Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source
Abstract
:1. Introduction
2. Properties of Fungal Proteins
2.1. Varieties of Fungus
2.2. The Composition of Fungal Proteins
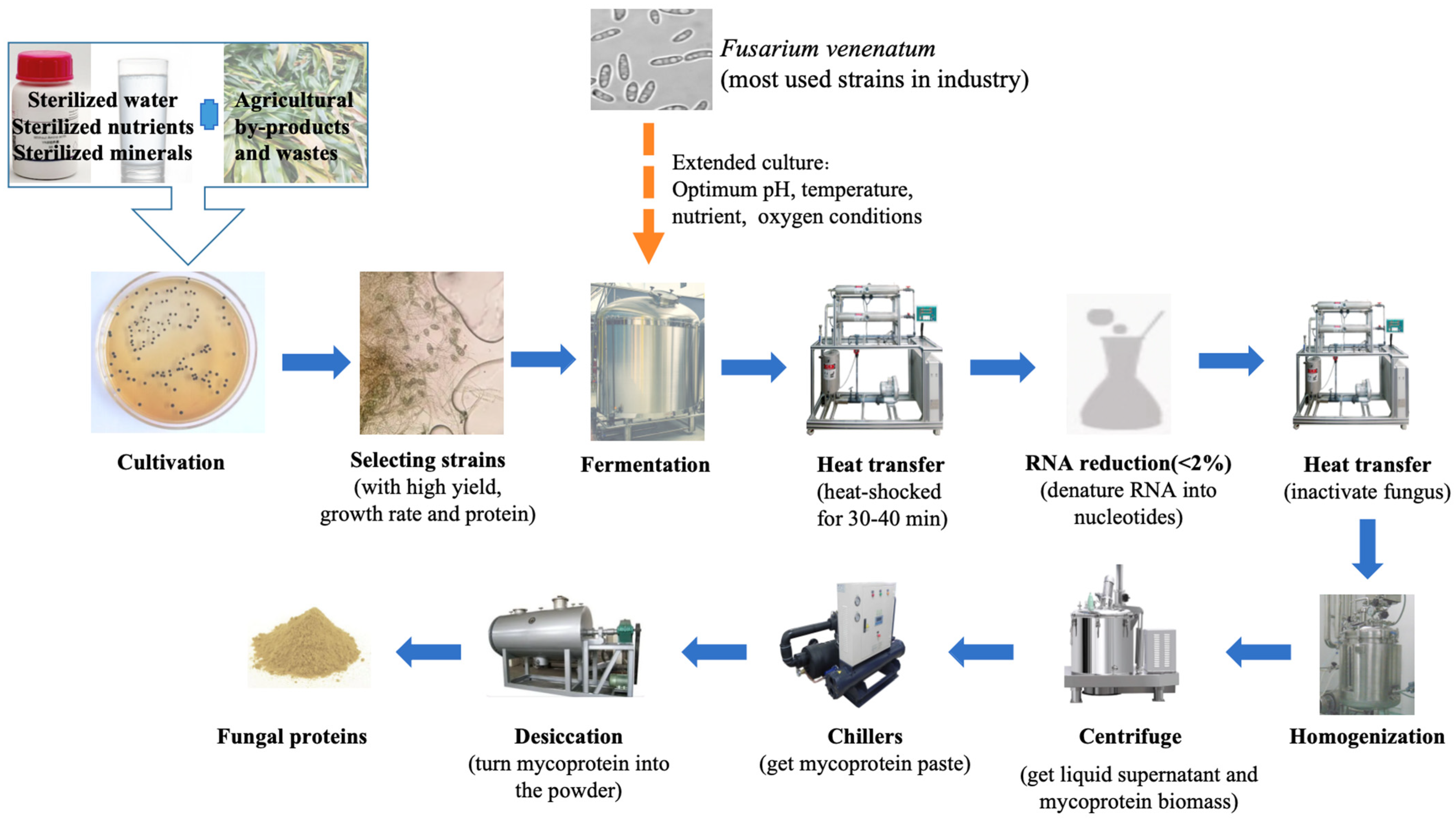
2.3. Extraction of Fungal Proteins
3. Nutritional Value of Fungus Proteins
3.1. Flavor Quality of Fungus Proteins
3.1.1. Amino Acid Content and Composition
3.1.2. Flavor Characteristics of Fungal Proteins
3.2. Nutritional Values of Fungus Proteins
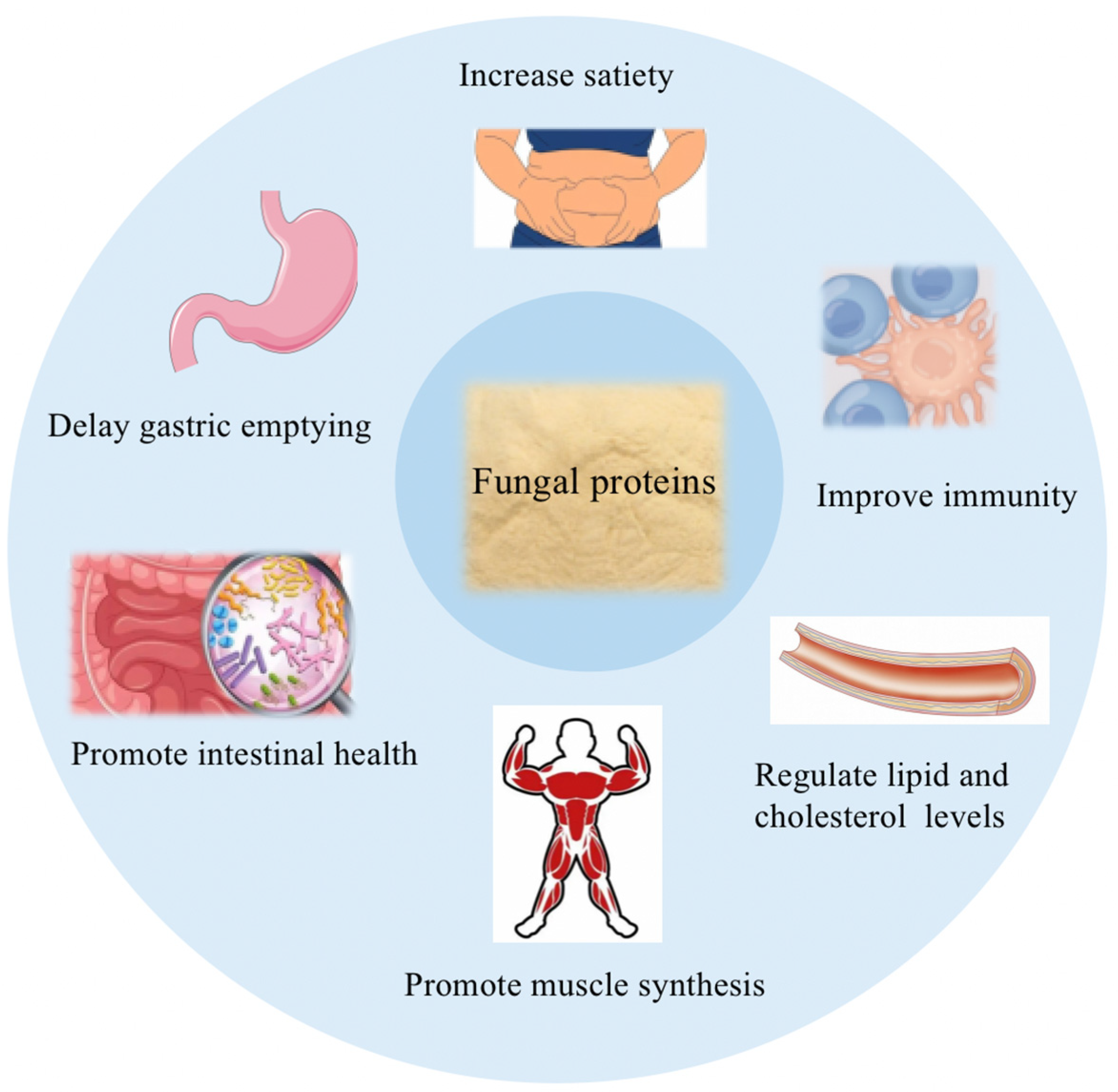
4. Bio-Functional Properties of Fungus Proteins
4.1. Digestive Properties of Fungus Proteins
4.2. Fungal Proteins Regulate Lipid and Cholesterol Levels
4.3. Fungal Proteins Improve Immunity
4.4. Fungal Proteins Promote Gut Health
5. Application of Fungal Proteins
5.1. Application of Fungal Proteins in Foods
5.2. Application of Fungal Proteins in Space Agriculture
6. Challenges and Future Trends
7. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- WS/T 578.1; 2017 Chinese Dietary Reference Intakes—Part 1: Macronutrient. National Health Commission of the People’s Republic of China: Beijing. China, 2018.
- Rizzoli, R.; Stevenson, J.C.; Bauer, J.M.; van Loon, L.J.; Walrand, S.; Kanis, J.A.; Cooper, C.; Brandi, M.L.; Diez-Perez, A.; Reginster, J.Y.; et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014, 79, 122–132. [Google Scholar] [CrossRef]
- Upcraft, T.; Tu, W.-C.; Johnson, R.; Finnigan, T.; Van Hung, N.; Hallett, J.; Guo, M. Protein from renewable resources: Mycoprotein production from agricultural residues. Green Chem. 2021, 23, 5150–5165. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S.F.S. Mycoproteins as safe meat substitutes. J. Clean. Prod. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Raghukumar, S. Fungi: Characteristics and Classification; Springer: Cham, Switzerland, 2017; Chapter 1; pp. 1–13. [Google Scholar] [CrossRef]
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Hadar, Y.; Dosoretz, C.G. Mushroom mycelium as a potential source of food flavour. Trends Food Sci. Technol. 1991, 2, 214–218. [Google Scholar] [CrossRef]
- Ward, O.P. Production of recombinant proteins by filamentous fungi. Biotechnol. Adv. 2012, 30, 1119–1139. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous fungi for future functional food and feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Cook, D.J. Food, Fermentation, and Micro-Organisms: Second Edition; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef]
- Niego, A.G.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K.D.; et al. The contribution of fungi to the global economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit. Rev. Food Sci. Nutr. 2014, 54, 64–83. [Google Scholar] [CrossRef]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Orso, P.B.; Bordin, K.; Hara, R.V.; Luciano, F.B. Toxicological effects of fumonisin B1 in combination with other Fusarium toxins. Food Chem. Toxicol. 2018, 121, 483–494. [Google Scholar] [CrossRef]
- Humpenöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected environmental benefits of replacing beef with microbial protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef]
- Colosimo, R.; Warren, F.J.; Finnigan, T.J.A.; Wilde, P.J. Protein bioaccessibility from mycoprotein hyphal structure: In vitro investigation of underlying mechanisms. Food Chem. 2020, 330, 127252. [Google Scholar] [CrossRef]
- Derbyshire, E. Fungal-derived mycoprotein and health across the lifespan: A narrative review. J. Fungi 2022, 8, 653. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Ainsworth, G.C. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CABI Bioscience, CAB International: Wallingford, UK, 2008. [Google Scholar]
- Ingold, C.T.; Hudson, H.J. Zygomycotina and Mastigomycotina. In The Biology of Fungi; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Dyer, P.S.; Ingram, D.S.; Johnstone, K. The control of sexual morphogenesis in the Ascomycotina. Biol. Rev. 1992, 67, 421–458. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts, a Taxonomic Study, 5th ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2011. [Google Scholar]
- EFSA. Regulation (EC) No 1924/2006 of the european parliament and of the council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, L 404/409. [Google Scholar]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2011, 42, 577–587. [Google Scholar] [CrossRef]
- Derbyshire, E.; Finnigan, T.J.A. Chapter 16—Mycoprotein: A futuristic portrayal. In Future Foods Global Trends, Opportunities, and Sustainability Challenges; Elsevier Academic Press: Cambridge, MA, USA, 2022; pp. 287–303. [Google Scholar]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Prakash, P.; Namasivayam, S.K.R. Anti oxidative and anti tumour activity of biomass extract of mycoprotein fusarium venenatum. Afr. J. Microbiol. Res. 2013, 7, 1697–1702. [Google Scholar] [CrossRef]
- Bahmani, Z.; Mavanes, F. Enrichment of Silver Carp (Hypophthalmichthys molitrix) sausage with (Fusarium venenatum) mycoprotein. Util. Cultiv. Aquat. 2021, 10, 11–25. [Google Scholar] [CrossRef]
- Ward, P.N. A Process for the Reduction of Nucleic Acid Content for a Fungus Imperfectus. WO Patent 95/23843, 8 September 1995. [Google Scholar]
- Raats, J. Meat (Substitutes) Comparing Environmental Impacts. A Case Study Comparing Quorn and Pork; University of Groningen: Groningen, The Netherlands, 2007. [Google Scholar]
- Zeng, B.; Nilsson, K.; Teixeira, P.G.; Bergenståhl, B. Study of mycoprotein extraction methods and its functional properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130800. [Google Scholar] [CrossRef]
- Lonchamp, J.; Stewart, K.; Munialo, C.D.; Evans, L.; Akintoye, M.; Gordon, S.; Clegg, P.S.; Willoughby, N.; Euston, S.R. Mycoprotein as novel functional ingredient: Mapping of functionality, composition and structure throughout the Quorn fermentation process. Food Chem. 2022, 396, 133736. [Google Scholar] [CrossRef]
- Corey, M.E.; Kerr, W.L.; Mulligan, J.H.; Lavelli, V. Phytochemical stability in dried apple and green tea functional products as related to moisture properties. LWT Food Sci. Technol. 2011, 44, 67–74. [Google Scholar] [CrossRef]
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56. [Google Scholar] [CrossRef]
- Stoffel, F.; Santana, W.D.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of edible mycoprotein using agroindustrial wastes: Influence on nutritional, chemical and biological properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision fermentation to advance fungal food fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
- Friedman, M. Nutritional value of proteins from different food sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The future of nutritious nonmeat protein, a symposium review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for future foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Coelho, M.O.C.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.O.; Jackman, S.R.; Blackwell, J.R.; Finnigan, T.J.A.; Stephens, F.B.; Dirks, M.L.; et al. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 318–333. [Google Scholar] [CrossRef]
- Edwards, D.G.; Cummings, J.H. The protein quality of mycoprotein. Proc. Nutr. Soc. 2010, 69, E331. [Google Scholar] [CrossRef]
- Coelho, M.O.C.; Monteyne, A.J.; Dunlop, M.V.; Harris, H.C.; Morrison, D.J.; Stephens, F.B.; Wall, B.T. Mycoprotein as a possible alternative source of dietary protein to support muscle and metabolic health. Nutr. Rev. 2020, 78, 486–497. [Google Scholar] [CrossRef]
- Miller, S.A.; Dwyer, J.T. Evaluating the safety and nutritional value of mycoprotein. Food Technol. 2001, 55, 42–47. [Google Scholar]
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 305–325. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, D.; Liu, X.; Jing, W. Amino acid content in milk and its nutritional value. China Dairy 2002, 2, 24–25. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sugano, M. Health Functions of Egg Protein. Foods 2022, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pu, D.; Zhang, J.; Hao, Z.; Zhao, X.; Sun, B.; Zhang, Y. Identification of novel umami peptides in chicken breast soup through a sensory-guided approach and molecular docking to the T1R1/T1R3 taste receptor. J. Agric. Food Chem. 2023, 71, 7803–7811. [Google Scholar] [CrossRef] [PubMed]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.S.; Singh, A. Single Cell Protein|Mycelial Fungi. In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 415–424. [Google Scholar] [CrossRef]
- Finnigan, T.J.A. Mycoprotein: Origins, production and properties. In Handbook of Food Proteins; Woodhead Publishing: Sawston, UK, 2011; pp. 335–352. [Google Scholar] [CrossRef]
- Kim, K.; Choi, B.; Lee, I.; Lee, H.; Kwon, S.; Oh, K.; Kim, A.Y. Bioproduction of mushroom mycelium of Agaricus bisporus by commercial submerged fermentation for the production of meat analogue. J. Sci. Food Agric. 2011, 91, 1561–1568. [Google Scholar] [CrossRef]
- Al-Dalain, S.Y.A. Utilization of mushroom fungi in processing of meat sausage. Res. Crops 2018, 19, 294. [Google Scholar] [CrossRef]
- Patinho, I.; Selani, M.M.; Saldaña, E.; Bortoluzzi, A.C.T.; Rios-Mera, J.D.; da Silva, C.M.; Kushida, M.M.; Contreras-Castillo, C.J. Agaricus bisporus mushroom as partial fat replacer improves the sensory quality maintaining the instrumental characteristics of beef burger. Meat Sci. 2021, 172, 108307. [Google Scholar] [CrossRef]
- Bordoni, A.; Laghi, L.; Babini, E.; Di Nunzio, M.; Picone, G.; Ciampa, A.; Valli, V.; Danesi, F.; Capozzi, F. The foodomics approach for the evaluation of protein bioaccessibility in processed meat upon in vitro digestion. Electrophoresis 2014, 35, 1607–1614. [Google Scholar] [CrossRef]
- Rinaldi, L.; Rioux, L.-E.; Britten, M.; Turgeon, S.L. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucan. Int. Dairy J. 2015, 46, 39–45. [Google Scholar] [CrossRef]
- Magro, A.E.A.; Silva, L.C.; Rasera, G.B.; de Castro, R.J. Solid-state fermentation as an efficient strategy for the biotransformation of lentils: Enhancing their antioxidant and antidiabetic potentials. Bioresour. Bioprocess. 2019, 6, 38. [Google Scholar] [CrossRef]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Fungal Cell Factories for Efficient and Sustainable Production of Proteins and Peptides. Microorganisms 2022, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Ng, T. Fungal Peptides with Ribonuclease and Ribosome Inactivating Activities. In Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 162–165. [Google Scholar] [CrossRef]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.-P. Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: A peptide-rich product with low RNA content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001, 393, 271–280. [Google Scholar] [CrossRef]
- Kaur, L.; Mao, B.; Beniwal, A.S.; Abhilasha Kaur, R.; Chian, F.M.; Singh, J. Alternative proteins vs animal proteins: The influence of structure and processing on their gastro-small intestinal digestion. Trends Food Sci. Technol. 2022, 122, 275–286. [Google Scholar] [CrossRef]
- Moore, D.; Chiu, S.W. Fungal products as food. In Bio-Exploitation of Filamentous Fungi; Pointing, S.B., Hyde, K.D., Eds.; Fungal Diversity Press: Hong Kong, China, 2001; Chapter 10; pp. 223–251. [Google Scholar]
- Warrilow, A.; Mellor, D.; McKune, A.; Pumpa, K. Dietary fat, fibre, satiation, and satiety—A systematic review of acute studies. Eur. J. Clin. Nutr. 2019, 73, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.V.; Kilroe, S.P.; Bowtell, J.L.; Finnigan, T.J.A.; Salmon, D.L.; Wall, B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: A dose-response study. Br. J. Nutr. 2017, 118, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Bottin, J.H.; Swann, J.R.; Cropp, E.; Chambers, E.S.; Ford, H.E.; Ghatei, M.A.; Frost, G.S. Mycoprotein reduces energy intake and postprandial insulin release without altering glucagon-like peptide-1 and peptide tyrosine-tyrosine concentrations in healthy overweight and obese adults: A randomised-controlled trial. Br. J. Nutr. 2016, 116, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Monteyne, A.; Dirks, M.; Finnigan, T.; Stephens, F.; Wall, B. Daily mycoprotein consumption for 1 week does not affect insulin sensitivity or glycaemic control but modulates the plasma lipidome in healthy adults: A randomised controlled trial. Br. J. Nutr. 2021, 125, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, R.; Mulet-Cabero, A.I.; Cross, K.L.; Haider, K.; Edwards, C.H.; Warren, F.J.; Finnigan, T.J.; Wilde, P.J. β-glucan release from fungal and plant cell walls after simulated gastrointestinal digestion. J. Funct. Foods 2021, 83, 104543. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Mcmillan, B. The impact of mycoprotein on blood cholesterol levels: A pilot study. Br. Food J. 2010, 112, 1092–1101. [Google Scholar] [CrossRef]
- Thomas, A.B.; Shetane, T.D.; Singha, R.G.; Nanda, R.K.; Poddar, S.S.; Shirsat, A. Employing Central Composite Design for Evaluation of Biomass Production by Fusarium venenatum: In Vivo Antioxidant and Antihyperlipidemic Properties. Appl. Biochem. Biotechnol. 2017, 183, 91–109. [Google Scholar] [CrossRef]
- Ou, C.C.; Hsiao, Y.M.; Wang, W.H.; Ko, J.L.; Lin, M.Y. Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-γ production. Food Agric. Immunol. 2009, 20, 319–332. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wang, X.F.; Zhou, X.W. Recent status and prospects of the fungal immunomodulatory protein family. Crit. Rev. Biotechnol. 2011, 31, 365–375. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Li, S.Y.; Shi, L.J.; Ding, Y.; Nie, Y.; Tang, X.M. Identification and functional characterization of a novel fungal immunomodulatory protein from Postia placenta. Food Chem. Toxicol. 2015, 78, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ejike, U.C.; Chan, C.J.; Okechukwu, P.N.; Lim, R.L.H. New advances and potentials of fungal immunomodulatory proteins for therapeutic purposes. Crit. Rev. Biotechnol. 2020, 40, 1172–1190. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsiao, Y.M.; Sheu, G.T.; Chang, J.T.; Wang, P.H.; Wu, M.F.; Shieh, G.J.; Hsu, C.P.; Ko, J.L. Nuclear translocation of telomerase reverse transcriptase and calcium signaling in repression of telomerase activity in human lung cancer cells by fungal immunomodulatory protein from Ganoderma tsugae. Biochem. Pharmacol. 2007, 74, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Farsi, D.N.; Gallegos, J.L.; Koutsidis, G.; Nelson, A.; Finnigan, T.J.; Cheung, W.; Muñoz-Muñoz, J.L.; Commane, D.M. Substituting meat for mycoprotein reduces genotoxicity and increases the abundance of beneficial microbes in the gut: Mycomeat, a randomised crossover control trial. Eur. J. Nutr. 2023, 62, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Chaoqun, Z.; Jian, Y.; Jian, L.; Jiyan, S.; Yizhen, X.; Guoxiao, L. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota. Front. Immunol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Gamarra-Castillo, O.; Echeverry-Montaña, N.; Marbello-Santrich, A.; Hernández-Carrión, M.; Restrepo, S. Meat Substitute Development from Fungal Protein (Aspergillus oryzae). Foods 2022, 11, 2940. [Google Scholar] [CrossRef]
- Whittaker, J.A.; Johnson, R.I.; Finnigan, T.J.A.; Avery, S.V.; Dyer, P.S. The Biotechnology of Quorn Mycoprotein: Past, Present and Future Challenges. In Grand Challenges in Fungal Biotechnology; Springer: Cham, Switzerland, 2020; pp. 59–79. [Google Scholar]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- Tamang, J.P.; Anupma, A.; Shangpliang, H.N.J. Ethno-microbiology of Tempe, an Indonesian fungal-fermented soybean food and Koji, a Japanese fungal starter culture. Curr. Opin. Food Sci. 2022, 48, 100912. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT Food Sci. Technol. 2020, 122, 109024. [Google Scholar] [CrossRef]
- Caron, T.; Piver, M.L.; Péron, A.C.; Lieben, P.; Lavigne, R.; Brunel, S.; Roueyre, D.; Place, M.; Bonnarme, P.; Giraud, T.; et al. Strong effect of Penicillium roqueforti populations on volatile and metabolic compounds responsible for aromas, flavor and texture in blue cheeses. Int. J. Food Microbiol. 2021, 354, 109174. [Google Scholar] [CrossRef] [PubMed]
- Younis, K.; Ashfaq, A.; Ahmad, A.; Anjum, Z.; Yousuf, O. A critical review focusing the effect of ingredients on the textural properties of plant-based meat products. J. Texture Stud. 2023, 54, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Stephan, A.; Ahlborn, J.; Zajul, M.; Zorn, H. Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: Functionality and sensory tests in comparison to commercial proteins and meat sausages. Eur. Food Res. Technol. 2018, 244, 913–924. [Google Scholar] [CrossRef]
- Jo, C.; Zhang, J.; Tam, J.M.; Church, G.M.; Khalil, A.S.; Segrè, D.; Tang, T.C. Unlocking the magic in mycelium: Using synthetic biology to optimize filamentous fungi for biomanufacturing and sustainability. Mater. Today Bio 2023, 19, 100560. [Google Scholar] [CrossRef]
- Denny, A.; Aisbitt, B.; Lunn, J. Mycoprotein and health. Nutr. Bull. 2008, 33, 298–310. [Google Scholar] [CrossRef]
- Bartholomai, B.M.; Ruwe, K.M.; Thurston, J.; Jha, P.; Scaife, K.; Simon, R.; Abdelmoteleb, M.; Goodman, R.E.; Farhi, M. Safety evaluation of Neurospora crassa mycoprotein for use as a novel meat alternative and enhancer. Food Chem. Toxicol. 2022, 168, 113342. [Google Scholar] [CrossRef]
- Dai, X.; Sharma, M.; Chen, J. Fungi in Sustainable Food Production; Series in Fungal Biology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Nout, M.J.R.; Aidoo, K.E. Asian fungal fermented food. In Industrial Applications; Springer: New York, NY, USA, 2002; pp. 23–47. [Google Scholar]
- Stahel, W.R. Circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Cortesão, M.; Schütze, T.; Marx, R.; Moeller, R.; Meyer, V. Fungal biotechnology in space: Why and how. In Grand Challenges in Fungal Biotechnology; Springer: Cham, Switzerland, 2020; pp. 501–535. [Google Scholar]
- Checinska Sielaff, A.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R.; et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 2019, 7, 50. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; De Gobba, C.; Zhang, L.; Bredie, W.L.; Lametsch, R. Production of taste enhancers from protein hydrolysates of porcine hemoglobin and meat using Bacillus amyloliquefaciens γ-glutamyltranspeptidase. J. Agric. Food Chem. 2020, 68, 11782–11789. [Google Scholar] [CrossRef] [PubMed]
- Furey, B.; Slingerland, K.; Bauter, M.R.; Dunn, C.; Goodman, R.E.; Koo, S. Safety evaluation of Fy Protein™ (Nutritional Fungi Protein), a macroingredient for human consumption. Food Chem. Toxicol. 2022, 166, 113005. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, P.F.; Andersson, D.; Ferreira, J.A.; Taherzadeh, M.J. Mycoprotein: Environmental impact and health aspects. World J. Microbiol. Biotechnol. 2019, 35, 147. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two faces of fermented foods-the benefits and threats of its consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef]
| Nutrients | Mycoprotein (Wet Weight, %) |
|---|---|
| Protein | 11.0 |
| Carbohydrate | 3.0 |
| Lipids | 2.90 |
| Dietary fiber | 6.0 |
| Vitamin B6 | 0.0001 |
| Vitamin B9 (folate) | 0.000114 |
| Riboflavin | 0.00026 |
| Choline | 0.00018 |
| Sodium | 0.005 |
| Manganese | 0.0049 |
| Magnesium | 0.049 |
| Calcium | 0.048 |
| Phosphorus | 0.29 |
| Potassium | 0.071 |
| Zinc | 0.0076 |
| Energy (kcals/100 g) | 85 |
| Amino Acids | Amino Acid Content (g/100 g Protein) | |||
|---|---|---|---|---|
| Mycoprotein | Milk Protein | Egg White | Soy Protein | |
| His | 3.50 | 2.37 | 2.10 | 2.40 |
| Ile | 5.70 | 4.57 | 4.40 | 4.00 |
| Leu | 8.60 | 9.66 | 7.30 | 7.00 |
| Lys | 8.30 | 7.78 | 6.10 | 5.50 |
| Met | 2.10 | 2.40 | 3.20 | 1.10 |
| Phe | 4.90 | 4.71 | 5.10 | 4.60 |
| Thr | 1.80 | 4.18 | 4.00 | 3.70 |
| Val | 6.20 | 5.33 | 5.80 | 4.10 |
| Cys | 0.80 | 0.90 | 2.50 | 1.10 |
| Arg | 7.30 | 3.50 | 5.00 | 6.90 |
| Tyr | 4.00 | 4.90 | 3.90 | 3.50 |
| Asp | 10.30 | 7.50 | 9.30 | 10.00 |
| Ser | 5.10 | 5.20 | 6.00 | 5.10 |
| Fungi | Fungal-Protein-Based Products | Source | Reference | |
|---|---|---|---|---|
| Mold (Aspergillus oryzae) | Miso |  | Soybean. | [95] |
| Aspergillus oryzae | Soy sauce |  | Soybean. | [96] |
| Rhizopus oligosporus | Tempeh |  | Rhizopus oligosporus is subjected to six-day solid-state fermentation in broad beans. | [97] |
| Penicillium roquefortii Penicillium camembertii | Blue cheeses |  | The source of mycelia can be milk, the natural environment, or starter cultures added to milk. | [98] |
| Fusarium | Dairy-free cream cheese |  | Fungal protein Fy™. | [47] |
| Aspergillus oryzae | Burgers—meat substitute |  | Aspergillus oryzae was cultivated using maltodextrin as a carbon source, supplemented with beet extract, carboxymethyl cellulose, and quinoa flour. | [92] |
| Fusarium | Meatless breakfast patties |  | Fungal protein Fy™ with all nine essential amino acids. | [99] |
| Fusarium venenatum | Animal meat substitutes |  | Fungal protein, egg white, and wheat flour. Rich in protein and fiber; soy-free. | [100] |
| Pleurotus sapidus | Vegetarian sausage |  | Pleurotus sapidus mycelium grown on isomaltulose molasses or apple pomace. | [101] |
| Mushroom mycelium | Plant-based bacon |  | Mushroom mycelium, salt, coconut oil, sugar, natural spices, concentrated beet juice. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Qiao, K.; Xiong, J.; Guo, H.; Zhang, Y. Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source. Foods 2023, 12, 4388. https://doi.org/10.3390/foods12244388
Li K, Qiao K, Xiong J, Guo H, Zhang Y. Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source. Foods. 2023; 12(24):4388. https://doi.org/10.3390/foods12244388
Chicago/Turabian StyleLi, Ku, Kaina Qiao, Jian Xiong, Hui Guo, and Yuyu Zhang. 2023. "Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source" Foods 12, no. 24: 4388. https://doi.org/10.3390/foods12244388
APA StyleLi, K., Qiao, K., Xiong, J., Guo, H., & Zhang, Y. (2023). Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source. Foods, 12(24), 4388. https://doi.org/10.3390/foods12244388






