese
3.7. Communication and Trends Relative to CPL Cheese on the Web
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilbery, B.; Kneafsey, M. Niche Markets and Regional Speciality Food Products in Europe: Towards a Research Agenda. Environ. Plan. Econ. Space 1999, 31, 2207–2222. [Google Scholar] [CrossRef]
- Renna, M.; Signore, A.; Santamaria, P. I Prodotti Agroalimentari Tradizionali (PAT), Espressione Del Territorio e Del Patrimonio Culturale Italiano. Italus Hortus 2018, 25, 1–13. [Google Scholar] [CrossRef]
- Pilone, V.; De Lucia, C.; Del Nobile, M.A.; Contò, F. Policy Developments of Consumer’s Acceptance of Traditional Products Innovation: The Case of Environmental Sustainability and Shelf Life Extension of a PGI Italian Cheese. Trends Food Sci. Technol. 2015, 41, 83–94. [Google Scholar] [CrossRef]
- ISMEA Rapporto Ismea-Qualivita on Italian PDO IGP TSG Agri-Food and Wine Production. 2022. Available online: https://www.qualivita.it/Attivita/Rapporto-Ismea-Qualivita-2022/ (accessed on 21 July 2023).
- Wägeli, S.; Janssen, M.; Hamm, U. Organic Consumers’ Preferences and Willingness-to-Pay for Locally Produced Animal Products: Preferences for Locally Produced Animal Products. Int. J. Consum. Stud. 2016, 40, 357–367. [Google Scholar] [CrossRef]
- Van Loo, E.J.; Grebitus, C.; Roosen, J. Explaining Attention and Choice for Origin Labeled Cheese by Means of Consumer Ethnocentrism. Food Qual. Prefer. 2019, 78, 103716. [Google Scholar] [CrossRef]
- Aurier, P.; Fort, F.; Sirieix, L. Exploring Terroir Product Meanings for the Consumer. Anthropol. Food 2005, 4, 187. [Google Scholar] [CrossRef]
- Ministerial Decree n. 350. Regolamento Recante Norme Per L’individuazione Dei Prodotti Tradizionali Di Cui All’articolo 8, Comma 1, Del Decreto Legislativo 30 Aprile 1998, n. 173. 1999. Available online: https://www.gazzettaufficiale.it/Eli/Id/1999/10/12/099G0423/Sg (accessed on 21 July 2023).
- Masaf Ministero Dell’agricoltura, Della Sovranità Alimentare e Delle Foreste. Decreto Ministeriale 22 Maggio 2023. Aggiornamento Dell’elenco Nazionale Dei Prodotti Agroalimentari Tradizionali Ai Sensi Dell’articolo 12, Comma 1, Della Legge 12 Dicembre 2016, n.238. (23A03302). G. U. Ser. Gen. 2023, 133, 1–19. [Google Scholar]
- EC 2013 European Commission. Report from the Commission to the European Parliament and the Council on the Case for a Local Farming and Direct Sales Labelling Scheme. 2013. Available online: https://op.europa.eu/En/Publication-Detail/-/Publication/Be106719-60e5-11e3-Ab0f-01aa75ed71a1 (accessed on 21 July 2023).
- FAO. First Global Forum of the UN Decade of Family Farming 2019–2028, 19–22 September 2022. 2022. Available online: https://www.fao.org/Events/Detail/First-Global-Forum-of-the-Un-Decade-of-Family-Farming/En (accessed on 21 July 2023).
- Dambrosio, A.; Ioanna, F.; Storelli, M.M.; Castiglia, D.; Giannico, F.; Colonna, M.A.; De Rosa, M.; Quaglia, N.C. Microbiological Quality and Safety of Cheeses Belonging to “Traditional Agri-Food Products” (T.A.P.) Produced in Southern Italy. J. Food Saf. 2018, 38, e12539. [Google Scholar] [CrossRef]
- Celano, G.; Costantino, G.; Calasso, M.; Randazzo, C.; Minervini, F. Distinctive Traits of Four Apulian Traditional Agri-Food Product (TAP) Cheeses Manufactured at the Same Dairy Plant. Foods 2022, 11, 425. [Google Scholar] [CrossRef]
- Di Grigoli, A.; Ponte, M.; Bonanno, A.; Maniaci, G.; Alabiso, M. Effects of Grazing Season on Physico-Chemical Characteristics and Fatty Acids of Nutritional Interest of Caciocavallo Palermitano Cheese. Animals 2022, 12, 544. [Google Scholar] [CrossRef]
- Di Trana, A.; Di Rosa, A.R.; Addis, M.; Fiori, M.; Di Grigoli, A.; Morittu, V.M.; Spina, A.A.; Claps, S.; Chiofalo, V.; Licitra, G.; et al. The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index. Animals 2022, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Bollettino Ufficiale Della Regione Basilicata (B.U.R.). Legge Regionale Del 4 Gennaio 1999; N.1., pp. 1–36. Available online: https://faolex.fao.org/docs/pdf/ita31541.pdf (accessed on 21 July 2023).
- Pizzillo, M. Il Caciocavallo Un Formaggio Sempre Diverso. In Il Caciocavallo Podolico e La Manteca. Un Grande Formaggio Del Sud; Ars Grafica: Potenza, Italy, 2001; pp. 45–64. [Google Scholar]
- Claps, S. Il Mondo Degli Aromi. In Il Caciocavallo Podolico e La Manteca. Un Grande Formaggio Del Sud; Ars Grafica: Potenza, Italy, 2001; pp. 77–98. [Google Scholar]
- Di Trana, A.; Marsico, G.; Muscio, A. Variazioni Quanti Qualitative Del Latte Di Bovina Di Razza Podolica: Influenza Del Sistema Di Allevamento. In Proceedings of the Atti XXVIII Simposio Internazionale Di Zootecnia, Milan, Italy, 14 May 1993; pp. 269–276. [Google Scholar]
- Perna, A.M.; Marsico, G.; Cosentino, C.; Santarsiere, L.A.; Freschi, P.; Gambacorta, E. La Produzione Quanti-Qualitativa Di Latte Nel Bovino Podolico in Allevamento Brado. In Proceedings of the 4th World Italian Beef Cattle Congress, Gubbio, Italy, 29 April–1 May 2005; pp. 457–462. [Google Scholar]
- Marsico, G.; Di Trana, A.; La Notte, E.; Muscio, A. L’influenza Del Sottosistema Di Allevamento Sulla Composizione Chimica e Su Quella Acidica Del Grasso Del Latte Di Bovine Podoliche. Arch. Vet. Ital. 1993, 44, 108–117. [Google Scholar]
- Lovallo, C.; Marchitelli, C.; Napolitano, F.; Claps, S.; Crisà, A. Sialyloligosaccharides Content in Mature Milk of Different Cow Breeds. Sustainability 2022, 14, 2805. [Google Scholar] [CrossRef]
- Perna, A.M.; Marsico, D.; Pistone, L.; Cosentino, E. Podolian Caciocavallo Cheese: Seasonal Variations of Food Quality Characteristics. Ital. J. Anim. Sci. 2003, 2 (Suppl. S1), 311–313. [Google Scholar]
- Busetta, G.; Garofalo, G.; Barbera, M.; Di Trana, A.; Claps, S.; Lovallo, C.; Franciosi, E.; Gaglio, R.; Settanni, L. Metagenomic, Microbiological, Chemical and Sensory Profiling of Caciocavallo Podolico Lucano Cheese. Food Res. Int. 2023, 169, 112926. [Google Scholar] [CrossRef] [PubMed]
- Schena, A.; Gambacorta, E.; Lamacchia, C.; Pati, S.; La Notte, E.; Faccia, M. Studio Dei Prodotti Della Lipolisi Nel Caciocavallo Podolico. In Proceedings of the VIII Congresso Italiano Di Scienza e Tecnologia Degli Alimenti, Milan, Italy, 7–8 May 2007. [Google Scholar]
- Villani, F.; Pepe, O.; Coppola, R.; Andolfi, R.; Coppola, S. Microbiological Aspects of Manufacture of Caciocavallo Podolico. Il Latte 1991, 16, 780–784. [Google Scholar]
- Cooren, F. Applying Socio-Semiotics to Organizational Communication: A New Approach. Manag. Commun. Q. 1999, 13, 294–304. [Google Scholar] [CrossRef]
- Ministry Of Agriculture and Forests. Approval of Official Methods of Analysis for Cheeses; DM 21/04/1986; Official Journal, 1986, 229, 02/10/1986 Met 2; Polygraphic Institute and State Mint: Rome, Italy, 1986. [Google Scholar]
- Ministry Of Agriculture and Forests. Approval of Official Methods of Analysis for Cheeses; DM 21/04/1986; Official Journal, 1986, 229, 02/10/1986 Met 4; Polygraphic Institute and State Mint: Rome, Italy, 1986. [Google Scholar]
- Ministry Of Agriculture and Forests. Approval of Methods of Analysis for Cereals and Derivatives; DM 23/07/1994; Official Journal, 186 SO114 10/08/1994 Suppl. 4; Polygraphic Institute and State Mint: Rome, Italy, 1994. [Google Scholar]
- Ministry Of Agriculture and Forests. Approval of Official Methods of Analysis for Cheeses; DM 21/04/1986; Official Journal, 1986, 229, 02/10/1986 Met 9; Polygraphic Institute and State Mint: Rome, Italy, 1986. [Google Scholar]
- Idda, I.; Spano, N.; Addis, M.; Galistu, G.; Ibba, I.; Nurchi, V.M.; Pilo, M.I.; Scintu, M.F.; Piredda, G.; Sanna, G. Optimization of a Newly Established Gas-Chromatographic Method for Determining Lactose and Galactose Traces: Application to Pecorino Romano Cheese. J. Food Compos. Anal. 2018, 74, 89–94. [Google Scholar] [CrossRef]
- UNI EN ISO 17294-2; Water Quality: Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Part 2: Determination Selected Elements Including Uranium Isotopes. Ente Nazionale Italiano Di Unificazione Database (UNI): Milan, Italy, 2016; pp. 1–31.
- UNI EN 13805; Standard Reference EN 13805:2014 Foodstuffs—Determination of Trace Elements—Pressure Digestion. European Committee for Standardization (CEN): Brussels, Belgium, 2014; pp. 1–7.
- Regulation on the Provisions of Food Information to Consumers: A Comparative Analysis of Its Enforcements in Relation to Member States; 1169/11/EU; OJEU: Strasbourg, France, 2011; Volume 304, pp. 18–63.
- Jiang, J.; Bjoerck, L.; Fondén, R.; Emanuelson, M. Occurrence of Conjugated Cis-9, Trans-11-Octadecadienoic Acid in Bovine Milk: Effects of Feed and Dietary Regimen. J. Dairy Sci. 1996, 79, 438–445. [Google Scholar] [CrossRef]
- ISO 15884: 2002; Milk Fat-Preparation of Fatty Acid Methyl Esters; ISO Methods 15884: 2002 (IDF 182: 2002). International Organization for Standardization: Geneva, Switzerland, 2002.
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of Fatty Acid Content in Sheep Milk by Mid-Infrared Spectrometry with a Selection of Wavelengths by Genetic Algorithms. LWT Food Sci. Technol. 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Panfili, G.; Manzi, P.; Pizzoferrato, L. High-Performance Liquid Chromatographic Method for the Simultaneous Determination of Tocopherols, Carotenes, and Retinol and Its Geometric Isomers in Italian Cheeses. Analyst 1994, 119, 1161. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Panfili, G.; Pizzoferrato, L. Normal and Reversed-Phase HPLC for More Complete Evaluation of Tocopherols, Retinols, Carotenes and Sterols in Dairy Products. Chromatographia 1996, 43, 89–93. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Effects of Catechin on the Phenolic Content and Antioxidant Properties of Low-Fat Cheese. Int. J. Food Sci. Technol. 2013, 48, 2448–2455. [Google Scholar] [CrossRef]
- ISO 14502; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent 21. ISO Method 14502-1; International Organization for Standardization: Geneva, Switzerland, 2007.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, D.; Di Trana, A.; Di Napoli, M.A.; Sepe, L.; Cecchini, S.; Rossi, R.; Claps, S. Comparison of Cheeses from Goats Fed 7 Forages Based on a New Health Index. J. Dairy Sci. 2019, 102, 6790–6801. [Google Scholar] [CrossRef]
- Kang, Z.-Q.; Yang, Y.; Xiao, B. Dietary Saturated Fat Intake and Risk of Stroke: Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 179–189. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of Fats and Carbohydrate Intake with Cardiovascular Disease and Mortality in 18 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of Dietary, Circulating, and Supplement Fatty Acids With Coronary Risk: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2014, 160, 398. [Google Scholar] [CrossRef]
- Di Rosa, A.; Leone, F.; Bressan, F.; Battaglia, G.; Veccia, T.; Chiofalo, V. Radio Frequency Heating of Milk–Effects on Quality, Safety, and Shelf Life Assessed Using Artificial Senses and Chemometric Tools. Electronics 2018, 7, 402. [Google Scholar] [CrossRef]
- Trabelsi, N.; Nalbone, L.; Di Rosa, A.R.; Ed-Dra, A.; Nait-Mohamed, S.; Mhamdi, R.; Giuffrida, A.; Giarratana, F. Marinated Anchovies (Engraulis Encrasicolus) Prepared with Flavored Olive Oils (Chétoui Cv.): Anisakicidal Effect, Microbiological, and Sensory Evaluation. Sustainability 2021, 13, 5310. [Google Scholar] [CrossRef]
- Albastaki, Y.A.; Albalooshi, F. (Eds.) Electronic Nose Technologies and Advances in Machine Olfaction. In Advances in Computer and Electrical Engineering; IGI Global: Hershey, PA, USA, 2018; ISBN 978-1-5225-3862-2. [Google Scholar]
- SYSTAT. SigmaPlot Statistics, Version 15; SYSTAT, Inc.: Evanston, IL, USA, 2022. [Google Scholar]
- Fallico, V.; McSweeney, P.L.H.; Siebert, K.J.; Horne, J.; Carpino, S.; Licitra, G. Chemometric Analysis of Proteolysis during Ripening of Ragusano Cheese. J. Dairy Sci. 2004, 87, 3138–3152. [Google Scholar] [CrossRef] [PubMed]
- Portnoi, P.A.; MacDonald, A. Determination of the Lactose and Galactose Content of Cheese for Use in the Galactosaemia Diet. J. Hum. Nutr. Diet. 2009, 22, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Gille, D.; Walther, B.; Badertscher, R.; Bosshart, A.; Brügger, C.; Brühlhart, M.; Gauch, R.; Noth, P.; Vergères, G.; Egger, L. Detection of Lactose in Products with Low Lactose Content. Int. Dairy J. 2018, 83, 17–19. [Google Scholar] [CrossRef]
- Dekker, P.; Koenders, D.; Bruins, M. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef]
- Natrella, G.; De Palo, P.; Maggiolino, A.; Faccia, M. A Study on Milk and Caciocavallo Cheese from Podolica Breed in Basilicata, Italy. Dairy 2023, 4, 482–496. [Google Scholar] [CrossRef]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef]
- Yehia, N.A.; Lai, K.Z.H.; Semnani-Azad, Z.; Blanco Mejia, S.; Bazinet, R.P.; Beaudry, J.L.; Hanley, A.J. Association of Branched Chain Fatty Acids with Cardiometabolic Disorders in Humans: A Systematic Review. Nutr. Rev. 2023, 81, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Thomas Brenna, J. Branched-Chain Fatty Acid Content of Foods and Estimated Intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.-D.G.; Kraft, J. Content and Composition of Branched-Chain Fatty Acids in Bovine Milk Are Affected by Lactation Stage and Breed of Dairy Cow. PLoS ONE 2016, 11, e0150386. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Liu, L.; Brenna, J.T.; Wu, Z.H.; Ma, L.; Bu, D.P. Odd- and Branched-Chain Fatty Acids in Milk Fat from Holstein Dairy Cows Are Influenced by Physiological Factors. Animal 2022, 16, 100545. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Function—An Update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors Affecting Odd- and Branched-Chain Fatty Acids in Milk: A Review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Bonanno, A.; Tornambè, G.; Bellina, V.; De Pasquale, C.; Mazza, F.; Maniaci, G.; Di Grigoli, A. Effect of Farming System and Cheesemaking Technology on the Physicochemical Characteristics, Fatty Acid Profile, and Sensory Properties of Caciocavallo Palermitano Cheese. J. Dairy Sci. 2013, 96, 710–724. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; De La Fuente, M.A. Milk Fatty Acids and Potential Health Benefits: An Updated Vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2007, 27, 104–119. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing Increases the Unsaturated Fatty Acid Concentration of Milk from Grass-fed Cows: A Review of the Contributing Factors, Challenges and Future Perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability 2020, 12, 3688. [Google Scholar] [CrossRef]
- Sofi, F.; Buccioni, A.; Cesari, F.; Gori, A.M.; Minieri, S.; Mannini, L.; Casini, A.; Gensini, G.F.; Abbate, R.; Antongiovanni, M. Effects of a Dairy Product (Pecorino Cheese) Naturally Rich in Cis-9, Trans-11 Conjugated Linoleic Acid on Lipid, Inflammatory and Haemorheological Variables: A Dietary Intervention Study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 117–124. [Google Scholar] [CrossRef]
- Pintus, S.; Murru, E.; Carta, G.; Cordeddu, L.; Batetta, B.; Accossu, S.; Pistis, D.; Uda, S.; Elena Ghiani, M.; Mele, M.; et al. Sheep Cheese Naturally Enriched in α-Linolenic, Conjugated Linoleic and Vaccenic Acids Improves the Lipid Profile and Reduces Anandamide in the Plasma of Hypercholesterolaemic Subjects. Br. J. Nutr. 2013, 109, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Gogna, S.; Kaur, J.; Sharma, K.; Bhadariya, V.; Singh, J.; Kumar, V.; Rasane, P.; Vipasha, V. A Systematic Review on the Role of Alpha Linolenic Acid (ALA) in Combating Non-Communicable Diseases (NCDs). Nutr. Food Sci. 2023, 53, 221–233. [Google Scholar] [CrossRef]
- Department of Health. Nutritional Aspects of Cardiovascular Disease; Report on Health and Social Subjects; Her Majesty’s Stationery Office (HMSO): London, UK, 1994; Volume 46, pp. 37–46. [Google Scholar]
- Calderón, F.; Chauveau-Duriot, B.; Pradel, P.; Martin, B.; Graulet, B.; Doreau, M.; Nozière, P. Variations in Carotenoids, Vitamins A and E, and Color in Cow’s Plasma and Milk Following a Shift from Hay Diet to Diets Containing Increasing Levels of Carotenoids and Vitamin E. J. Dairy Sci. 2007, 90, 5651–5664. [Google Scholar] [CrossRef] [PubMed]
- Agabriel, J. Alimentation des Bovins, Ovins et Caprins. Besoins des Animaux. Valeur des Aliments. Tables INRA; Quae Editions: Versailles, France, 2007; ISBN 978-2-7592-0020-7. [Google Scholar]
- Revilla, I.; Lobos-Ortega, I.; Vivar-Quintana, A.; González-Martín, M.I.; Hernández-Hierro, J.M.; González-Pérez, C. Variations in the Contents of Vitamins A and E during the Ripening of Cheeses with Different Compositions. Czech J. Food Sci. 2014, 32, 342–347. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk Nutritional Composition and Its Role in Human Health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Fletouris, D.J.; Botsoglou, N.A.; Psomas, I.E.; Mantis, A.I. Rapid Determination of Cholesterol in Milk and Milk Products by Direct Saponification and Capillary Gas Chromatography. J. Dairy Sci. 1998, 81, 2833–2840. [Google Scholar] [CrossRef]
- Marletta, L.; Camilli, E. Tabelle Di Composizione Degli Alimenti, Aggiornamento 2019. 2019. Available online: https://www.alimentinutrizione.it/Sezioni/Tabelle-Nutrizionali (accessed on 25 July 2023).
- Danieli, P.P.; Lopez, A.; Bellagamba, F.; Vetturini, T.; Bernabucci, U.; Ronchi, B.; Moretti, V.M.; Basiricò, L. Effects of Season and Management on Fatty Acid Profile, ACE-Inhibitory Activity and Anti-Oxidant Properties of Italian Alpine Cheeses. Ital. J. Anim. Sci. 2022, 21, 1021–1033. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A Battery of Translational Biomarkers for the Assessment of the in Vitro and in Vivo Antioxidant Action of Plant Polyphenolic Compounds: The Biomarker Issue. Curr. Opin. Toxicol. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The Effects of Dietary Consumption of Plants Secondary Compounds on Small Ruminants’ Products Quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Kuhnen, S.; Moacyr, J.R.; Mayer, J.K.; Navarro, B.B.; Trevisan, R.; Honorato, L.A.; Maraschin, M.; Pinheiro Machado Filho, L.C. Phenolic Content and Ferric Reducing-Antioxidant Power of Cow’s Milk Produced in Different Pasture-Based Production Systems in Southern Brazil: Phenolic Content and Ferric Reducing-Antioxidant Power of Cow’s Milk Produced in Southern Brazil. J. Sci. Food Agric. 2014, 94, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; Quaranta, E.; Fedele, V.; Rubino, R.; Pizza, C. Flavonoids and Terpenoids in Goats Milk in Relation to Forage Intake. Ital. J. Food Sci. 2006, 18, 85–92. [Google Scholar]
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdagué, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-Absorbing Compounds in Milk Are Related to Forage Polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla Forage (Sulla coronarium L.) on the Oxidative Status and Milk Polyphenol Content in Goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef]
- Cabiddu, A.; Molle, G.; Decandia, M. Influenza Del Sistema Di Allevamento Caprino Sulla Composizione Chimica Del Latte Con Particolare Riferimento Alla Composizione Acidica, Vitaminica e Fenolica. In Proceedings of the 5 Congresso Lattiero-Caseario AITeL Latte e Derivati: Ricerca, Innovazione e Valorizzazione, Bari, Italy, 9 September 2016. [Google Scholar]
- Chávez-Servín, J.L.; Andrade-Montemayor, H.M.; Velázquez Vázquez, C.; Aguilera Barreyro, A.; García-Gasca, T.; Ferríz Martínez, R.A.; Olvera Ramírez, A.M.; De La Torre-Carbot, K. Effects of Feeding System, Heat Treatment and Season on Phenolic Compounds and Antioxidant Capacity in Goat Milk, Whey and Cheese. Small Rumin. Res. 2018, 160, 54–58. [Google Scholar] [CrossRef]
- Hilario, M.C.; Puga, C.D.; Ocaña, A.N.; Romo, F.P.-G. Antioxidant Activity, Bioactive Polyphenols in Mexican Goats’ Milk Cheeses on Summer Grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Trabalza-Marinucci, M.; Codini, M.; Orru, M.; Ortenzi, R.; Forte, C.; Ceccarini, M.R.; Valiani, A. Evaluation of the Antioxidant Properties and Oxidative Stability of Pecorino Cheese Made from the Raw Milk of Ewes Fed Rosmarinus Officinalis L. Leaves. Int. J. Food Sci. Technol. 2015, 50, 558–565. [Google Scholar] [CrossRef]
- Jeangros, B.; Scehovic, J.; Schubiger, F.X.; Lehmann, J.; Daccord, R.; Arrigo, Y. Valeur Nutritive Des Plantes Des Prairies 4. Composés Phénoliques. Rev. Suisse Agric. 2001, 33, 147–151. [Google Scholar]
- Piluzza, G.; Bullitta, S. The Dynamics of Phenolic Concentration in Some Pasture Species and Implications for Animal Husbandry. J. Sci. Food Agric. 2010, 90, 1452–1459. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Olagaray, K.E.; Bradford, B.J. Plant Flavonoids to Improve Productivity of Ruminants—A Review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Fraisse, D.; Carnat, A.; Viala, D.; Pradel, P.; Besle, J.-M.; Coulon, J.-B.; Felgines, C.; Lamaison, J.-L. Polyphenolic Composition of a Permanent Pasture: Variations Related to the Period of Harvesting. J. Sci. Food Agric. 2007, 87, 2427–2435. [Google Scholar] [CrossRef]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant Activity of Cheddar Cheeses at Different Stages of Ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant Properties of Milk and Dairy Products: A Comprehensive Review of the Current Knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. In ACS Symposium Series; Andreescu, S., Hepel, M., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 1–37. ISBN 978-0-8412-2683-8. [Google Scholar]
- Elsayed Azab, A.; Adwas, A.A.; Ibrahim Elsayed, A.S.; Adwas, A.A.; Ibrahim Elsayed, A.S.; Quwaydir, F.A. Oxidative Stress and Antioxidant Mechanisms in Human Body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Lucas, A.; Agabriel, C.; Martin, B.; Ferlay, A.; Verdier-Metz, I.; Coulon, J.-B.; Rock, E. Relationships between the Conditions of Cow’s Milk Production and the Contents of Components of Nutritional Interest in Raw Milk Farmhouse Cheese. Le Lait 2006, 86, 177–202. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Gambacorta, E.; Simonetti, A. The Influence of Casein Haplotype on Quality, Coagulation, and Yield Traits of Milk from Italian Holstein Cows. J. Dairy Sci. 2016, 99, 3288–3294. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. In Vitro and in Vivo Antioxidant Potential of Milks, Yoghurts, Fermented Milks and Cheeses: A Narrative Review of Evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Santos, S.; Royo, L.J.; Martínez-Fernández, A.; Chocarro, C.; Vicente, F. The Mode of Grass Supply to Dairy Cows Impacts on Fatty Acid and Antioxidant Profile of Milk. Foods 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements Used Worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Přikryl, J.; Hájek, T.; Švecová, B.; Salek, R.N.; Černíková, M.; Červenka, L.; Buňka, F. Antioxidant Properties and Textural Characteristics of Processed Cheese Spreads Enriched with Rutin or Quercetin: The Effect of Processing Conditions. LWT 2018, 87, 266–271. [Google Scholar] [CrossRef]
- Puchau, B.; Zulet, M.Á.; De Echávarri, A.G.; Hermsdorff, H.H.M.; Martínez, J.A. Dietary Total Antioxidant Capacity: A Novel Indicator of Diet Quality in Healthy Young Adults. J. Am. Coll. Nutr. 2009, 28, 648–656. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of Cheese Ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Upadhyay, R.; Sehwag, S.; Mishra, H.N. Electronic Nose Guided Determination of Frying Disposal Time of Sunflower Oil Using Fuzzy Logic Analysis. Food Chem. 2017, 221, 379–385. [Google Scholar] [CrossRef]
- Álvarez, S.; Fresno, M. Effect of the Ripening Period and Intravarietal Comparison on Chemical, Textural and Sensorial Characteristics of Palmero (PDO) Goat Cheese. Animals 2020, 11, 58. [Google Scholar] [CrossRef]
- Pillonel, L.; Badertscher, R.; Bütikofer, U.; Casey, M.; Dalla Torre, M.; Lavanchy, P.; Meyer, J.; Tabacchi, R.; Bosset, J. Analytical Methods for the Determination of the Geographic Origin of Emmentaler Cheese. Main Framework of the Project; Chemical, Biochemical, Microbiological, Colour and Sensory Analyses. Eur. Food Res. Technol. 2002, 215, 260–267. [Google Scholar] [CrossRef]
- Paulson, B.M.; Mcmahon, D.J.; Oberg, C.J. Influence of Sodium Chloride on Appearance, Functionality, and Protein Arrangements in Nonfat Mozzarella Cheese. J. Dairy Sci. 1998, 81, 2053–2064. [Google Scholar] [CrossRef]
- Pinto, M.S.; De Carvalho, A.F.; Pires, A.C.D.S.; Campos Souza, A.A.; Fonseca Da Silva, P.H.; Sobral, D.; De Paula, J.C.J.; De Lima Santos, A. The Effects of Nisin on Staphylococcus Aureus Count and the Physicochemical Properties of Traditional Minas Serro Cheese. Int. Dairy J. 2011, 21, 90–96. [Google Scholar] [CrossRef]
- Pinho, O.; Mendes, E.; Alves, M.M.; Ferreira, I.M.P.L.V.O. Chemical, Physical, and Sensorial Characteristics of “Terrincho” Ewe Cheese: Changes During Ripening and Intravarietal Comparison. J. Dairy Sci. 2004, 87, 249–257. [Google Scholar] [CrossRef]
- Bárcenas, P.; Elortondo, F.J.P.; Salmerón, J.; Albisu, M. Sensory Profile of Ewe’s Milk Cheeses. Food Sci. Technol. Int. 2001, 7, 347–353. [Google Scholar] [CrossRef]
- Carvalho, G.P.; Santos, R.; Fino, A.; Ferreira, P.; Rodrigues, F.M.; Dias, J. Evolution during Three Ripening Stages of Évora Cheese. Foods 2020, 9, 1140. [Google Scholar] [CrossRef]
- Braghieri, A.; Piazzolla, N.; Romaniello, A.; Paladino, F.; Ricciardi, A.; Napolitano, F. Effect of Adjuncts on Sensory Properties and Consumer Liking of Scamorza Cheese. J. Dairy Sci. 2015, 98, 1479–1491. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese Ripening: A Review on Modern Technologies towards Flavor Enhancement, Process Acceleration and Improved Quality Assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Puleo, S.; Braghieri, A.; Condelli, N.; Piasentier, E.; Di Monaco, R.; Favotto, S.; Masi, P.; Napolitano, F. Pungency Perception and Liking for Pasta Filata Cheeses in Consumers from Different Italian Regions. Food Res. Int. 2020, 138, 109813. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, G.; Sanjuán, A.I. Territorial Food Product Perceptions in Greece and Spain: A Logit Modelling Approach. J. Food Prod. Mark. 2006, 11, 41–62. [Google Scholar] [CrossRef]
- Holt, D.B. Why Do Brands Cause Trouble? A Dialectical Theory of Consumer Culture and Branding. J. Consum. Res. 2002, 29, 70–90. [Google Scholar] [CrossRef]
- Russo, V.; Bilucaglia, M.; Circi, R.; Bellati, M.; Valesi, R.; Laureanti, R.; Licitra, G.; Zito, M. The Role of the Emotional Sequence in the Communication of the Territorial Cheeses: A Neuromarketing Approach. Foods 2022, 11, 2349. [Google Scholar] [CrossRef]
- Russo, V.; Zito, M.; Bilucaglia, M.; Circi, R.; Bellati, M.; Marin, L.E.M.; Catania, E.; Licitra, G. Dairy Products with Certification Marks: The Role of Territoriality and Safety Perception on Intention to Buy. Foods 2021, 10, 2352. [Google Scholar] [CrossRef] [PubMed]
- van Ittersum, K.; Meulenberg, M.T.G.; Van Trijp, H.; Candel, M.J.J.M. Consumers’ Appreciation of Regional Certification Labels: A Pan-European Study. J. Agric. Econ. 2007, 58, 1–23. [Google Scholar] [CrossRef]







| Parameter | Description |
|---|---|
| Shape | Pear shape with a slightly elongated head |
| Weight | Weight ranging from 2 to 3 kg |
| Diameter | The largest diameter of this cheese is between 20 and 22 cm |
| Height | Height, including the head, between 25 and 30 cm |
| Surface | Characterised by a thin and smooth crust in less seasoned products, with cracks that increase with seasoning |
| Colour | Straw yellow in less ripened cheese, darker yellow colour in more ripened cheese |
| Texture | Homogeneous and soft texture with rare holes in young products, firmer and grainy structure in more ripened ones |
| Taste | Sweet, oily, salty, slightly spicy taste in less seasoned cheese, various levels of spiciness in more ripened cheese |
| Smell | Fresh grass, berries, fruity, floral, wet straw, hay; seasoned cheese is very aromatic |
| Ripening Time | Moisture | Protein | Fat | Lactose | Ash | NaCl |
|---|---|---|---|---|---|---|
| 6 months | 33 ± 1 | 31 ± 1 | 30 ± 1 | 0 | 5.9 ± 0.3 | 2.3 ± 0.2 |
| 12 months | 29 ± 1 | 32 ± 1 | 31.7 ± 1.5 | 0 | 6.6 ± 0.3 | 2.6 ± 0.2 |
| Significance | ns | ns | ns | ns | ns |
| Fatty Acids | Ripening Time | Significance | |
|---|---|---|---|
| 6 Months | 12 Months | ||
| C4:0 | 1.02 ± 0.04 | 1.1 ± 0.1 | ns |
| C6:0 | 0.64 ± 0.04 | 0.69 ± 0.05 | ns |
| C7:0 | 0.004 ± 0.0001 | 0.005 ± 0.0001 | ns |
| C8:0 | 0.32 ± 0.02 | 0.37 ± 0.03 | ns |
| C10:0 | 0.66 ± 0.05 | 0.8 ± 0.1 | ns |
| C11:0 | 0.08 ± 0.01 | 0.09 ± 0.01 | ns |
| C12:0 | 0.7 ± 0.1 | 0.9 ± 0.1 | ns |
| iso C13:0 | 0.016 ± 0.001 | 0.017 ± 0.002 | ns |
| anteiso C13:0 | 0.021 ± 0.002 | 0.021 ± 0.002 | ns |
| iso C14:0 | 0.07 ± 0.01 | 0.06 ± 0.01 | ns |
| C14:0 | 2.7 ± 0.1 | 3.2 ± 0.2 | ns |
| iso C15:0 | 0.018 ± 0.0005 | 0.019 ± 0.002 | ns |
| anteiso C15:0 | 0.12 ± 0.02 | 0.13 ± 0.01 | ns |
| C14:1c9 | 0.22 ± 0.02 | 0.21 ± 0.03 | ns |
| C15:0 | 0.39 ± 0.05 | 0.45 ± 0.03 | ns |
| iso C16:0 | 0.12 ± 0.01 | 0.12 ± 0.01 | ns |
| C16:0 | 7.8 ± 0.4 | 8.6 ± 1 | ns |
| iso C17:0 | 0.13 ± 0.01 | 0.15 ± 0.02 | ns |
| C16:1 trans9 | 0.028 ± 0.003 | 0.04 ± 0.01 | ns |
| C16:1 cis7 | 0.07 ± 0.01 | 0.068 ± 0.005 | ns |
| anteiso C17:0 | 0.17 ± 0.02 | 0.19 ± 0.02 | ns |
| C16:1 cis9 | 0.38 ± 0.03 | 0.34 ± 0.03 | ns |
| C17:0 | 0.24 ± 0.03 | 0.27 ± 0.02 | ns |
| iso C18:0 | 0.021 ± 0.003 | 0.021 ± 0.003 | ns |
| C17:1 cis10 | 0.08 ± 0.01 | 0.067 ± 0.003 | ns |
| C18:0 | 3.4 ± 0.1 | 3.8 ± 0.5 | ns |
| C18:1 trans4 | 0.004 ± 0.001 | 0.004 ± 0.0005 | ns |
| C18:1 trans5 | 0.004 ± 0.001 | 0.003 ± 0.001 | ns |
| C18:1 trans6 + C18:1 trans8 | 0.065 ± 0.005 | 0.07 ± 0.01 | ns |
| C18:1 trans9 | 0.072 ± 0.005 | 0.07 ± 0.01 | ns |
| C18:1 trans10 | 0.08 ± 0.02 | 0.08 ± 0.01 | ns |
| C18:1 trans11 (VA) | 0.6 ± 0.1 | 0.9 ± 0.3 | ns |
| C18:1 trans12 | 0.09 ± 0.01 | 0.08 ± 0.01 | ns |
| C18:1 trans13 + C18:1 trans14 | 0.18 ± 0.01 | 0.21 ± 0.03 | ns |
| C18:1 cis9 | 5.8 ± 0.2 | 5.2 ± 0.2 | ns |
| C18:1 trans15 + C18:1 cis10 | 0.3 ± 0.1 | 0.16 ± 0.05 | ns |
| C18:1 cis11 | 0.16 ± 0.01 | 0.14 ± 0.01 | ns |
| C18:1 cis12 | 0.05 ± 0.13 | 0.04 ± 0.01 | ns |
| C18:1 cis13 | 0.022 ± 0.001 | 0.020 ± 0.001 | ns |
| C18:1 trans16 + C18:1 c14 | 0.101 ± 0.005 | 0.12 ± 0.02 | ns |
| C18:2 trans9 trans12 | 0.014 ± 0.004 | 0.02 ± 0.01 | ns |
| C18:2 cis9 trans13 | 0.050 ± 0.003 | 0.05 ± 0.01 | ns |
| C18:2 cis9 trans12 + C18:2 trans8 cis12 | 0.021 ± 0.001 | 0.022 ± 0.003 | ns |
| C18:1 cis16 | 0.023 ± 0.001 | 0.024 ± 0.003 | ns |
| C18:2 trans9 cis12 | 0.009 ± 0.001 | 0.014 ± 0.003 | ns |
| C18:2 trans11 cis15 | 0.09 ± 0.03 | 0.12 ± 0.04 | ns |
| C18:2 cis9 cis12 (LA) n6 | 0.5 ± 0.1 | 0.4 ± 0.1 | ns |
| C18:2 cis9 cis15 n3 | 0.006 ± 0.001 | 0.006 ± 0.001 | ns |
| C20:0 | 0.0721 ± 0.01 | 0.08 ± 0.01 | ns |
| C18:3 cis6 cis9 cis12 n6 | 0.008 ± 0.001 | 0.006 ± 0.001 | ns |
| C20:1 cis9 | 0.003 ± 0.001 | 0.004 ± 0.001 | ns |
| C20:1 cis11 | 0.016 ± 0.002 | 0.013 ± 0.002 | ns |
| C18:3 cis9 cis12 cis15 n3 | 0.20 ± 0.03 | 0.28 ± 0.04 | ns |
| C18:2 cis9 trans11 (CLA) (RU) | 0.30 ± 0.04 | 0.4 ± 0.1 | ns |
| C18:2 trans9 cis11 (CLA) | 0.022 ± 0.003 | 0.029 ± 0.005 | ns |
| C18:2 trans10 cis12 + C21:0 (CLA) | 0.003 ± 0.001 | 0.003 ± 0.001 | ns |
| C18:2 cis9 cis11 (CLA) | 0.015 ± 0.004 | 0.02 ± 0.01 | ns |
| C18:2 trans12 trans14 + C18:2 cis11 cis13 (CLA) | 0.002 ± 0.001 | 0.006 ± 0.003 | ns |
| C18:2 trans11 trans13 (CLA) | 0.006 ± 0.001 | 0.009 ± 0.002 | ns |
| C18:2 trans9 trans11 (CLA) | 0.012 ± 0.001 | 0.008 ± 0.001 | ns |
| C20:2 cis11 cis14 n6 | 0.007 ± 0.001 | 0.007 ± 0.001 | ns |
| C20:3 cis5 cis8 cis11 | 0.013 ± 0.003 | 0.02 ± 0.01 | ns |
| C22:0 | 0.032 ± 0.005 | 0.04 ± 0.01 | ns |
| C20:4 cis5 cis8 cis11 cis14 n6 | 0.038 ± 0.003 | 0.032 ± 0.005 | ns |
| C23:0 | 0.015 ± 0.002 | 0.020 ± 0.004 | ns |
| C24:0 | 0.020 ± 0.003 | 0.025 ± 0.004 | ns |
| C20:5 c5c8c11c14c17 n3 (EPA) | 0.016 ± 0.003 | 0.023 ± 0.002 | ns |
| C24:1c15 | 0.004 ± 0.001 | 0.005 ± 0.001 | ns |
| C26:0 | 0.011 ± 0.002 | 0.014 ± 0.003 | ns |
| C22:5 cis7 cis10 cis13 cis16 cis19 n3 (DPA) | 0.031 ± 0.004 | 0.044 ± 0.004 | ns |
| C22:6 cis4 cis7 cis10 cis13 cis16 cis19 n3 (DHA) | 0.003 ± 0.001 | 0.005 ± 0.001 | ns |
| BCFAs | 0.68 ± 0.03 | 0.74 ± 0.04 | ns |
| OCFAs | 0.72 ± 0.03 | 0.82 ± 0.04 | ns |
| ΣOBCFAs | 1.4 ± 0.1 | 1.6 ± 0.1 | ns |
| SCFAs (C4-C8) | 1.98 ± 0.05 | 2.2 ± 0.1 | ns |
| MCFAs (C10-C16) | 13.5 ± 0.4 | 14.7 ± 0.5 | ns |
| LCFAs (C17-C26) | 13.1 ± 0.3 | 13.3 ± 0.4 | ns |
| SFAs | 19 ± 1 | 21 ± 1 | ns |
| MUFAs | 8.4 ± 0.2 | 7.8 ± 0.4 | ns |
| PUFAs | 1.40 ± 0.05 | 1.5 ± 0.2 | ns |
| Σn6 | 0.6 ± 0.1 | 0.5 ± 0.1 | ns |
| Σn3 | 0.25 ± 0.04 | 0.36 ± 0.05 | ns |
| PUFA-n6/n3 | 2.8 ± 0.9 | 1.4 ± 0.3 | ns |
| Ripening Time | Total Retinol | α-Tocopherol | Cholesterol |
|---|---|---|---|
| 6 months | 2.0 ± 0.1 | 9 ± 1 | 872 $ ± 55 |
| 12 months | 1.9 ± 0.2 | 7 ± 2 | 1089 & ± 83 |
| Significance | ns | ns | 0.057 |
| Ripening Time | TPC (g GAE/kg of Cheese) | CAT | |
|---|---|---|---|
| FRAP | TEAC | ||
| (mmol FeSO4/kg of Cheese) | (mmol TROLOX/kg of Cheese) | ||
| 6 months | 4.1 ± 0.2 | 1.4 ± 0.1 | 67 ± 3 |
| 12 months | 5 ± 1 | 1.4 ± 0.2 | 69 ± 9 |
| Significance | ns | ns | ns |
| Index | Ripening Time | Significance | ||
|---|---|---|---|---|
| 6 Months | 12 Months | |||
| HPI 1 | Health-Promoting Index | 0.51 ± 0.03 | 0.44 ± 0.05 | ns |
| GHIC-7 2 | General Health Index of Cheese-7 | 32 ± 3 | 39 ± 4 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Trana, A.; Sabia, E.; Di Rosa, A.R.; Addis, M.; Bellati, M.; Russo, V.; Dedola, A.S.; Chiofalo, V.; Claps, S.; Di Gregorio, P.; et al. Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication. Foods 2023, 12, 4339. https://doi.org/10.3390/foods12234339
Di Trana A, Sabia E, Di Rosa AR, Addis M, Bellati M, Russo V, Dedola AS, Chiofalo V, Claps S, Di Gregorio P, et al. Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication. Foods. 2023; 12(23):4339. https://doi.org/10.3390/foods12234339
Chicago/Turabian StyleDi Trana, Adriana, Emilio Sabia, Ambra Rita Di Rosa, Margherita Addis, Mara Bellati, Vincenzo Russo, Alessio Silvio Dedola, Vincenzo Chiofalo, Salvatore Claps, Paola Di Gregorio, and et al. 2023. "Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication" Foods 12, no. 23: 4339. https://doi.org/10.3390/foods12234339
APA StyleDi Trana, A., Sabia, E., Di Rosa, A. R., Addis, M., Bellati, M., Russo, V., Dedola, A. S., Chiofalo, V., Claps, S., Di Gregorio, P., & Braghieri, A. (2023). Caciocavallo Podolico Cheese, a Traditional Agri-Food Product of the Region of Basilicata, Italy: Comparison of the Cheese’s Nutritional, Health and Organoleptic Properties at 6 and 12 Months of Ripening, and Its Digital Communication. Foods, 12(23), 4339. https://doi.org/10.3390/foods12234339