Enhancing Nutritional Profile of Pasta: The Impact of Sprouted Pseudocereals and Cushuro on Digestibility and Health Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pasta Making
2.3. Simplex Centroid Mixture Design
2.4. Nutritional Composition
2.5. Phytic Acid (PA)
2.6. Total Soluble Phenolic Compounds (TSPC)
2.7. γ-Aminobutyric Acid (GABA)
2.8. Oxygen Radical Antioxidant Capacity (ORAC)
2.9. Determination of Optimal Cooking Time
2.10. Simulated Gastrointestinal Digestion
2.11. Determination of Mineral Bioaccessibility
2.12. Determination of GI
2.13. Statistics
3. Results and Discussion
3.1. Nutritional Quality of SQF, SKF, and CuF Is Remarkably Higher Than Refined WF
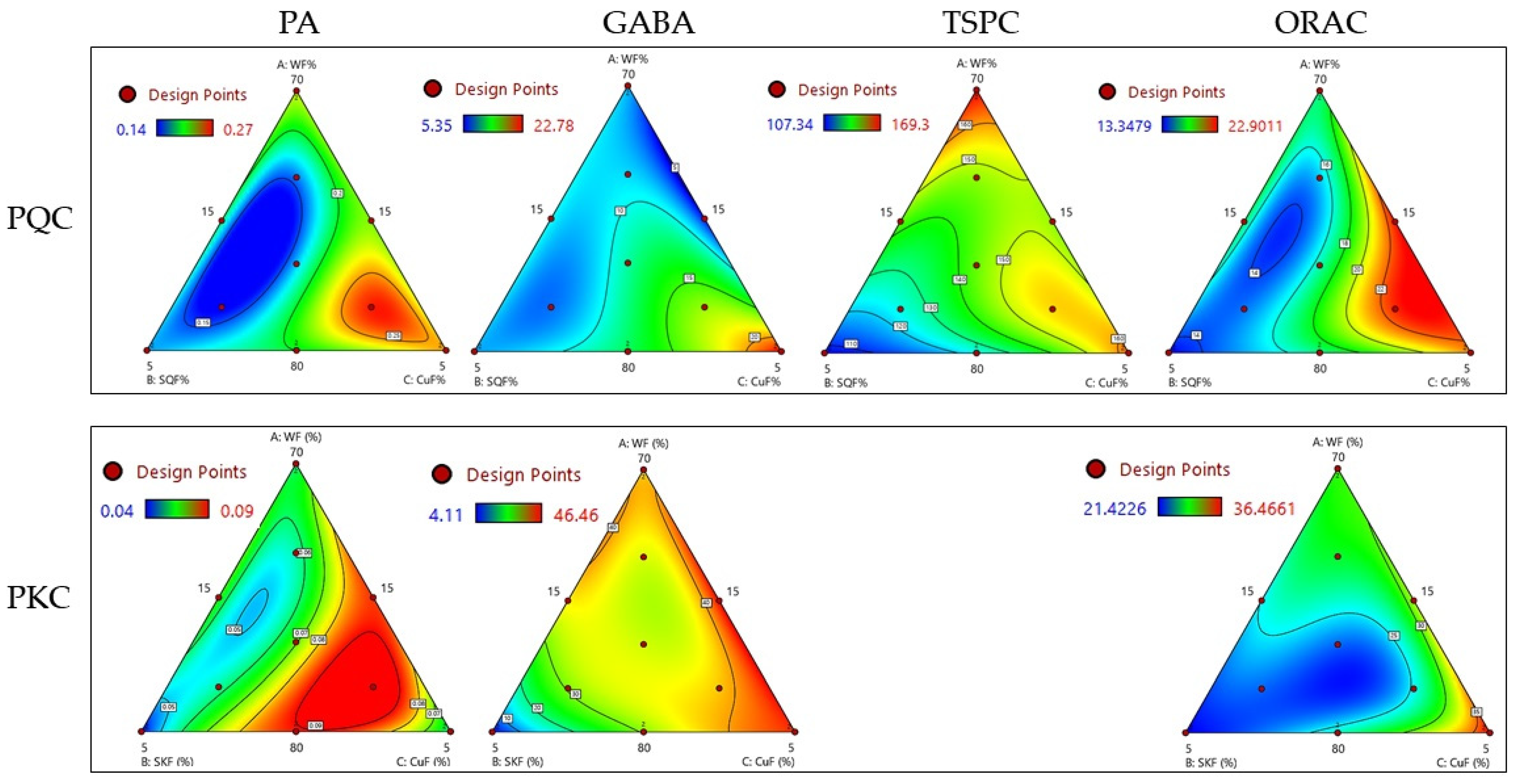
3.2. Modelization of the Effect of Substitution Ratio of WF with Sprouted Pseudocereals and Cushuro on PA, GABA, TSPC, and Antioxidant Activity in Pasta
3.3. Optimal Supplementation Ratio of WF with SQF/SKF and CuF Blends Improved the Nutrional Quality and Functional Value of Wheat-Based Pasta
3.4. Effect of Cooking on Nutritional Composition and Bioactive Value of Optimized Pasta Types
3.5. Optimal WF Supplementation with SQF and CuF Reduces Starch Digestion and GI in Pasta
3.6. Optimal WF Supplementation with SQF and CuF Reduces Starch Digestion and GI in Pasta
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo-Lerma, J.; Crespo-Escobar, P.; Martinez-Barona, S.; Fornes-Ferrer, V.; Donat, E.; Ribes-Koninckx, C. Differences in the macronutrient and dietary fibre profile of gluten-free products as compared to their gluten-containing counterparts. Eur. J. Clin. Nutr. 2019, 73, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Nilusha, R.; Jayasinghe, J.; Perera, O.; Perera, P. Development of pasta products with nonconventional ingredients and their effect on selected quality characteristics: A brief overview. Int. J. Food Sci. 2019, 2019, 6750726. [Google Scholar] [CrossRef] [PubMed]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the Chemical and Technological Characteristics of Wholemeal Flours Obtained from Amaranth (Amaranthus sp.), Quinoa (Chenopodium quinoa) and Buckwheat (Fagopyrum sp.) Seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Hernández, A.; Beta, T.; Loarca-Piña, G.; Castaño-Tostado, E.; Nieto-Barrera, J.O.; Mendoza, S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016, 72, 84–90. [Google Scholar] [CrossRef]
- Corpus-Gomez, A.; Alcantara-Callata, M.; Celis-Teodoro, H.; Echevarria-Alarcón, B.; Paredes-Julca, J.; Paucar-Menacho, L.M. Cushuro (Nostoc sphaericum): Hábitat, características fisicoquímicas, composición nutricional, formas de consumo y propiedades medicinales. Agroind. Sci. 2021, 11, 231–238. [Google Scholar] [CrossRef]
- Méndez-Ancca, S.; Pepe-Victoriano, R.; Gonzales, H.H.S.; Zambrano-Cabanillas, A.W.; Marín-Machuca, O.; Rojas, J.C.Z.; Maquera, M.M.; Huanca, R.F.; Aguilera, J.G.; Zuffo, A.M.; et al. Physicochemical Evaluation of Cushuro (Nostoc sphaericum Vaucher ex Bornet & Flahault) in the Region of Moquegua for Food Purposes. Foods 2023, 12, 1939. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L. Microalgae: From staple foodstuff to avant-garde cuisine. Int. J. Gastron. Food Sci. 2020, 21, 100221. [Google Scholar] [CrossRef]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Peñas, E.; Dueñas, M.; Frias, J.; Martínez-Villaluenga, C. Optimizing germination conditions to enhance the accumulation of bioactive compounds and the antioxidant activity of kiwicha (Amaranthus caudatus) using response surface methodology. LWT Food Sci. Technol. 2017, 76, 245–252. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Al-Jumayi, H.A.O.; Elhendy, H.A. Effect of germination on the nutritional profile of quinoa (Cheopodium quinoa Willd.) seeds and its anti-anemic potential in Sprague–Dawley male albino rats. Cereal Chem. 2021, 98, 315–327. [Google Scholar] [CrossRef]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Miano, A.C.; Obregón, J.; Soriano-Colchado, J.; Barraza-Jáuregui, G. Malting process as an alternative to obtain high nutritional quality quinoa flour. J. Cereal Sci. 2019, 90, 102858. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Repo-Carrasco-Valencia, R.; Arribas, S.M.; Gonzalez, M.C.; Condezo-Hoyos, L. Effect of germination on total phenolic compounds, total antioxidant capacity, Maillard reaction products and oxidative stress markers in canihua (Chenopodium pallidicaule). J. Cereal Sci. 2012, 56, 410–417. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Pico, J.; Pismag, R.Y.; Laudouze, M.; Martinez, M.M. Systematic evaluation of the Folin–Ciocalteu and Fast Blue BB reactions during the analysis of total phenolics in legumes, nuts and plant seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Schmiele, M.; Lavado-Cruz, A.A.; Verona-Ruiz, A.L.; Mollá, C.; Peñas, E.; Frias, J.; Simpalo-Lopez, W.D.; Castillo-Martínez, W.E.; Martínez-Villaluenga, C. Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties. Foods 2022, 11, 3259. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Simpalo-López, W.D.; Castillo-Martínez, W.E.; Esquivel-Paredes, L.J.; Martínez-Villaluenga, C. Improving Nutritional and Health Benefits of Biscuits by Optimizing Formulations Based on Sprouted Pseudocereal Grains. Foods 2022, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Menacho, L.M.; Simpalo-López, W.D.; Castillo-Martínez, W.E.; Esquivel-Paredes, L.J.; Martínez-Villaluenga, C. Reformulating Bread Using Sprouted Pseudo-cereal Grains to Enhance Its Nutritional Value and Sensorial Attributes. Foods 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, J.; Acosta-Coral, K.; Paucar-Menacho, L.M. Quinoa (Chenopodium quinoa): Nutritional composition and bioactive compounds of grain and leaf, and impact of heat treatment and germination. Sci. Agropecu. 2022, 13, 209–220. [Google Scholar] [CrossRef]
- Bala, M.; Tushir, S.; Garg, M.; Meenu, M.; Kaur, S.; Sharma, S.; Mann, S. Wheat Milling and Recent Processing Technologies: Effect on Nutritional Properties, Challenges, and Strategies. In Wheat Science: Nutritional and Anti-Nutritional Properties, Processing, Storage, Bioactivity, and Product Development; CRC Press: Boca Raton, FL, USA, 2023; pp. 219–256. [Google Scholar]
- Thakur, P.; Kumar, K.; Ahmed, N.; Chauhan, D.; Eain Hyder Rizvi, Q.U.; Jan, S.; Singh, T.P.; Dhaliwal, H.S. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Curr. Res. Food Sci. 2021, 4, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Kim-Anne, L.Ê.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Rao, J.; Chen, B. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Crit. Rev. Food Sci. Nutr. 2020, 60, 740–759. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Sreenivasulu, N.; Alseekh, S.; Sartagoda, K.J.D.; Usadel, B.; Fernie, A.R. Metabolomics and machine learning technique revealed that germination enhances the multi-nutritional properties of pigmented rice. Commun. Biol. 2023, 6, 1000. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Miyatake, K.; Morita, N. Total phenolic compounds and antioxidant capacity of wheat graded flours by polishing method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Pedrali, D.; Giupponi, L.; De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Mateos-Aparicio, I. The quinoa variety influences the nutritional and antioxidant profile rather than the geographic factors. Food Chem. 2023, 402, 133531. [Google Scholar] [CrossRef]
- Chen, W.; Xu, D. Phytic acid and its interactions in food components, health benefits, and applications: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104201. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, Z.; Zhu, M.; Teng, C.; Zou, L.; Liu, R.; Zhang, L.; Yang, X.; Ren, G.; Qin, P. The gluten structure, starch digestibility and quality properties of pasta supplemented with native or germinated quinoa flour. Food Chem. 2023, 399, 133976. [Google Scholar] [CrossRef] [PubMed]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef]
- Teterycz, D.; Sobota, A.; Starek, A. Possibility of using wheat germ and wheat germ protein isolate for high-protein pasta production. Cereal Chem. 2023, 100, 299–309. [Google Scholar] [CrossRef]
- Sobota, A.; Zarzycki, P. Effect of Pasta Cooking Time on the Content and Fractional Composition of Dietary Fiber. J. Food Qual. 2013, 36, 127–132. [Google Scholar] [CrossRef]
- Duijsens, D.; Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How postharvest variables in the pulse value chain affect nutrient digestibility and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5067–5096. [Google Scholar] [CrossRef]
- Duijsens, D.; Alfie Castillo, A.I.; Verkempinck, S.H.E.; Pälchen, K.; Hendrickx, M.E.; Grauwet, T. In vitro macronutrient digestibility and mineral bioaccessibility of lentil-based pasta: The influence of cellular intactness. Food Chem. 2023, 423, 136303. [Google Scholar] [CrossRef]
- Gwala, S.; Kyomugasho, C.; Wainaina, I.; Rousseau, S.; Hendrickx, M.; Grauwet, T. Ageing, dehulling and cooking of Bambara groundnuts: Consequences for mineral retention and in vitro bioaccessibility. Food Funct. 2020, 11, 2509–2521. [Google Scholar] [CrossRef]
- Singh, P.; Prasad, S. A review on iron, zinc and calcium biological significance and factors affecting their absorption and bioavailability. J. Food Compos. Anal. 2023, 123, 105529. [Google Scholar] [CrossRef]
- Rousseau, S.; Celus, M.; Duijsens, D.; Gwala, S.; Hendrickx, M.; Grauwet, T. The impact of postharvest storage and cooking time on mineral bioaccessibility in common beans. Food Funct. 2020, 11, 7584–7595. [Google Scholar] [CrossRef]
- Sirisoontaralak, P.; Nakornpanom, N.N.; Koakietdumrongkul, K.; Panumaswiwath, C. Development of quick cooking germinated brown rice with convenient preparation and containing health benefits. LWT Food Sci. Technol. 2015, 61, 138–144. [Google Scholar] [CrossRef]
- Toyoizumi, T.; Kosugi, T.; Toyama, Y.; Nakajima, T. Effects of high-temperature cooking on the gamma-aminobutyric acid content and antioxidant capacity of germinated brown rice (Oryza sativa L.). CyTA J. Food 2021, 19, 360–369. [Google Scholar] [CrossRef]
- Conti, V.; Piccini, C.; Romi, M.; Salusti, P.; Cai, G.; Cantini, C. Pasta Enriched with Carrot and Olive Leaf Flour Retains High Levels of Accessible Bioactives after In Vitro Digestion. Foods 2023, 12, 3540. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Pompei, F.; Bonfini, M.; Mustafa, A.M.; Sagratini, G.; Wang, Z.; Vittadini, E. Quality of wholemeal pasta made with pigmented and ancient wheats. Int. J. Gastron. Food Sci. 2023, 31, 100665. [Google Scholar] [CrossRef]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.-S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, Z.; Yang, Q.; Xiao, Z.; Lu, X. Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chem. 2019, 294, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Barreto, F.F.; Miñano, H.A.; Alvarez-Ramirez, J.; Bello-Pérez, L.A. Structural, functional, and chemical properties of small starch granules: Andean quinoa and kiwicha. Food Hydrocoll. 2021, 120, 106883. [Google Scholar] [CrossRef]
- Yao, M.; Li, M.; Dhital, S.; Tian, Y.; Guo, B. Texture and digestion of noodles with varied gluten contents and cooking time: The view from protein matrix and inner structure. Food Chem. 2020, 315, 126230. [Google Scholar] [CrossRef]
- Dodi, R.; Bresciani, L.; Biasini, B.; Cossu, M.; Scazzina, F.; Taddei, F.; D’Egidio, M.G.; Dall’Asta, M.; Martini, D. Traditional and Non-Conventional Pasta-Making Processes: Effect on In Vitro Starch Digestibility. Foods 2021, 10, 921. [Google Scholar] [CrossRef]
- Wang, J.; Brennan, M.A.; Brennan, C.S.; Serventi, L. Predictive Glycaemic Response of Pasta Enriched with Juice, Puree, and Pomace from Red Cabbage and Spinach. Nutrients 2022, 14, 4575. [Google Scholar] [CrossRef]
- Koehnlein, E.A.; Koehnlein, É.M.; Corrêa, R.C.G.; Nishida, V.S.; Correa, V.G.; Bracht, A.; Peralta, R.M. Analysis of a whole diet in terms of phenolic content and antioxidant capacity: Effects of a simulated gastrointestinal digestion. Int. J. Food Sci. Nutr. 2016, 67, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Galani, Y.J.H.; Orfila, C.; Gong, Y.Y. A review of micronutrient deficiencies and analysis of maize contribution to nutrient requirements of women and children in Eastern and Southern Africa. Crit. Rev. Food Sci. Nutr. 2022, 62, 1568–1591. [Google Scholar] [CrossRef] [PubMed]

| Units | WF | SQF | SKF | CuF | |
|---|---|---|---|---|---|
| Starch | g/100 g | 70.81 ± 0.69 a | 55.84± 0.52 b | 44.69 ± 0.04 c | 0.16 ± 0.03 d |
| TDF | g/100 g | 8.50 ± 0.78 c | 18.84 ± 1.20 b | 23.06 ± 0.67 a | 19.77 ± 0.57 b |
| IDF | g/100 g | 0.73 ± 0.26 d | 12.34 ± 1.06 b | 16.18 ± 0.60 a | 5.53 ± 0.26 c |
| SDF | g/100 g | 7.77 ± 0.53 b | 6.14 ± 0.14 d | 6.87 ± 0.07 c | 14.23 ± 0.33 a |
| Protein | g/100 g | 12.44 ± 0.02 d | 23.36 ± 2.38 b | 13.87 ± 0.03 c | 46.76 ± 4.80 a |
| Fat | g/100 g | 0.90 ± 0.09 b | 6.55 ± 0.11 a | 6.77 ± 0.06 a | 0.68 ± 0.01 c |
| Ash | g/100 g | 0.52 ± 0.07 d | 3.66 ± 0.11 b | 2.09 ± 0.02 c | 6.17 ± 1.78 a |
| K | mg/100 g | 152.37 ± 2.58 a | 523.59 ± 9.20 b | 653.75 ± 13.63 a | 112.39 ± 1.66 d |
| Na | mg/100 g | 1.26 ± 0.39 c | 14.71 ± 0.34 b | 8.03 ± 0.66 c | 453.42 ± 2.65 a |
| Fe | mg/100 g | 5.59 ± 0.41 c | 4.45 ± 0.20 d | 8.36 ± 0.35 b | 24.68 ± 0.67 a |
| Zn | mg/100 g | 1.26 ± 0.03 c | 4.54 ± 0.07 b | 5.99 ± 0.13 a | 4.78 ± 0.17 b |
| Mg | mg/100 g | 38.09 ± 2.88 d | 153.27 ± 9.87 c | 279.42 ± 16.39 a | 215.36 ± 1.21 b |
| Mn | mg/100 g | 1.03 ± 0.04 d | 1.79 ± 0.05 c | 2.15 ± 0.03 b | 17.24 ± 0.78 a |
| Ca | mg/100 g | 17.09 ± 1.01 d | 129.39 ± 3.22 c | 206.14 ± 2.50 b | 2161.69 ± 64.86 a |
| PA | g/100 g | 0.17 ± 0.00 c | 0.58 ± 0.01 b | 1.21 ± 0.07 a | 0.05 ± 0.00 d |
| GABA | mg/100 g | 0.38 ± 0.00 b | 5.15 ± 0.43 a | 5.03 ± 0.02 a | 0.42 ± 0.01 b |
| TSPC | mg GAE/100 g | 55.40 ± 5.56 d | 525.50 ± 38.14 a | 144.72 ± 2.09 c | 306.48 ± 22.91 b |
| ORAC | μmol TE/g | 17.40 ± 1.94 c | 45.30 ± 3.96 a | 35.44 ± 4.55 b | 15.46 ± 1.41 c |
| Pasta Type | Dependent Variables | Mathematical Models | p-Value | R2 (pred) | R2 (adj) |
|---|---|---|---|---|---|
| PQC | PA | y = 0.23A + 0.16B + 0.25C − 0.08AC + 4.94A2BC − 3.84AB2C + 5.88ABC2 | 0.000 | 0.997 | 0.99 |
| GABA | y = 8.76A + 8.22B + 22.87C − 40.42AC − 11.86BC + 365.14ABC2 | 0.014 | 0.98 | 0.96 | |
| TSPC | y = 169.53A + 107.57B + 163.41C −77.58AC − 49.03BC + 1320.92ABC2 | 0.021 | 0.99 | 0.98 | |
| ORAC | y = 16.66A + 13.31B + 21.60C + 8.82AB + 14.51AC − 367.85A2BC − 124.45AB2C + 248.29ABC2 | 0.001 | 0.99 | 0.98 | |
| PKQ | PA | y = 0.06A + 0.04B + 0.06C + 0.07AB + 0.11AC + 0.16BC − 2.00A2BC | 0.013 | 0.94 | 0.83 |
| GABA | y = 37.91A + 4.15B + 44.63C + 62.26AB + 21.41AC + 24.57BC − 772.47A2BC + 584.84AB2C − 382.48ABC2 | 0.000 | 0.99 | 0.99 | |
| TSPC | y = 215.12A + 174.92B + 160.65C | 0.022 | 0.50 | 0.41 | |
| ORAC | y = 29.69A + 21.33B + 36.38C − 10.25BC − 482.142ABC2 | 0.026 | 0.97 | 0.95 |
| Pasta Type | Response Variables | Criteria | Importance Level | Optimum Desirability (D) | Optimal Formulation | Predicted Values | Experimental Values |
|---|---|---|---|---|---|---|---|
| PQC | PA | minimize | 5 | 0.593 | 79.5% WF 13% SQF 8% CuF | 0.25 | 0.27 ± 0.01 |
| GABA | maximize | 5 | 20.28 | 17.70 ± 0.19 | |||
| TSPC | maximize | 5 | 160.08 | 158.94 ± 7.98 | |||
| ORAC | maximize | 5 | 22.03 | 22.10 ± 0.73 | |||
| PKC | PA | minimize | 5 | 0.693 | 70% WF 15% SKF 15% CuF | 0.06 | 0.06 ± 0.01 |
| GABA | maximize | 5 | 37.91 | 37.87 ± 0.65 | |||
| TSPC | maximize | 5 | 215.12 | 227.06 ± 11.53 | |||
| ORAC | maximize | 5 | 29.64 | 29.79 ± 2.87 |
| Parameter | Units | RAW | COOKED | ||||
|---|---|---|---|---|---|---|---|
| C | oPQC | oPKC | C | oPQC | oPKC | ||
| Starch | g/100 g | 62.95 ± 2.03 ab | 50.88 ± 3.34 bc | 42.94 ± 0.18 c | 68.57 ± 7.68 a | 59.40 ± 1.54 bc | 44.73 ± 3.54 c |
| Fat | g/100 g | 1.63 ± 0.03 cd | 2.53 ± 0.02 a | 2.19 ± 0.07 ab | 1.99 ± 0.16 bc | 1.87 ± 0.14 bcd | 1.38 ± 0.16 e |
| Protein | g/100 g | 12.88 ± 0.01 d | 14.90 ± 0.09 b | 14.97 ± 0.10 b | 14.08 ± 0.32 c | 17.04 ± 0.11 a | 16.92 ± 0.08 a |
| TDF | g/100 g | 5.50 ± 0.56 d | 8.16 ± 0.27 c | 19.89 ± 0.12 a | 1.95 ± 0.27 e | 12.58 ± 1.10 b | 19.23 ± 0.32 a |
| IDF | g/100 g | 2.33 ± 0.21 c | 5.25 ± 0.12 b | 10.37 ± 0.20 a | 1.40 ± 0.20 c | 10.95 ± 0.81 a | 11.44 ± 0.74 a |
| SDF | g/100 g | 3.17 ± 0.35 c | 2.91 ± 0.15 cd | 9.52 ± 0.31 a | 0.55 ± 0.08 e | 1.63 ± 0.29 de | 7.79 ± 0.41 b |
| Ash | g/100 g | 0.62 ± 0.10 c | 1.40 ± 0.10 b | 1.69 ± 0.07 a | 1.36 ± 0.01 b | 1.29 ± 0.08 b | 1.67 ± 0.11 a |
| K | mg/100 g | 129.34 ± 5.91 b | 205.62 ± 7.48 a | 204.17 ± 9.64 a | 45.46 ± 1.71 d | 101.8 ±2.89 c | 106.76 ± 4.09 c |
| Na | mg/100 g | 22.61 ± 0.23 b | 40.75 ± 2.30 a | 42.57 ± 0.41 a | 11.46 ± 1.94 c | 23.41 ±3.99 b | 14.90 ± 0.51 c |
| Fe | mg/100 g | 22.83 ± 0.51 c | 28.32 ± 0.86 ab | 29.44 ± 0.93 ab | 25.95 ± 1.28 bc | 32.12 ± 1.31 a | 32.04 ± 6.18 a |
| Mg | mg/100 g | 35.48 ± 0.95 d | 80.57 ± 1.32 c | 90.71 ± 1.84 b | 38.35 ± 0.97 d | 86.97 ± 2.39 b | 101.44 ± 0.73 a |
| Mn | mg/100 g | 0.69 ± 0.01 e | 11.78 ± 0.42 b | 8.00 ± 0.33 d | 0.80 ± 0.03 e | 13.77 ± 0.23 a | 9.35 ± 0.20 c |
| Zn | mg/100 g | 1.08 ± 0.35 e | 4.22 ± 0.13 b | 1.82 ± 0.03 d | 1.20 ± 0.05 d | 5.14 ± 0.14 a | 2.18 ± 0.04 c |
| Ca | mg/100 g | 31.24 ± 1.57 c | 215.62 ± 4.72 b | 383.07 ± 28.8 a | 25.17 ± 0.87 c | 241.13 ± 32.14 b | 231.90 ± 20.11 b |
| PA | g/100 g | 0.02 ± 0.00 d | 0.09 ± 0.01 c | 0.06 ± 0.01 cd | 0.08 ± 0.01 c | 0.17 ± 0.02 b | 0.23 ± 0.00 a |
| GABA | mg/100 g | 1.48 ± 0.04 d | 1.77 ± 0.02 c | 3.79 ± 0.06 a | 0.49± 0.03 f | 0.86± 0.02 e | 2.21± 0.04 b |
| TSPC | mg GAE/100 g | 121.03 ± 6.12 e | 158.94 ± 7.98 c | 227.06 ± 11.53 a | 87.37 ± 6.04 f | 146.46 ± 2.38 d | 186.46 ± 10.75 b |
| ORAC | µmol TE/g | 3.42 ± 0.18 e | 22.10 ± 0.73 b | 29.79 ± 2.87 a | 2.48 ±0.43 f | 12.92 ± 0.34 d | 15.99 ± 2.90 c |
| C | oPQC | oPKC | |
|---|---|---|---|
| Undigested | |||
| GABA (mg/100 g) | 4.94 ± 0.29 cA | 8.61 ± 0.16 bA | 22.11 ± 0.44 aA |
| TSPC (mg GAE/100 g) | 67.37 ± 6.04 cA | 146.46 ± 2.38 bB | 186.46 ± 10.75 aB |
| ORAC (µmol TE/g) | 1.33 ± 0.51 cC | 12.92 ± 0.34 bC | 15.99 ± 2.90 aC |
| Gastric phase | |||
| GABA (mg/100 g) | 3.97 ± 0.36 cA | 7.21 ± 0.13 bAB | 18.70 ± 0.56 aB |
| TSPC (mg GAE/100 g) | 27.77 ± 16.8 bB | 134.38 ± 6.28 aC | 139.58 ± 22.63 aC |
| ORAC (µmol TE/g) | 21.73 ± 1.74 bB | 25.54 ± 0.67 aB | 26.45 ± 1.69 aB |
| Intestinal Phase | |||
| GABA (mg/100 g) | 5.12 ± 0.27 bA | 6.69 ± 0.55 bB | 16.26 ± 0.69 aB |
| TSPC (mg GAE/100 g) | 70.89 ± 4.61 bA | 261.86 ± 11.94 aA | 257.72 ± 14.59 aA |
| ORAC (µmol TE/g) | 142.34 ± 4.92 bA | 171.06 ± 9.53 aA | 164.93 ± 22.74 aA |
| Soluble Fe (mg/100 g) | 2.13 ± 0.09 c | 2.76 ± 0.14 a | 2.52 ± 0.03 b |
| Fe bioaccessibility (%) | 8.20 ± 0.35 a | 7.52 ± 0.09 b | 7.35 ± 0.11 b |
| Soluble Ca (mg/100 g) | 16.81 ± 0.74 c | 93.50 ± 3.77 a | 58.40 ± 1.07 b |
| Ca bioaccessibility (%) | 66.77 ± 2.95 a | 40.32 ± 1.62 b | 24.22 ± 0.45 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar-Menacho, L.M.; Vásquez Guzmán, J.C.; Simpalo-Lopez, W.D.; Castillo-Martínez, W.E.; Martínez-Villaluenga, C. Enhancing Nutritional Profile of Pasta: The Impact of Sprouted Pseudocereals and Cushuro on Digestibility and Health Potential. Foods 2023, 12, 4395. https://doi.org/10.3390/foods12244395
Paucar-Menacho LM, Vásquez Guzmán JC, Simpalo-Lopez WD, Castillo-Martínez WE, Martínez-Villaluenga C. Enhancing Nutritional Profile of Pasta: The Impact of Sprouted Pseudocereals and Cushuro on Digestibility and Health Potential. Foods. 2023; 12(24):4395. https://doi.org/10.3390/foods12244395
Chicago/Turabian StylePaucar-Menacho, Luz María, Juan Carlos Vásquez Guzmán, Wilson Daniel Simpalo-Lopez, Williams Esteward Castillo-Martínez, and Cristina Martínez-Villaluenga. 2023. "Enhancing Nutritional Profile of Pasta: The Impact of Sprouted Pseudocereals and Cushuro on Digestibility and Health Potential" Foods 12, no. 24: 4395. https://doi.org/10.3390/foods12244395
APA StylePaucar-Menacho, L. M., Vásquez Guzmán, J. C., Simpalo-Lopez, W. D., Castillo-Martínez, W. E., & Martínez-Villaluenga, C. (2023). Enhancing Nutritional Profile of Pasta: The Impact of Sprouted Pseudocereals and Cushuro on Digestibility and Health Potential. Foods, 12(24), 4395. https://doi.org/10.3390/foods12244395








