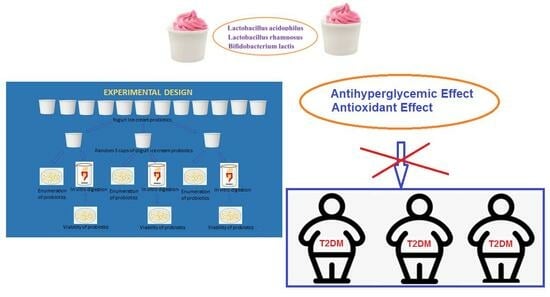
Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yogurt Ice Cream Preparation
2.3. Nutritional Information for the Yogurt Ice Cream
2.4. In Vitro Digestion
2.5. Preparation of Yogurt Ice Cream Probiotic Water Extracts (YIEs)
2.6. Microbiological Analysis
2.7. α-Amylase Inhibition Assay
2.8. α-Glucosidase Inhibition Assay
2.9. Antioxidant Activity by DPPH Inhibition Assay
2.10. FRAP Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Information for the Yogurt Ice Cream
3.2. Viability of Probiotics in Yogurt Ice Cream before and after In Vitro Digestion
3.3. Inhibition of α-Amylase and α-Glucosidase Activities by Probiotic Yogurt Ice Cream
3.4. Antioxidant Activity of Probiotics in Yogurt Ice Cream
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probiotics, Prebiotics, and Synbiotics Added to Dairy Products: Uses and applications to manage type 2 diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Mortazavian, A.M.; Khosrokhavar, R.; Gomes da Cruz, A. Probiotic Ice Cream: Viability of Probiotic Bacteria and Sensory Properties. Ann. Microbiol. 2011, 61, 411–424. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, B.; Hyun, C.K. Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance through Alleviating ER Stress and Suppressing Macrophage Activation in db/db Mice. J. Clin. Biochem. Nutr. 2015, 56, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.Y.; Utra, U.; Ahmad, R.; Rather, I.A.; Park, Y.H. Evaluation of Probiotic Potential and Anti-hyperglycemic Properties of A Novel Lactobacillus Strain Isolated from Water Kefir Grains. Food Sci. Biotechnol. 2018, 27, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Guilhem, P.; Gilbert, R.G.; Kuchel, P.W. Digestion of Starch: In Vivo and In Vitro Kinetic Models Used to Characterise Oligosaccharide or Glucose Release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Mársico, E.T.; Campelo, P.H.; Pimentel, T.C.; Cristina Silva, M.; et al. Probiotic Fermented Whey-Milk Beverages: Effect of Different Probiotic Strains on the Physicochemical Characteristics, Biological Activity, and Bioactive Peptides. Food Res. Int. 2023, 164, 112396. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Masoodi, F. Himalayan Cheese (Kalari/Kradi) Fermented with Different Probiotic Strains: In vitro Investigation of Nutraceutical Properties. LWT 2019, 104, 53–60. [Google Scholar] [CrossRef]
- Rajendiran, D.; Packirisamy, S.; Gunasekaran, K. A Review on Role of Antioxidants in Diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 48–53. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani Abdollahi, M. A Review on the Role of Antioxidants in the Management of Diabetes and Its Complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. Antioxidant Properties of Probiotic Fermented Milk Supplemented with Chestnut Flour (Castanea sativa Mill). Food Process. Preserv. 2017, 41, e13156. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Díaz-López, A.; Babio, N. Yogurt and Diabetes: Overview of Recent Observational Studies. J. Nutr. 2017, 147, 1452S–1461S. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Saeting, O.; Chandarajoti, K.; Phongphisutthinan, A.; Hongsprabhas, P.; Sae-Tan, S. Water Extract of Mungbean (Vigna radiata L.) Inhibits Protein Tyrosine Phosphatase-1B in Insulin-Resistant HepG2 Cells. Molecules 2021, 26, 1452. [Google Scholar] [CrossRef]
- Shori, A.; Baba, A. Comparative Antioxidant Activity, Proteolysis and In Vitro α-amylase and α-Glucosidase Inhibition of Allium sativum-Yogurts Made from Cow and Camel Milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J.S. Development of a Novel Probiotic Milk Product with Enhanced Antioxidant Properties Using Mango Peel as a Fermentation Substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Shi, B.M. Milk in The Prevention and Management of Type 2 Diabetes: The Potential Role of Milk Proteins. Diabetes Metab. Res. Rev. 2019, 35, e3187. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of Dairy Foods and Diabetes Incidence: A Dose-Response Meta-Analysis of Observational Studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Znamirowska-Piotrowska, A.; Buniowska-Olejnik, M.; Pawlos, M. Sheep Milk Symbiotic Ice Cream: Effect of Inulin and Apple Fiber on the Survival of Five Probiotic Bacterial Strains during Simulated In Vitro Digestion Conditions. Nutrients 2022, 14, 4454. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Aslan Azizi, A.; Javadi, M.; Solmaz Mahdipour, S.; Hanie Ejtahed, H. Factors Influencing Probiotic Survival in Ice Cream: A Review. Int. J. Dairy Sci. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Magariños, H.; Selaive, S.; Costa, M.; Flores, M.; Pizarro, O. Viability of Probiotic Microorganisms (Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis Bb-12) in Ice Cream. Int. J. Dairy. Technol. 2007, 60, 128–134. [Google Scholar] [CrossRef]
- Rosak, C.; Mertes, G. Critical Evaluation of The Role of Acarbose in the Treatment of Diabetes: Patient Considerations. Diabetes Metab. Syndr. Obes. 2012, 5, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Wihansaha, R.R.S.; Ariefb, I.I.; Batubarac, I. Anti-diabetic Potency and Characteristics of Probiotic Goat-Milk Yogurt Supplemented with Roselle Extract during Cold Storage. Trop. Anim. Sci. J. 2018, 41, 191–199. [Google Scholar] [CrossRef]
- da Cruz, A.G.; Alonso Buriti, A.C.; de Souza, C.H.B.; Fonseca Faria, J.A.; Isay Saad, S.M. Probiotic Cheese: Health Benefits, Technological and Stability Aspects. Trends Food Sci. Technol. 2009, 20, 344–354. [Google Scholar] [CrossRef]
- Sekar Sudharhsan, S.; Sivaprakasam Senthilkumar, S.; Ranjith, K. Physical and Nutritional Factors Affecting the Production of Amylase from Species of Bacillus Isolated from Spoiled Food Waste. Afr. J. Biotechnol. 2007, 6, 430–435. [Google Scholar]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-Derived Non-Phenolic α-Amylase and α-Glucosidase Inhibitors for Controlling Starch Digestion Rate and Guiding Diabetes-Friendly Recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Screening for Potential Novel Probiotics With Dipeptidyl Peptidase IV-Inhibiting Activity for Type 2 Diabetes Attenuation in vitro and in vivo. Front. Microbiol. 2020, 10, 2855. [Google Scholar] [CrossRef] [PubMed]
- Zahrani, A.J.A.; Shori, A.B. Viability of Probiotics and Antioxidant Activity of Soy and Almond Milk Fermented with Selected Strains of Probiotic Lactobacillus spp. LWT 2023, 176, 114531. [Google Scholar] [CrossRef]
- Abubakr, M.A.S.; Hassan, Z.; Imdakim, M.M.A.; Sharifah, N.R.S.A. Antioxidant Activity of Lactic Acid Bacteria (LAB) Fermented Skim Milk as Determined by 1, 1-Diphenyl-2-Picrylhydrazyl (DPPH) and Ferrous Chelating Activity (FCA). Afr. J. Microbiol. Res. 2012, 6, 6358–6364. [Google Scholar]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and Its Functionally Valuable Products—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Najgebauer-Lejko, D. Effect of Green Tea Supplementation on the Microbiological, Antioxidant, and Sensory Properties of Probiotic Milks. Dairy. Sci. Technol. 2014, 94, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Bule, M.; Ullah, R.; Nadeem, M.; Asif, S.; Niaz, K. The Antioxidant Components of Milk and Their Role in Processing, Ripening, and Storage: Functional Food. Vet. World 2019, 12, 12–33. [Google Scholar] [CrossRef]
- Akan, E. The Effect of Fermentation Time and Yogurt Bacteria on the Physicochemical, Microbiological and Antioxidant Properties of Probiotic Goat Yogurts. An. Acad. Bras. Cienc. 2022, 94, e20210875. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In Vitro Digestion Models Suitable for Foods: Opportunities for New Fields of Application and Challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef]

| Yogurt Ice Cream | Viable Probiotic Bacterial Counts in Yogurt Ice Cream (log CFU/g of Ice Cream) | |||
|---|---|---|---|---|
| Simulated In Vitro Digestive Stage | ||||
| Before Digestion | Oral Phase | Gastric Phase | Intestinal Phase | |
| YI-Control | ND | ND | ND | ND |
| YI-LA-5 | 6.61 ± 0.02 Ab | 5.12 ± 0.02 Bb | 4.65 ± 0.09 Cb | 4.11 ± 0.04 Db |
| YI-BB-12 | 6.23 ± 0.03 Ac | 4.53 ± 0.57 Bb | 3.11 ± 0.12 Cc | ND |
| YI-LGG | 7.68 ± 0.04 Aa | 6.35 ± 0.09 Ba | 6.23 ± 0.08 Ba | 6.04 ± 0.03 Ca |
| Yogurt Ice Cream | α-Amylase Inhibition (%) | α-Glucosidase Inhibition (%) |
|---|---|---|
| YIE-LA-5 | 19.31 ± 0.30 b | 16.37 ± 0.32 a |
| YIE-BB-12 | 16.14 ± 0.70 c | 11.54 ± 0.21 c |
| YIE-LGG | 41.37 ± 0.61 a | 14.41 ± 0.26 b |
| Yogurt Ice Cream | DPPH (mg Trolox Equivalent/g of Sample) | FRAP (mg Trolox Equivalent/g of Sample) |
|---|---|---|
| YIE-CT | 21.40 ± 0.40 b | 51.47 ± 0.23 d |
| YIE-LA-5 | 21.40 ± 0.37 b | 97.63 ± 0.25 a |
| YIE-BB-12 | 23.95 ± 0.28 a | 67.31 ± 0.17 b |
| YIE-LGG | 23.50 ± 0.33 a | 54.55 ± 0.40 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talearngkul, R.; Sae-tan, S.; Sirivarasai, J. Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after In Vitro Digestion. Foods 2023, 12, 4373. https://doi.org/10.3390/foods12234373
Talearngkul R, Sae-tan S, Sirivarasai J. Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after In Vitro Digestion. Foods. 2023; 12(23):4373. https://doi.org/10.3390/foods12234373
Chicago/Turabian StyleTalearngkul, Rinrada, Sudathip Sae-tan, and Jintana Sirivarasai. 2023. "Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after In Vitro Digestion" Foods 12, no. 23: 4373. https://doi.org/10.3390/foods12234373
APA StyleTalearngkul, R., Sae-tan, S., & Sirivarasai, J. (2023). Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after In Vitro Digestion. Foods, 12(23), 4373. https://doi.org/10.3390/foods12234373