Reduction and Growth Inhibition of Listeria monocytogenes by Use of Anti-Listerial Nisin, P100 Phages and Buffered Dry Vinegar Fermentates in Standard and Sodium-Reduced Cold-Smoked Salmon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Overview and Study Design
2.2. Bacterial Strains and Culture Conditions
2.3. Antimicrobial Compounds for L. monocytogenes Growth Inhibition and Reduction
2.4. Production and Preparation of Cold-Smoked Salmon
2.5. Contamination of Salmon with L. monocytogenes and Antimicrobial Treatments
2.6. Culture-Dependent and Independent Microbial Analyses
2.7. Statistical Analyses
3. Results
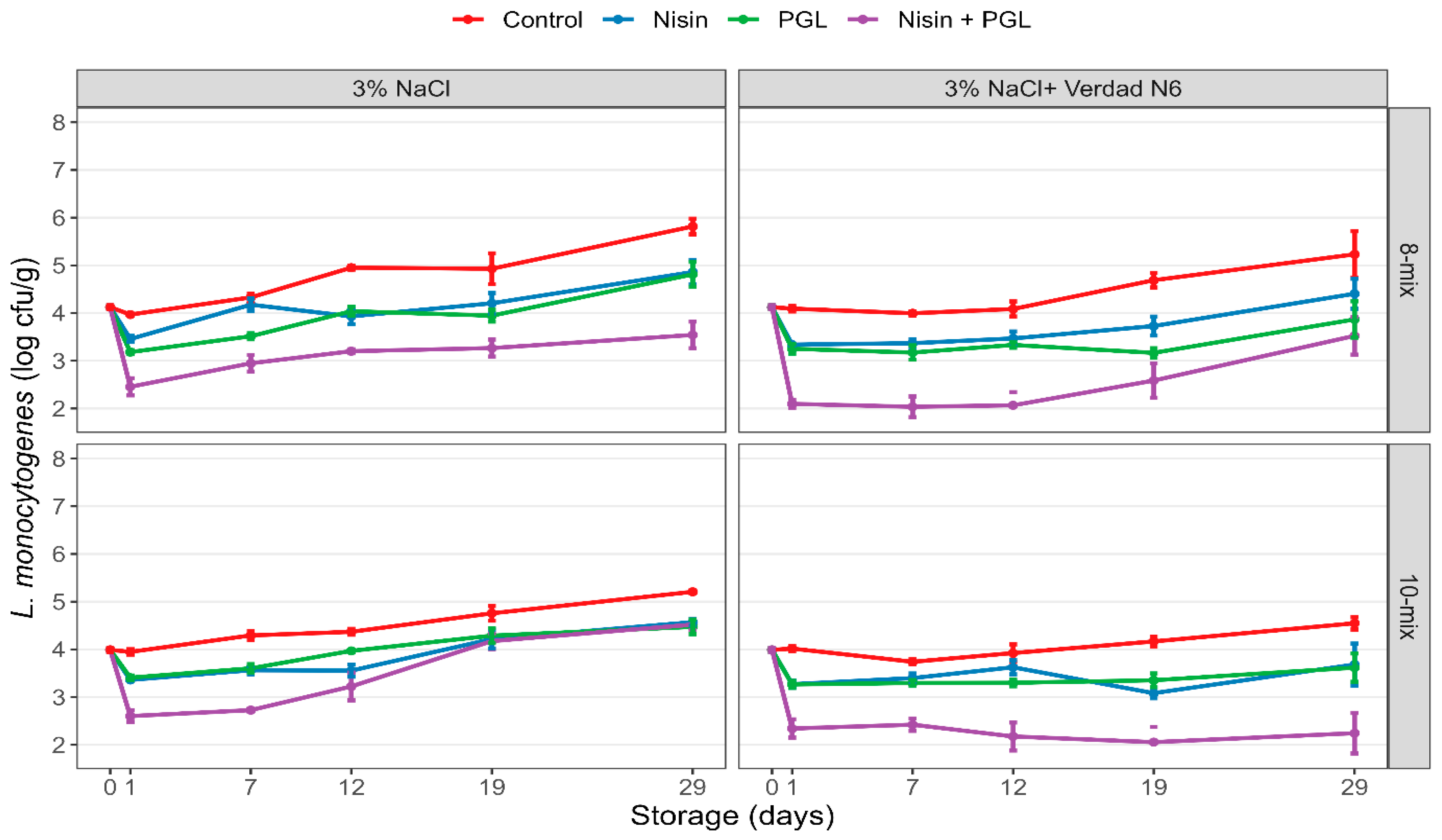
3.1. Experiment 1: Effects of Nisin and Phages on L. monocytogenes in CS Salmon with and without Preservative Fermentate Verdad N6
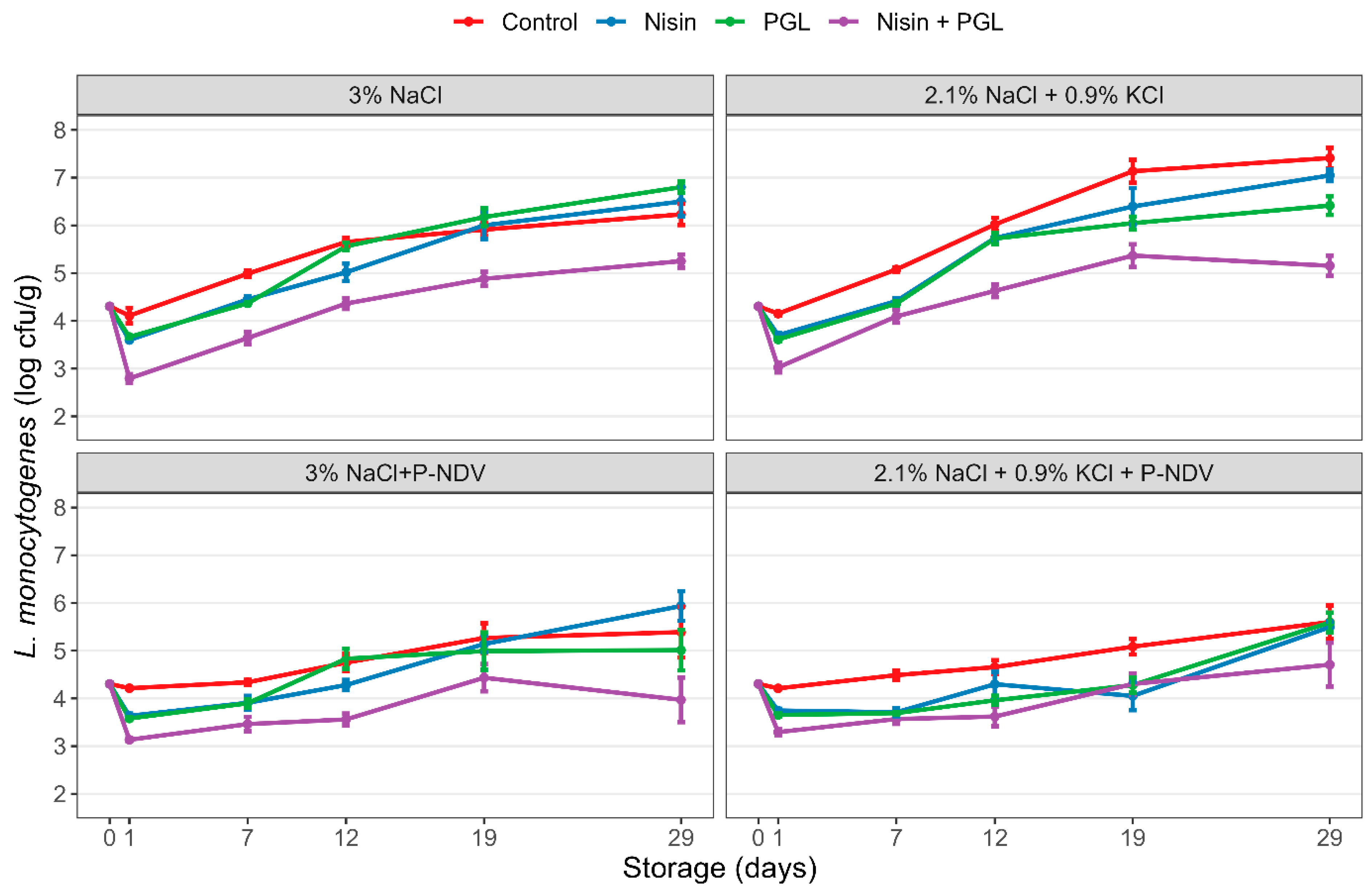
3.2. Experiment 2: Effects of Nisin and Phages on L. monocytogenes in Mildly Smoked CS Salmon with and without KCl-Based Sodium Reduction
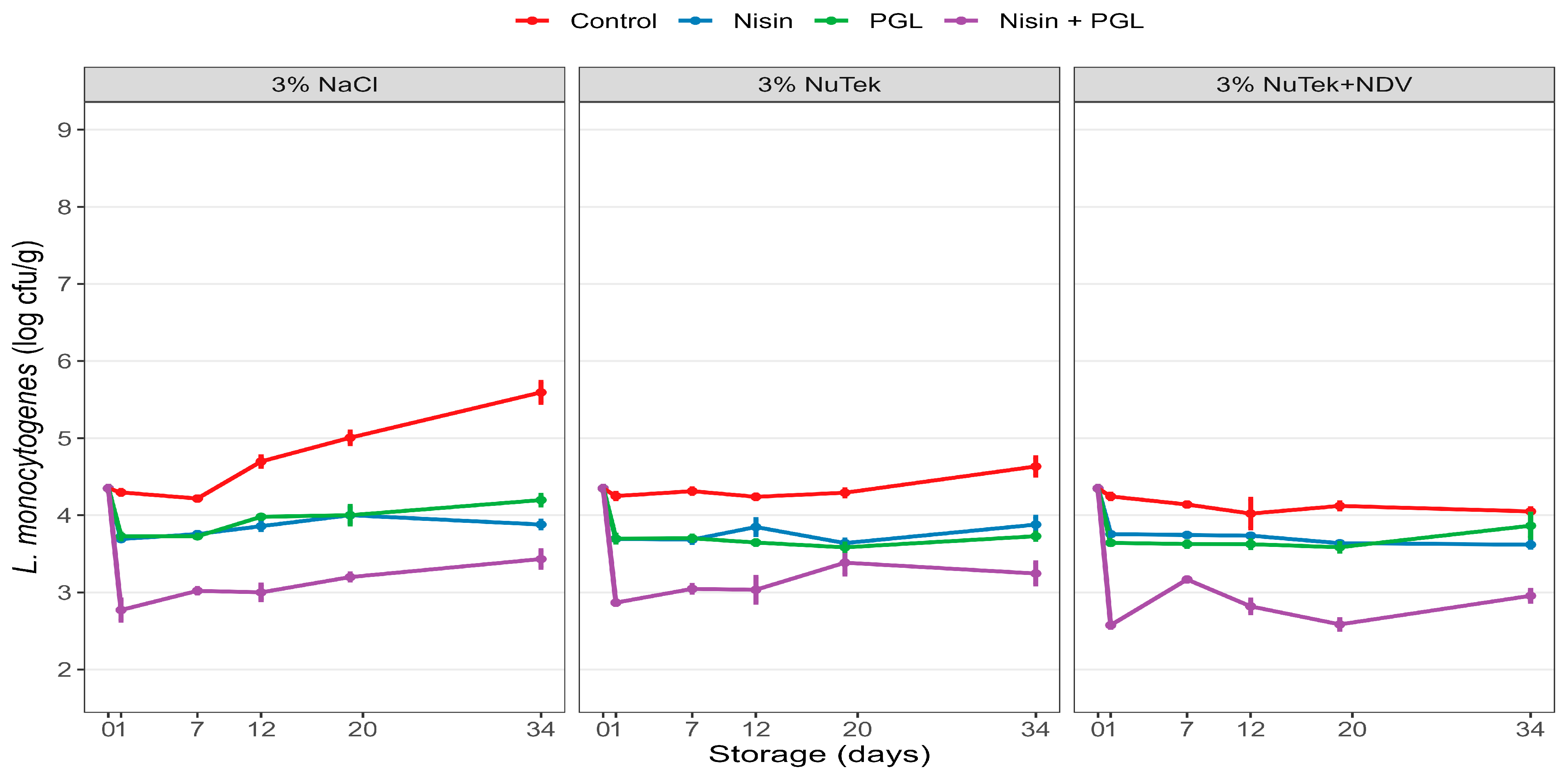
3.3. Experiment 3: Effects of Nisin and Phages on L. monocytogenes in Industrially Produced Standard and Sodium-Reduced CS Salmon Produced with and without Preservative Fermentate P-NDV
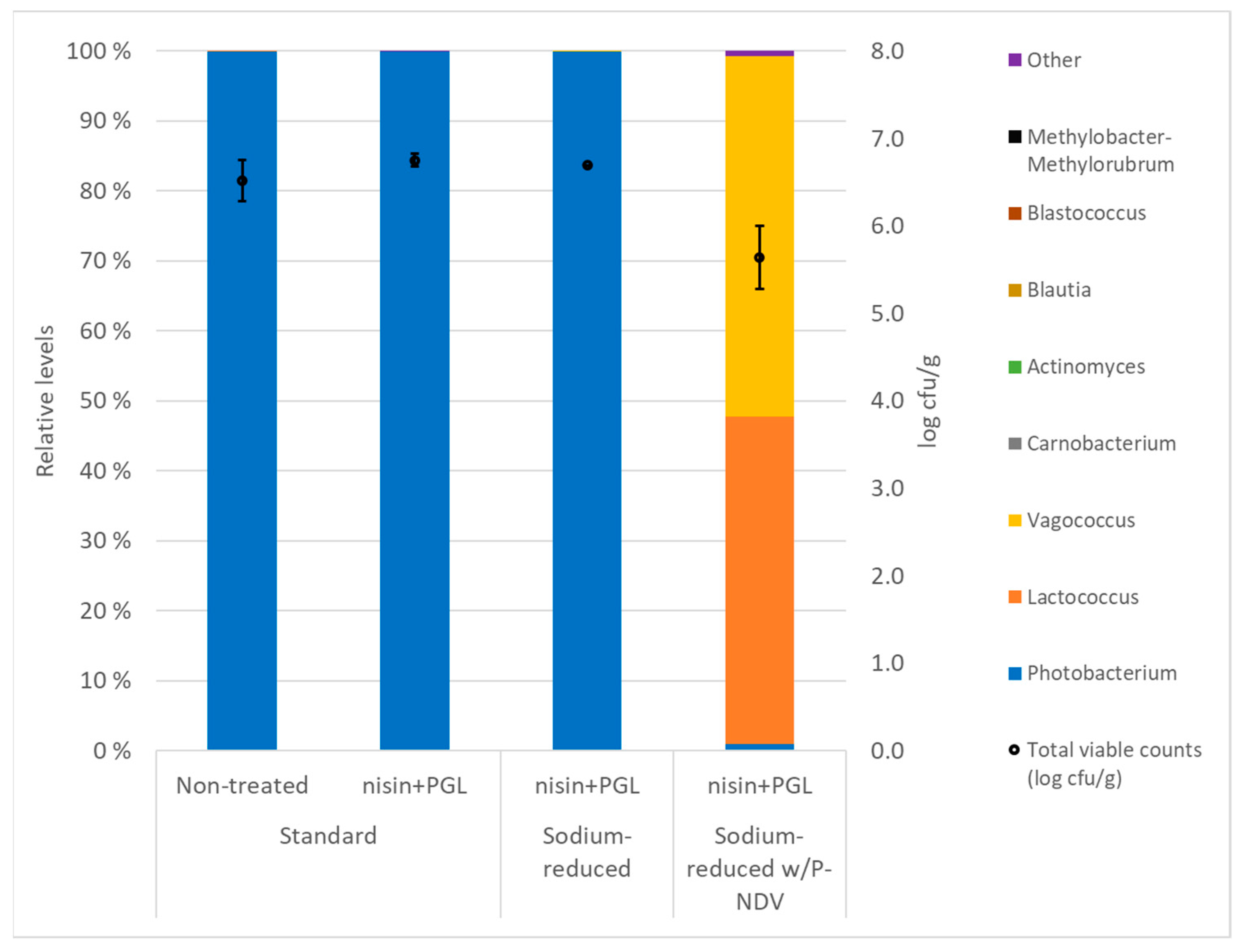
3.4. Total Viable Counts, Microbiota and pH in CS Salmon Produced with and without Preservative Fermentate P-NDV and Treated with Nisin and PGL Phages and Nisin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Hoffmann, S.; Batz, M.B.; Morris, J.G. Annual Cost of Illness and Quality-Adjusted Life Year Losses in the United States Due to 14 Foodborne Pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escamez, P.S.F.; Girones, R.; Herman, L.; Koutsoumanis, K.; Norrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef] [PubMed]
- Rørvik, L.M.; Aase, B.; Alvestad, T.; Caugant, D.A. Molecular epidemiological survey of Listeria monocytogenes in seafoods and seafood-processing plants. Appl. Environ. Microbiol. 2000, 66, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Osek, J. Prevalence, genetic diversity and antimicrobial resistance of Listeria monocytogenes isolated from fresh and smoked fish in Poland. Food Microbiol. 2017, 64, 164–171. [Google Scholar] [CrossRef]
- Vivant, A.L.; Garmyn, D.; Piveteau, P. Listeria monocytogenes, a down-to-earth pathogen. Front. Cell. Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef]
- Fagerlund, A.; Idland, L.; Heir, E.; Moretro, T.; Aspholm, M.; Lindback, T.; Langsrud, S. Whole-Genome Sequencing Analysis of Listeria monocytogenes from Rural, Urban, and Farm Environments in Norway: Genetic Diversity, Persistence, and Relation to Clinical and Food Isolates. Appl. Environ. Microbiol. 2022, 88, 6. [Google Scholar] [CrossRef]
- Fagerlund, A.; Wagner, E.; Moretro, T.; Heir, E.; Moen, B.; Rychli, K.; Langsrud, S. Pervasive Listeria monocytogenes Is Common in the Norwegian Food System and Is Associated with Increased Prevalence of Stress Survival and Resistance Determinants. Appl. Environ. Microbiol. 2022, 88, 18. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Møretrø, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria monocytogenes in Aquatic Food Products-A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gillesberg Lassen, S.; Ethelberg, S.; Björkman, J.T.; Jensen, T.; Sørensen, G.; Kvistholm Jensen, A.; Müller, L.; Nielsen, E.M.; Mølbak, K. Two listeria outbreaks caused by smoked fish consumption—using whole-genome sequencing for outbreak investigations. Clin. Microbiol. Infect. 2016, 22, 620–624. [Google Scholar] [CrossRef]
- Lüth, S.; Boone, I.; Kleta, S.; Al Dahouk, S. Analysis of RASFF notifications on food products contaminated with Listeria monocytogenes reveals options for improvement in the rapid alert system for food and feed. Food Control 2019, 96, 479–487. [Google Scholar] [CrossRef]
- Soon, J.M.; Brazier, A.K.M.; Wallace, C.A. Determining common contributory factors in food safety incidents–A review of global outbreaks and recalls 2008–2018. Trends Food Sci. Technol. 2020, 97, 76–87. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready-to-eat foods in the EU, 2010–2011 Part A: Listeria monocytogenes prevalence estimates. EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Acciari, V.A.; Torresi, M.; Iannetti, L.; Scattolini, S.; Pomilio, F.; Decastelli, L.; Colmegna, S.; Muliari, R.; Bossu, T.; Proroga, Y.; et al. Listeria monocytogenes in Smoked Salmon and Other Smoked Fish at Retail in Italy: Frequency of Contamination and Strain Characterization in Products from Different Manufacturers. J. Food Prot. 2017, 80, 271–278. [Google Scholar] [CrossRef]
- Gombas, D.E.; Chen, Y.H.; Clavero, R.S.; Scott, V.N. Survey of Listeria monocytogenes in ready-to-eat foods. J. Food Prot. 2003, 66, 559–569. [Google Scholar] [CrossRef]
- Cepanec, K.; Vugrinec, S.; Cvetkovic, T.; Ranilovic, J. Potassium Chloride-Based Salt Substitutes: A Critical Review with a Focus on the Patent Literature. Compr. Rev. Food Sci. Food Saf. 2017, 16, 881–894. [Google Scholar] [CrossRef]
- World Health Organization. Sodium Reduction—Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 2 October 2023).
- Munoz, I.; Guardia, M.D.; Arnau, J.; Dalgaard, P.; Bover, S.; Fernandes, J.O.; Monteiro, C.; Cunha, S.C.; Goncalves, A.; Nunes, M.L.; et al. Effect of the sodium reduction and smoking system on quality and safety of smoked salmon (Salmo salar). Food Chem. Toxicol. 2020, 143. [Google Scholar] [CrossRef]
- Almli, V.L.; Hersleth, M. Salt replacement and injection salting in smoked salmon evaluated from descriptive and hedonic sensory perspectives. Aquac. Int. 2013, 21, 1091–1108. [Google Scholar] [CrossRef]
- Giese, E.; Meyer, C.; Ostermeyer, U.; Lehmann, I.; Fritsche, J. Sodium reduction in selected fish products by means of salt substitutes. Eur. Food Res. Technol. 2019, 245, 1651–1664. [Google Scholar] [CrossRef]
- Heir, E.; Jacobsen, M.; Gaarder, M.O.; Berget, I.; Dalgaard, P.; Jensen, M.R.; Holck, A.L. Microbial Safety and Sensory Analyses of Cold-Smoked Salmon Produced with Sodium-Reduced Mineral Salts and Organic Acid Salts. Foods 2022, 11, 1483. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.G. Bacteriophage control of foodborne bacteria. J. Food Prot. 2005, 68, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Shehzadi, U.; Islam, F.; Afzaal, M.; Ali, R.; Ali, Y.A.; Chauhan, A.; Biswas, S.; Khurshid, S.; Usman, I.; et al. Bacteriophages and food safety: An updated overview. Food Sci. Nutr. 2023, 11, 3621–3630. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Sarkar, T.; Pati, S.; Basu, D.; Kari, Z.A.; Wei, L.S.; Smaoui, S.; Goh, K.W.; et al. Bacteriocin: A natural approach for food safety and food security. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14. [Google Scholar]
- Lasagabaster, A.; Jimenez, E.; Lehnherr, T.; Miranda-Cadena, K.; Lehnherr, H. Bacteriophage biocontrol to fight Listeria outbreaks in seafood. Food Chem. Toxicol. 2020, 145. [Google Scholar] [CrossRef]
- Atterbury, R.J. Bacteriophage biocontrol in animals and meat products. Microb. Biotechnol. 2009, 2, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.; Berg, J. Inhibition of Listeria monocytogenes in Cooked Ham by Virulent Bacteriophages and Protective Cultures. Appl. Environ. Microbiol. 2009, 75, 6944–6946. [Google Scholar] [CrossRef] [PubMed]
- Bigot, B.; Lee, W.J.; McIntyre, L.; Wilson, T.; Hudson, J.A.; Billington, C.; Heinemann, J.A. Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 2011, 28, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, M.M.; Wouters, J.; Hagens, S.; Hugenholtz, J. Bacteriophage P100 application to control Listeria monocytogenes on smeared cheese. Milchwiss.-Milk Sci. Int. 2007, 62, 284–287. [Google Scholar]
- Oliveira, M.; Vinas, I.; Colas, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.; Stasiewicz, M.J.; Wiedmann, M.; Boor, K.J.; Bergholz, T.M. Efficacy of different antimicrobials on inhibition of Listeria monocytogenes growth in laboratory medium and on cold-smoked salmon. Int. J. Food Microbiol. 2013, 165, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Stasiewicz, M.J.; Murray, D.; Boor, K.J.; Wiedmann, M.; Bergholz, T.M. Optimization of combinations of bactericidal and bacteriostatic treatments to control Listeria monocytogenes on cold-smoked salmon. Int. J. Food Microbiol. 2014, 179, 1–9. [Google Scholar] [CrossRef]
- Chen, R.X.; Skeens, J.; Orsi, R.H.; Wiedmann, M.; Guariglia-Oropeza, V. Pre-growth conditions and strain diversity affect nisin treatment efficacy against Listeria monocytogenes on cold-smoked salmon. Int. J. Food Microbiol. 2020, 333. [Google Scholar] [CrossRef]
- Duffes, F.; Leroi, F.; Boyaval, P.; Dousset, X. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4°C. Int. J. Food Microbiol. 1999, 47, 33–42. [Google Scholar] [CrossRef]
- Szabo, E.A.; Cahill, M.E. Nisin and ALTATM 2341 inhibit the growth of Listeria monocytogenes on smoked salmon packaged under vacuum or 100% CO2. Lett. Appl. Microbiol. 1999, 28, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Shen, Q.; Nannapaneni, R. Reduction of Listeria monocytogenes in cold-smoked salmon by bacteriophage P100, nisin and lauric arginate, singly or in combinations. Int. J. Food Sci. Technol. 2014, 49, 1918–1924. [Google Scholar] [CrossRef]
- Heir, E.; Liland, K.H.; Carlehog, M.; Hoick, A.L. Reduction and inhibition of Listeria monocytogenes in cold-smoked salmon by Verdad N6, a buffered vinegar fermentate, and UV-C treatments. Int. J. Food Microbiol. 2019, 291, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.; Liland, K.H.; Carlehog, M.; Heir, E. Reductions of Listeria monocytogenes on cold-smoked and raw salmon fillets by UV-C and pulsed UV light. Innov. Food Sci. Emerg. Technol. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Tocmo, R.; Krizman, K.; Khoo, W.J.; Phua, L.K.; Kim, M.J.; Yuk, H.G. Listeria monocytogenes in Vacuum-Packed Smoked Fish Products: Occurrence, Routes of Contamination, and Potential Intervention Measures. Compr. Rev. Food. Sci. Food Saf. 2014, 13, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Pedros-Garrido, S.; Clemente, I.; Calanche, J.B.; Condon-Abanto, S.; Beltran, J.A.; Lyng, J.G.; Brunton, N.; Bolton, D.; Whyte, P. Antimicrobial activity of natural compounds against listeria spp. and their effects on sensory attributes in salmon (Salmo salar) and cod (Gadus morhua). Food Control 2020, 107. [Google Scholar] [CrossRef]
- Rudi, K.; Zimonja, M.; Hannevik, S.E.; Dromtorp, S.M. Multiplex real-time single nucleotide polymorphism detection and quantification by quencher extension. Biotechniques 2006, 40, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Fugett, E.; Fortes, E.; Nnoka, C.; Wiedmann, M. International life sciences institute north America Listeria monocytogenes strain collection: Development of standard Listeria monocytogenes strain sets for research and validation studies. J. Food Prot. 2006, 69, 2929–2938. [Google Scholar] [CrossRef]
- Cantinelli, T.; Chenal-Francisque, V.; Diancourt, L.; Frezal, L.; Leclercq, A.; Wirth, T.; Lecuit, M.; Brisse, S. “Epidemic Clones” of Listeria monocytogenes Are Widespread and Ancient Clonal Groups. J. Clin. Microbiol. 2013, 51, 3770–3779. [Google Scholar] [CrossRef]
- Mejlholm, O.; Gunvig, A.; Borggaard, C.; Blom-Hanssen, J.; Mellefont, L.; Ross, T.; Leroi, F.; Else, T.; Visser, D.; Dalgaard, P. Predicting growth rates and growth boundary of Listeria monocytogenes—An international validation study with focus on processed and ready-to-eat meat and seafood. Int. J. Food Microbiol. 2010, 141, 137–150. [Google Scholar] [CrossRef]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. Br. Med. J. 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J.; et al. Global Sodium Consumption and Death from Cardiovascular Causes. New Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.; Fahimi, S.; Singh, G.M.; Khatibzadeh, S.; Micha, R.; Powles, J.; Mozaffarian, D. Cost effectiveness of a government supported policy strategy to decrease sodium intake: Global analysis across 183 nations. Br. Med. J. 2017, 356, i6699. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Nannapaneni, R.; Hagens, S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog. Dis. 2010, 7, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Vongkamjan, K.; Roof, S.; Stasiewicz, M.J.; Wiedmann, M. Persistent Listeria monocytogenes subtypes isolated from a smoked fish processing facility included both phage susceptible and resistant isolates. Food Microbiol. 2013, 35, 38–48. [Google Scholar] [CrossRef]
- Fister, S.; Fuchs, S.; Stessl, B.; Schoder, D.; Wagner, M.; Rossmanith, P. Screening and characterisation of bacteriophage P100 insensitive Listeria monocytogenes isolates in Austrian dairy plants. Food Control 2016, 59, 108–117. [Google Scholar] [CrossRef]
- Denes, T.; den Bakker, H.C.; Tokman, J.I.; Guldimann, C.; Wiedmann, M. Selection and Characterization of Phage-Resistant Mutant Strains of Listeria monocytogenes Reveal Host Genes Linked to Phage Adsorption. Appl. Environ. Microbiol. 2015, 81, 4295–4305. [Google Scholar] [CrossRef]
- Brown, P.; Chen, Y.; Parsons, C.; Brown, E.; Loessner, M.J.; Shen, Y.; Kathariou, S. Whole Genome Sequence Analysis of Phage-Resistant Listeria monocytogenes Serotype 1/2a Strains from Turkey Processing Plants. Pathogens 2021, 10, 199. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of Environmental Factors on Phage–Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-To-Eat Foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, 231. [Google Scholar]
- Chen, R.X.; Skeens, J.W.; Wiedmann, M.; Guariglia-Oropeza, V. The efficacy of nisin against Listeria monocytogenes on cold-smoked salmon at natural contamination levels is concentration-dependent and varies by serotype. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Katla, T.; Naterstad, K.; Vancanneyt, M.; Swings, J.; Axelsson, L. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl. Environ. Microbiol. 2003, 69, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Bergis, H.; Bonanno, L.; Assére, A.; Lombard, B. EURL Lm Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 5 October 2023).
- Heir, E.; Solberg, L.E.; Carlehog, M.; Moen, B.; Jensen, M.R.; Holck, A.L. Improved control of Listeria monocytogenes during storage of raw salmon by treatment with the fermentate Verdad N6 and nisin. Int. J. Food Microbiol. 2021, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Sumrall, E.T.; Shen, Y.; Keller, A.P.; Rismondo, J.; Pavlou, M.; Eugster, M.R.; Boulos, S.; Disson, O.; Thouvenot, P.; Kilcher, S.; et al. Phage resistance at the cost of virulence: Listeria monocytogenes serovar 4b requires galactosylated teichoic acids for InlB-mediated invasion. PLoS Pathog. 2019, 15. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Wiernasz, N.; Leroi, F.; Chevalier, F.; Cornet, J.; Cardinal, M.; Rohloff, J.; Passerini, D.; Skirnisdóttir, S.; Pilet, M.F. Salmon Gravlax Biopreservation With Lactic Acid Bacteria: A Polyphasic Approach to Assessing the Impact on Organoleptic Properties, Microbial Ecosystem and Volatilome Composition. Front. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Hagens, S.; Loessner, M.J. Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 2007, 76, 513–519. [Google Scholar] [CrossRef]
- Hagens, S.; Loessner, M.J. Bacteriophage for Biocontrol of Foodborne Pathogens: Calculations and Considerations. Curr. Pharm. Biotechnol. 2010, 11, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Rizo, A.; Fuentes, A.; Barat, J.M.; Fernandez-Segovia, I. Development of a novel smoke-flavoured salmon product by sodium replacement using water vapour permeable bags. J. Sci. Food Agric. 2018, 98, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Rizo, A.; Fuentes, A.; Fernandez-Segovia, I.; Barat, J.M. Development of a novel smoke-flavoured trout product: An approach to sodium reduction and shelf life assessment. J. Food Eng. 2017, 211, 22–29. [Google Scholar] [CrossRef]
| Variables | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| Site of production | Pilot plant | Pilot plant | Industrial smokehouse |
| Standard CS salmon | 3% NaCl | 3% NaCl | 3% NaCl + 0.6% sucrose |
| Sodium-reduced CS salmon | Not included | 2.1% NaCl + 0.9% KCl | 3% NuTek 78300 |
| Preservative salt (fermentate) | Without or with Verdad N6 | Without or with P-NDV | Without or with P-NDV in sodium-reduced CS salmon |
| Smoking | Moderate | Mild | Moderate |
| L. monocytogenes mix 2 | 8-strain and 10-strain | 10-strain | 10-strain |
| Storage temperature | 4 °C and 8 °C | 4 °C | 4 °C |
| Storage time | 29 days | 29 days | 34 days |
| Strain No. | Serotype | MLVA/ST 2 | Source 3 | Other Designations; Reference |
|---|---|---|---|---|
| MF3860 | 1/2a | 6-10-5-16-6/20 | Salmon processing, Plant S4 | [10] |
| MF3939 | 1/2a | 5-8-15-10-6/14 | Salmon processing, Plant S3 | [10] |
| MF4001 | 1/2a | 5-8-15-10-6/14 | Salmon processing, Plant S2 | [10] |
| MF4077 | 1/2a | 6-9-18-16-6/8 | Salmon processing, Plant S1 | [10] |
| MF4588 | 1/2a | 7-7-10-10-6/7 | Salmon processing, Plant S1 | [10] |
| MF4804 | 1/2a | 6-7-14-10-6/121 | Salmon processing, Plant S2 | [10] |
| MF2184 | 1/2b | 7-8-0-16-0/3 | Meat processing, outbreak | 2583/92; [48] |
| MF3009 | 1/2b | n.d./5 | Cattle | FSL J2-064; [49] https://www.ncbi.nlm.nih.gov/nuccore/AARO00000000.2/ (accessed on 3 October 2023) |
| MF3039 | 4b | n.d./6 | Human, cerebrospinal fluid, outbreak | FSL N1-227; [50] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3889766/ (accessed on 3 October 2023) |
| MF3710 | 4b | 7-7-20-6-10/n.d. | Human, cerebrospinal fluid | CCUG3998; Culture Collection University of Gothenburg |
| Experiment No. 1 | Treatment 2 | Reductions (log) in L. monocytogenes Levels during Storage 3 | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 12 | Day 19 | Day 29 | ||
| Exp. 1 | Nisin | 0.7 (**) 4 | 0.5 (.) | 0.7 (**) | 0.8 (***) | 0.8 (***) |
| PGL | 0.7 (**) | 0.7 (**) | 0.7 (**) | 1.0 (***) | 1.0 (***) | |
| Nisin + PGL | 1.6 (***) | 1.6 (***) | 1.7 (***) | 1.6 (***) | 1.7 (***) | |
| Exp. 2 | Nisin | 0.5 (*) | 0.6 (**) | 0.4 (*) | 0.5 (*) | −0.1 (ns) |
| PGL | 0.6 (**) | 0.6 (***) | 0.3 (ns) | 0.5 (*) | 0.2 (ns) | |
| Nisin + PGL | 1.1 (***) | 1.0 (***) | 1.2 (***) | 1.1 (***) | 1.4 (***) | |
| Treatment 1,2 | Overall Reductions (log) in L. monocytogenes in Three Types of CS Salmon during Storage 3 | ||
|---|---|---|---|
| Standard (3% NaCl) | Sodium-Reduced (3% NuTek) | Sodium-Reduced (3% NuTek) + P-NDV | |
| Nisin | 0.9 | 0.6 | 0.4 |
| PGL | 0.8 | 0.7 | 0.5 |
| Nisin + PGL | 1.7 | 1.2 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heir, E.; Jensen, M.R.; Aasli, A.W.; Berget, I.; Holck, A.L. Reduction and Growth Inhibition of Listeria monocytogenes by Use of Anti-Listerial Nisin, P100 Phages and Buffered Dry Vinegar Fermentates in Standard and Sodium-Reduced Cold-Smoked Salmon. Foods 2023, 12, 4391. https://doi.org/10.3390/foods12244391
Heir E, Jensen MR, Aasli AW, Berget I, Holck AL. Reduction and Growth Inhibition of Listeria monocytogenes by Use of Anti-Listerial Nisin, P100 Phages and Buffered Dry Vinegar Fermentates in Standard and Sodium-Reduced Cold-Smoked Salmon. Foods. 2023; 12(24):4391. https://doi.org/10.3390/foods12244391
Chicago/Turabian StyleHeir, Even, Merete Rusås Jensen, Anette Wold Aasli, Ingunn Berget, and Askild Lorentz Holck. 2023. "Reduction and Growth Inhibition of Listeria monocytogenes by Use of Anti-Listerial Nisin, P100 Phages and Buffered Dry Vinegar Fermentates in Standard and Sodium-Reduced Cold-Smoked Salmon" Foods 12, no. 24: 4391. https://doi.org/10.3390/foods12244391
APA StyleHeir, E., Jensen, M. R., Aasli, A. W., Berget, I., & Holck, A. L. (2023). Reduction and Growth Inhibition of Listeria monocytogenes by Use of Anti-Listerial Nisin, P100 Phages and Buffered Dry Vinegar Fermentates in Standard and Sodium-Reduced Cold-Smoked Salmon. Foods, 12(24), 4391. https://doi.org/10.3390/foods12244391






