The Antioxidant Potential of Various Wheat Crusts Correlates with AGE Content Independently of Acrylamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bread Crust and Bread Crust Extracts
2.3. Cell Culture
2.4. RNA Extraction, Reverse-Transcription, and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analysis
2.6. AGE-Fluorescence Measurements
2.7. Determination of Asparagine Content
2.8. Determination of Acrylamide Content by LC-ESI-MS/MS Analysis
2.8.1. Sample Preparation for LC-ESI-MS/MS Analysis
2.8.2. LC-ESI-MS/MS Analysis
2.9. Mass Spectrometric Analyses of the Soluble WBCEs
2.9.1. Protein Extraction for Comparative Proteome Analysis
2.9.2. In-Solution Digest of Proteins and Peptide Purification
2.9.3. Mass Spectrometry Analysis
2.9.4. Data Analysis
3. Results
3.1. Analysis of Wheat Flour and Wheat Bread Crust Extracts (WBCEs)
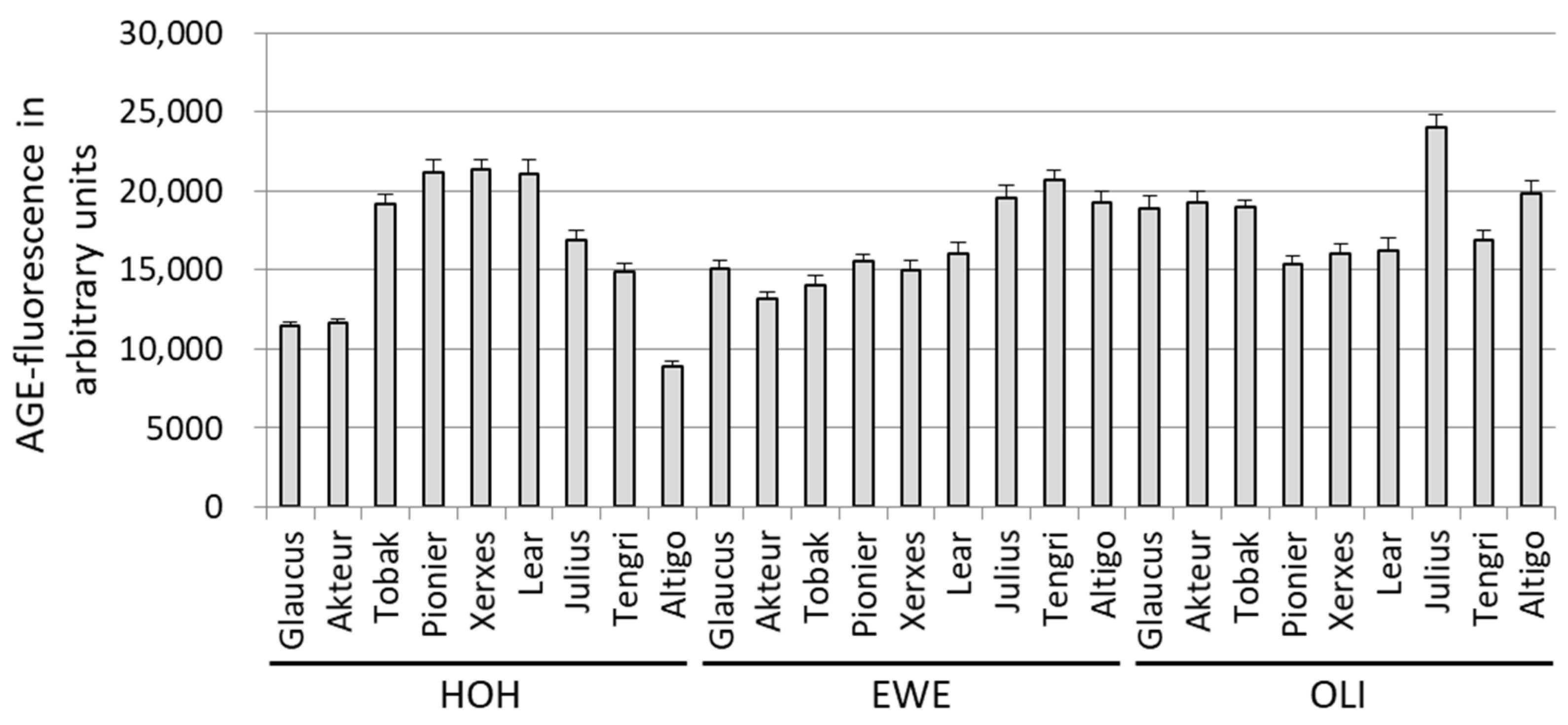
3.1.1. AGE-Related Fluorescence
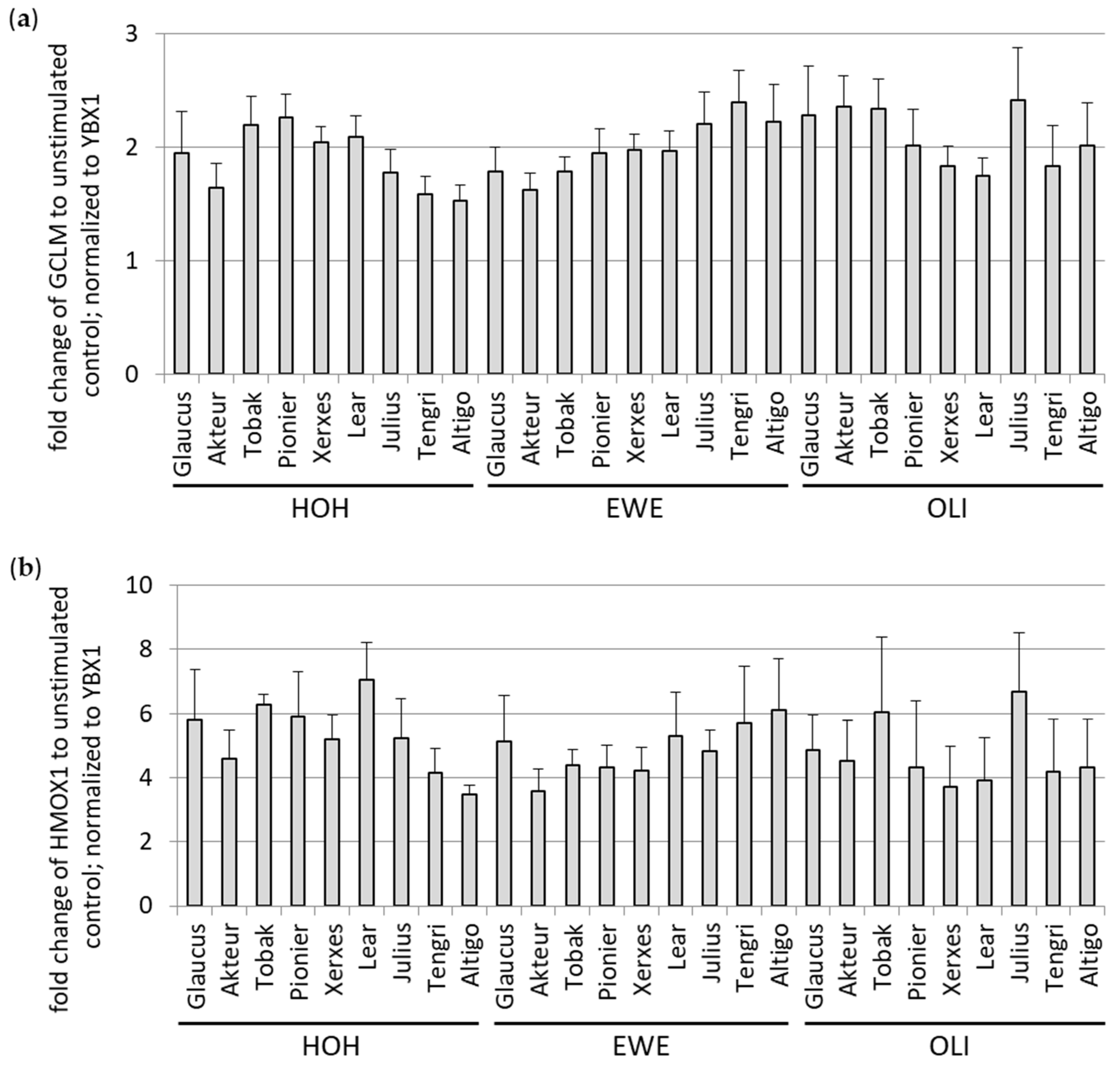
3.1.2. Antioxidant Potential of Soluble WBCEs
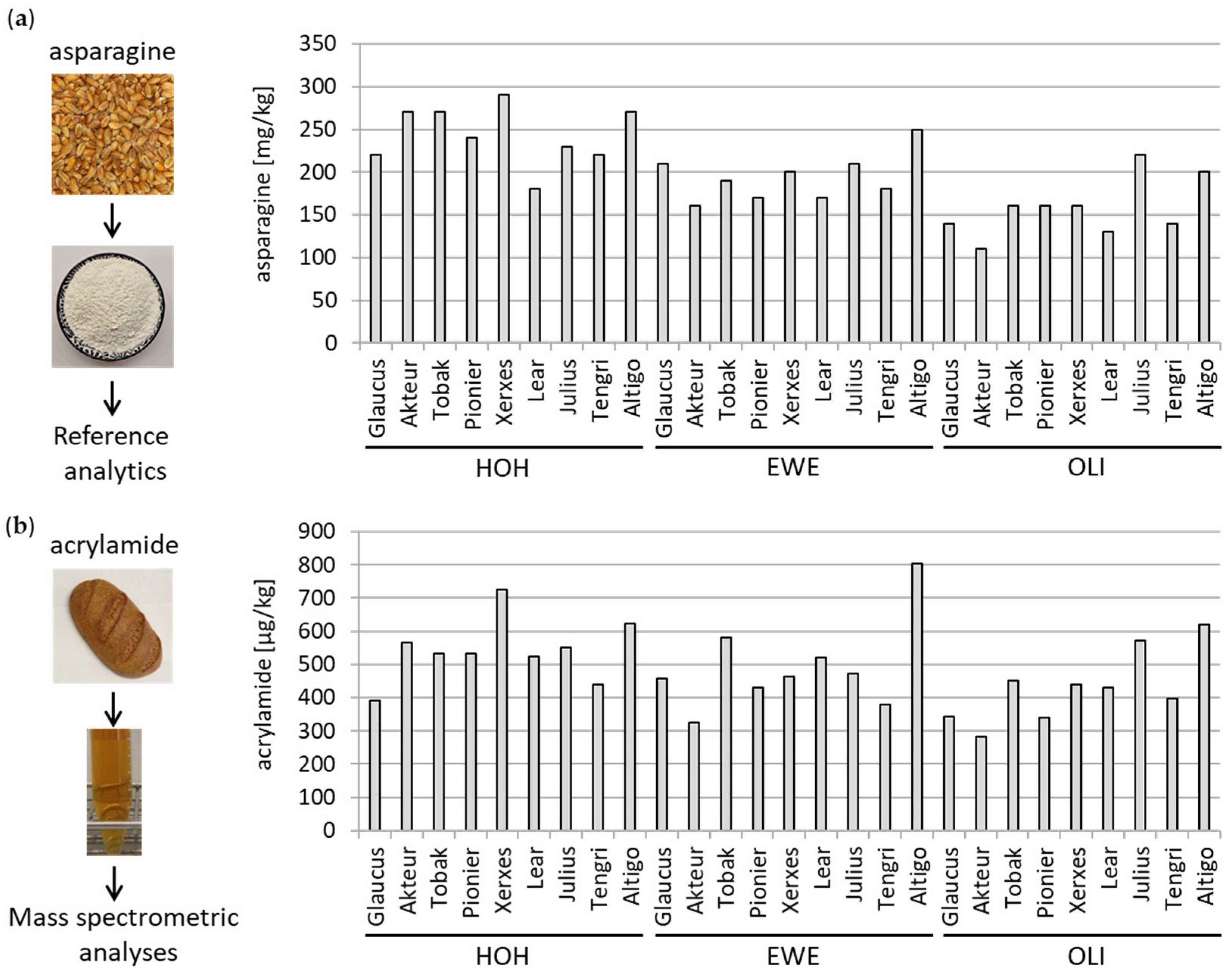
3.1.3. Determination of Asparagine and Acrylamide
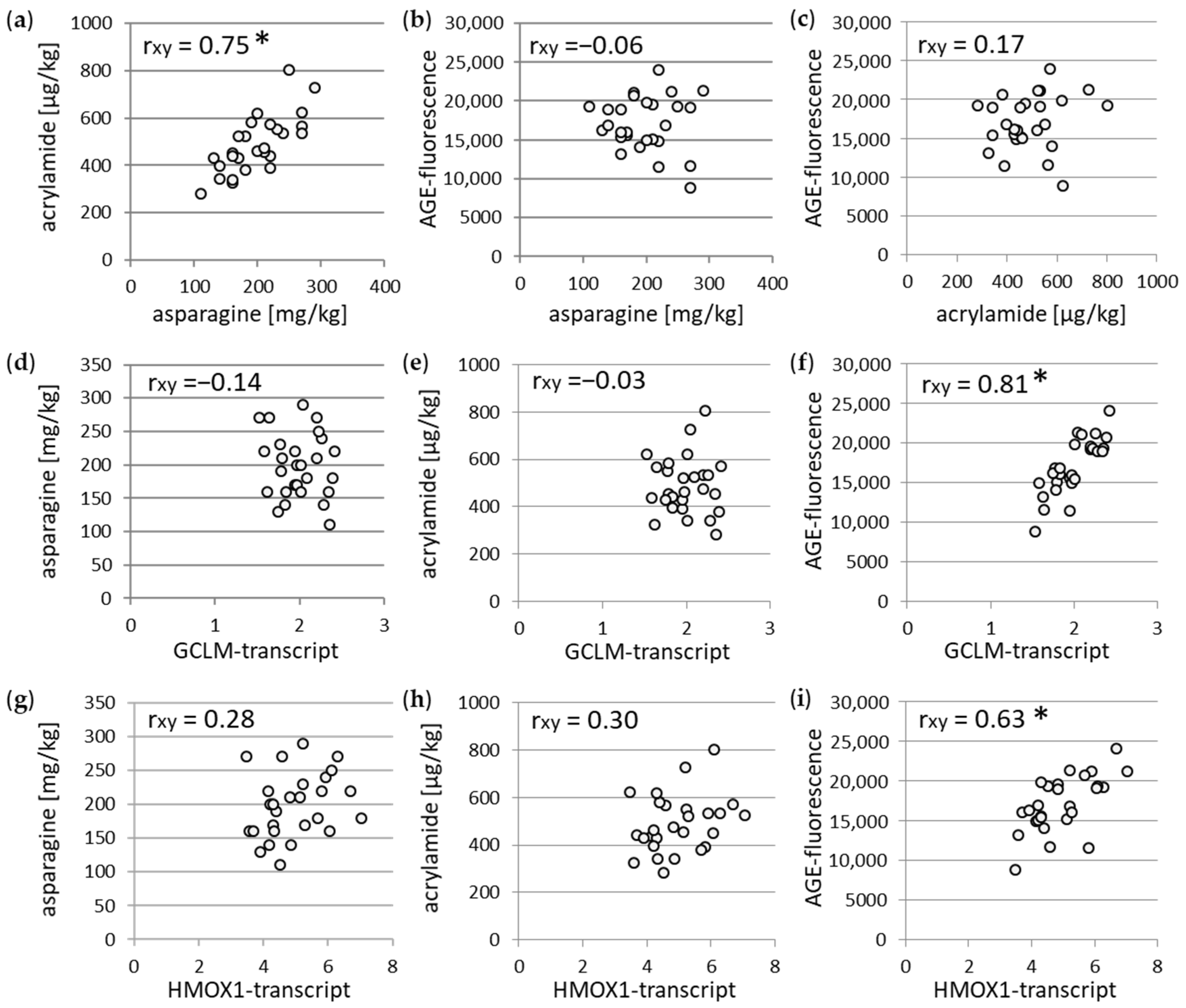
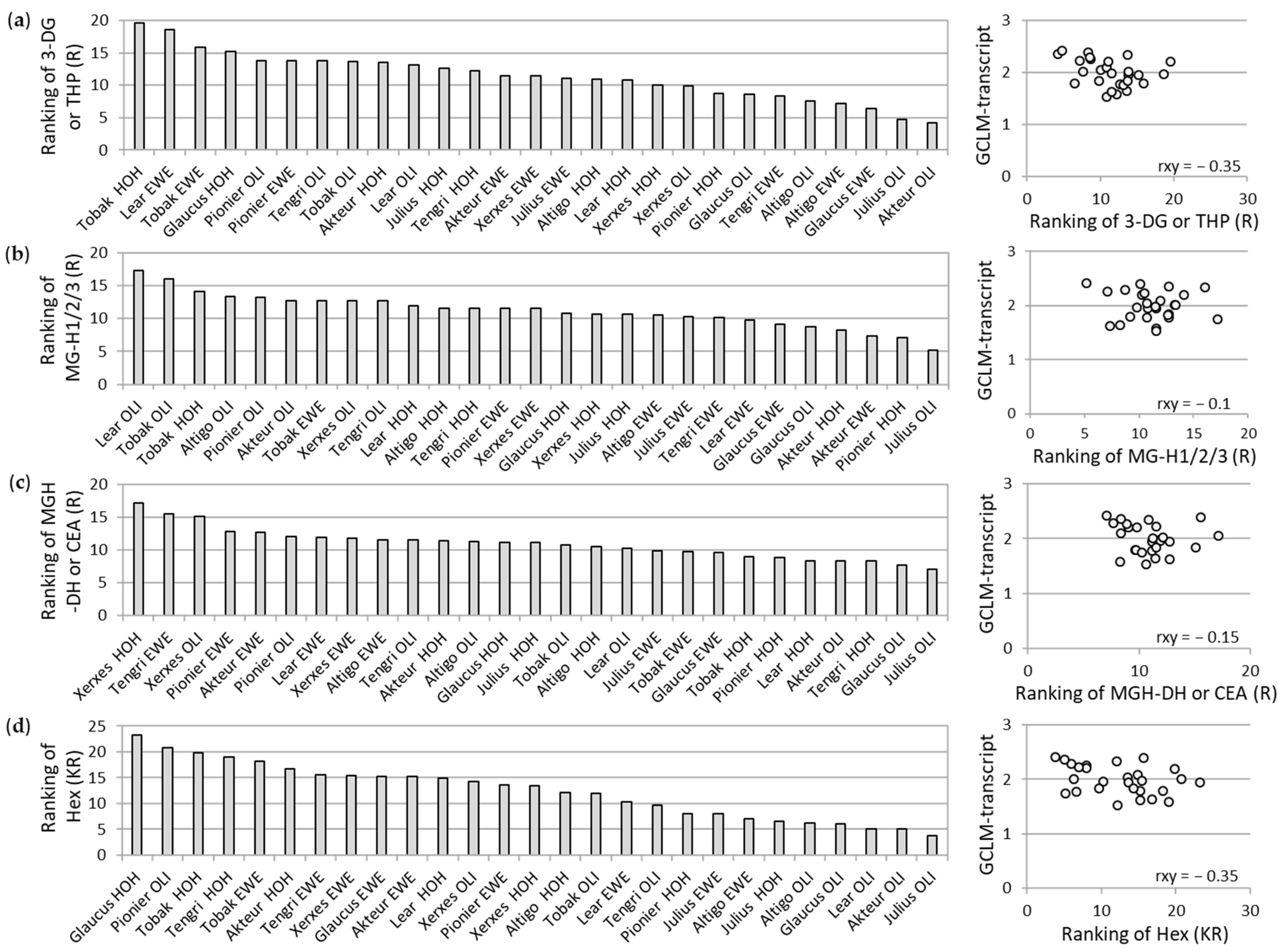
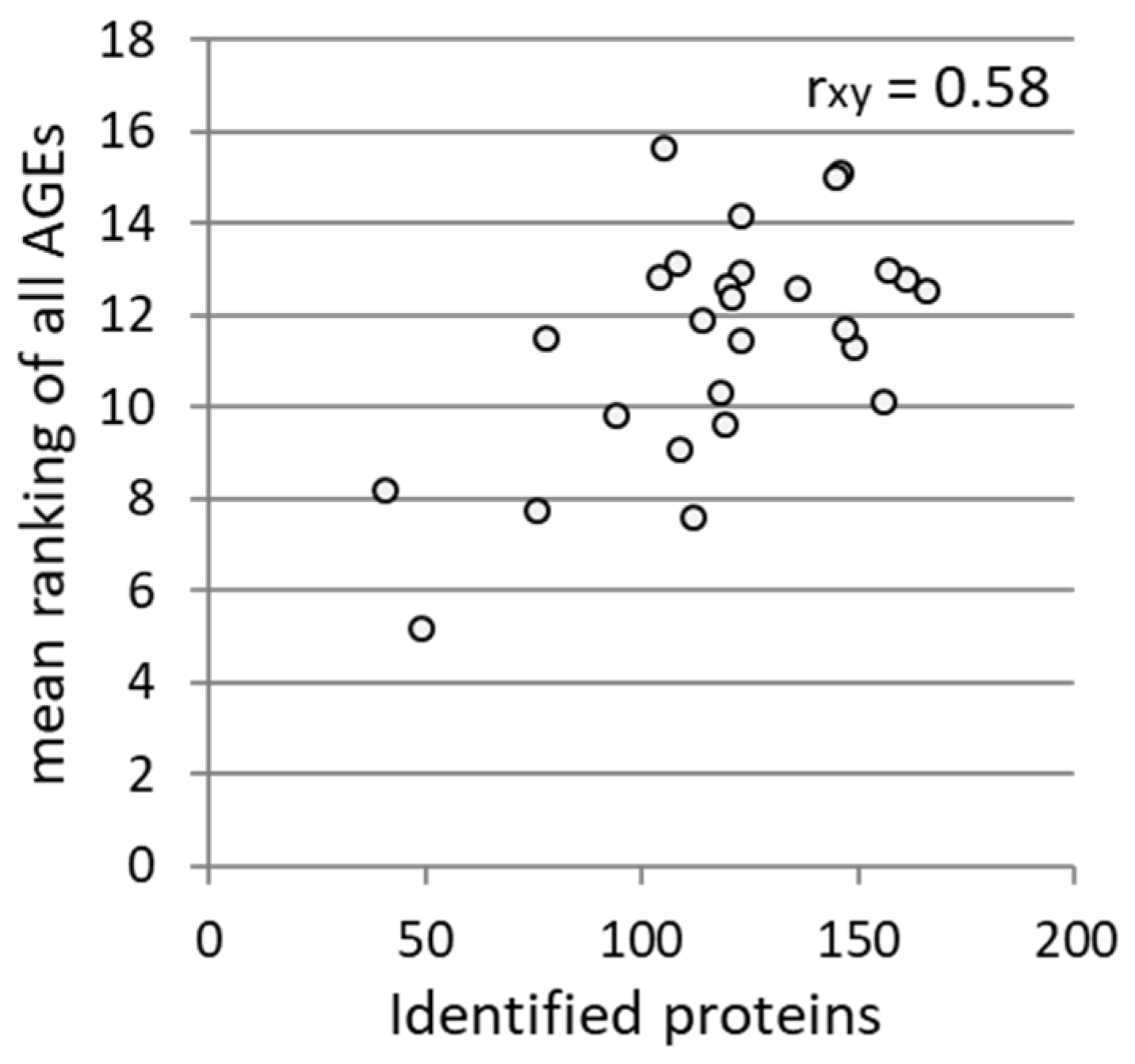
3.2. Association between Different Parameters of Wheat Flour and WBCEs
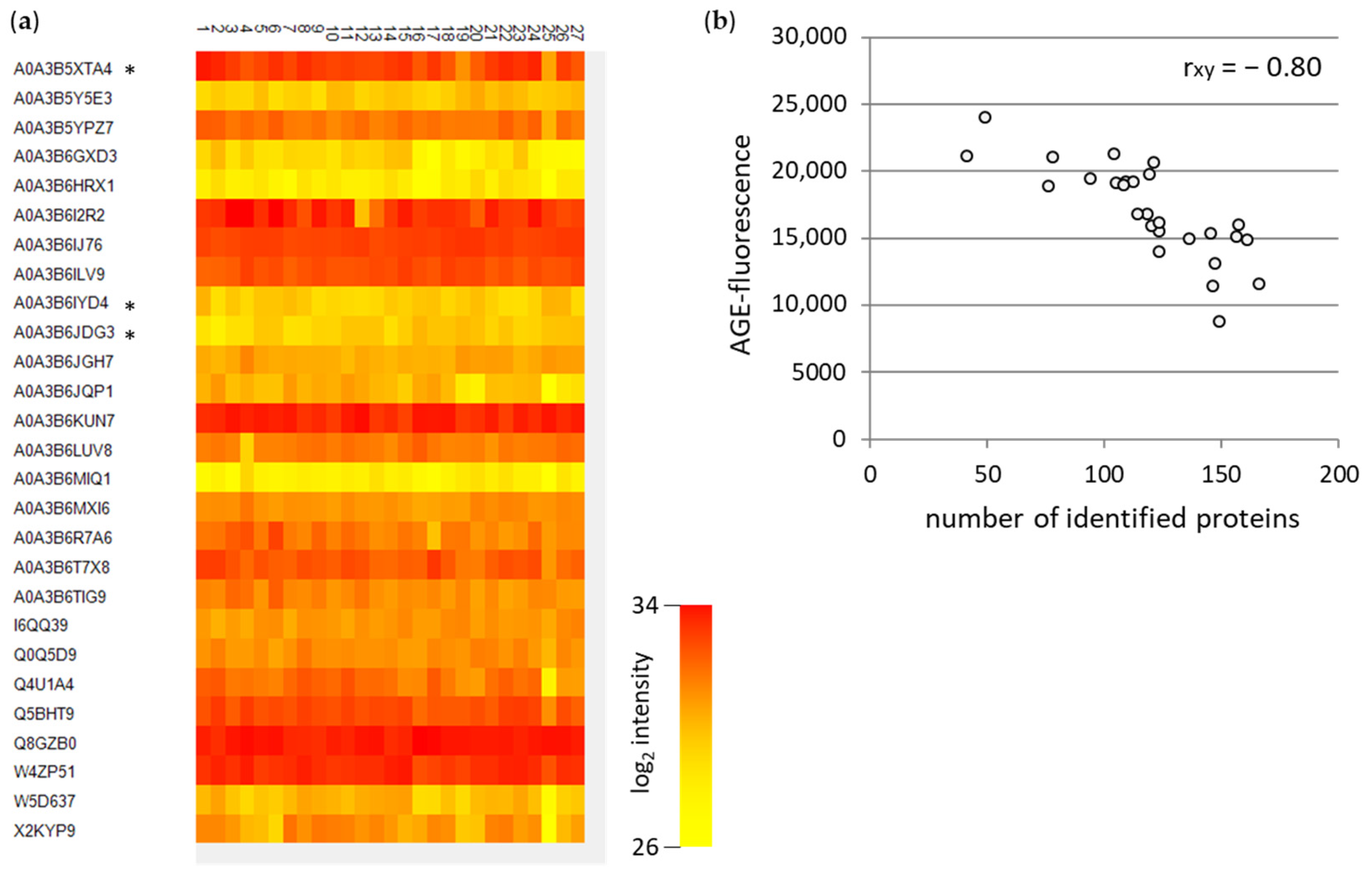
3.3. Mass Spectrometric Analyses of WBCEs
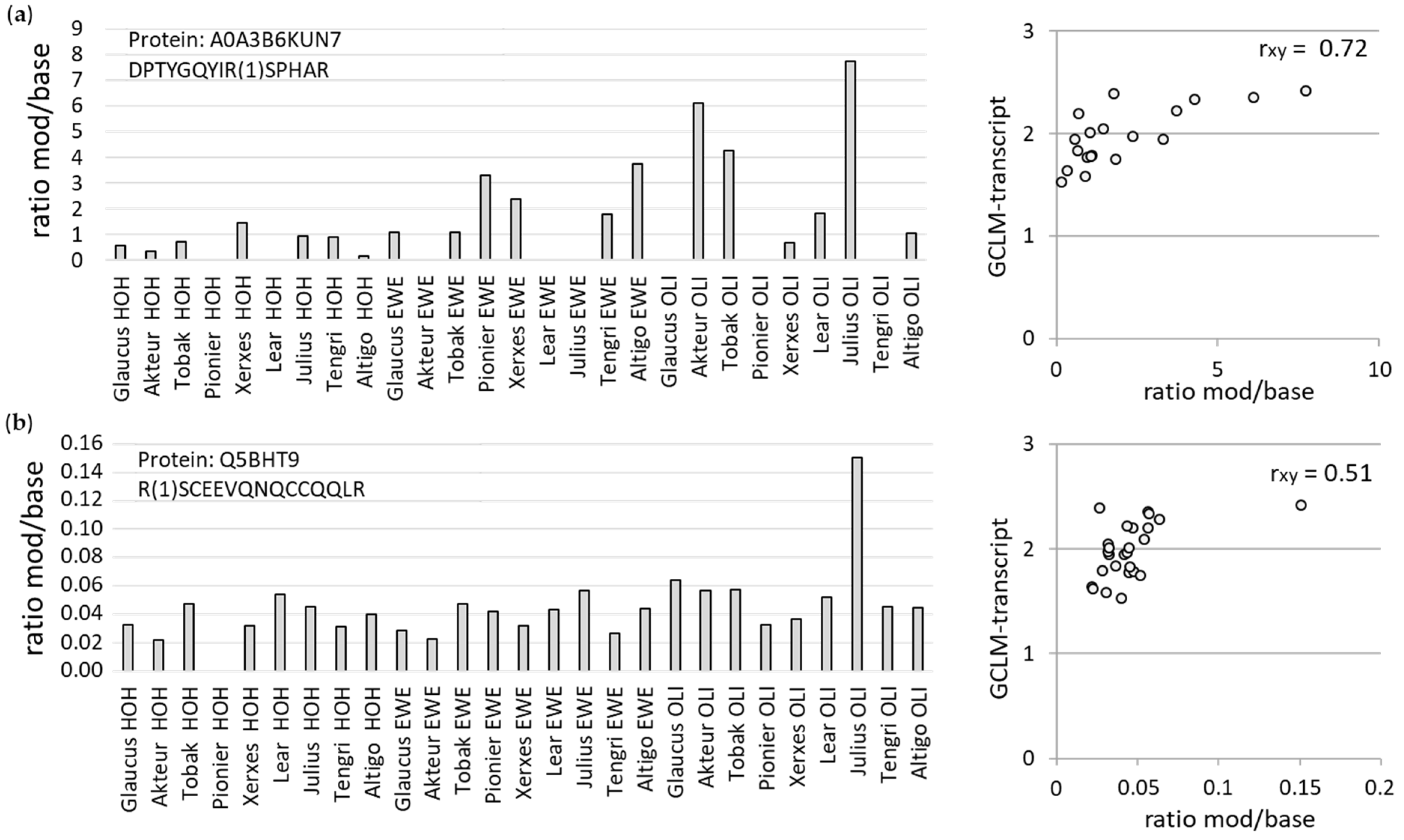
3.3.1. Mass Spectrometric Analyses of Specific AGE Modifications in WBCEs
3.3.2. Mass Spectrometric Analyses of AGE Modifications at Specific Peptides
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-DG | 3-deoxyglucosone |
| AGEs | advanced glycation end products |
| BCE | bread crust extract |
| CEA | carboxyethylarginine |
| EWE | Eckartsweier |
| FA | formic acid |
| FCS | fetal calf serum |
| GCLM | glutamate-cysteine ligase modifier subunit |
| HeLa | human cervical carcinoma |
| Hex | hexose |
| HMOX1 | heme oxygenase 1 |
| HOH | Hohenheim |
| HPLC | high performance liquid chromatography |
| HPLC-ESI-MS/MS | high performance liquid chromatography electrospray tandem mass spectrometry |
| LFQ | label-free quantification |
| MG-H1/2/3 | methylglyoxal-derived hydroimidazolones |
| MGH-DH | dihydroxyimidazolidine |
| Mod | modified |
| MRM | multiple reaction monitoring |
| NRF2 | nuclear factor E2-related factor 2 |
| OLI | Oberer Lindenhof |
| PBS | phosphate-buffered saline |
| qRT-PCR | quantitative Real time-PCR |
| TFA | trifluoroacetic acid |
| THP | tetrahydropyrimidine |
| WBCE | soluble extract from whole-grain wheat bread crust |
| YBX1 | Y-box binding protein 1 |
References
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Qiao, Z.; Sang, S.; Zhang, J.; Zhan, S.; Wu, Z.; Liu, L. Research advances of advanced glycation end products in milk and dairy products: Formation, determination, control strategy and immunometabolism via gut microbiota. Food Chem. 2023, 417, 135861. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef]
- Rice, J.M. The carcinogenicity of acrylamide. Mutat Res. 2005, 580, 3–20. [Google Scholar] [CrossRef]
- Wächter, K.; Navarrete Santos, A.; Großkopf, A.; Baldensperger, T.; Glomb, M.A.; Szabó, G.; Simm, A. AGE-Rich Bread Crust Extract Boosts Oxidative Stress Interception via Stimulation of the NRF2 Pathway. Nutrients 2021, 13, 3874. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Schwär, M.; Loganathan, S.; Wächter, K.; Georgevici, A.-I.; Kraft, P.; Mayer, T.; Simm, A.; Karck, M.; Szabó, G. Bread crust extract protects rats’ vascular grafts from in vitro ischemia/reperfusion injury. J. Funct. Foods 2022, 95, 105187. [Google Scholar] [CrossRef]
- Wächter, K.; Gohde, B.; Szabó, G.; Simm, A. Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo. Nutrients 2022, 14, 4790. [Google Scholar] [CrossRef]
- Sarion, C.; Codină, G.G.; Dabija, A. Acrylamide in Bakery Products: A Review on Health Risks, Legal Regulations and Strategies to Reduce Its Formation. Int. J. Environ. Res. Public Health 2021, 18, 4332. [Google Scholar] [CrossRef]
- Claus, A.; Carle, R.; Schieber, A. Acrylamide in cereal products: A review. J. Cereal Sci. 2008, 47, 118–133. [Google Scholar] [CrossRef]
- Hedegaard, R.V.; Granby, K.; Frandsen, H.; Thygesen, J.; Skibsted, L.H. Acrylamide in bread. Effect of prooxidants and antioxidants. Eur. Food Res. Technol. 2008, 227, 519–525. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Man, Y.; Liu, Y. Reduction of acrylamide content in bread crust by starch coating. J. Sci. Food Agric. 2018, 98, 336–345. [Google Scholar] [CrossRef]
- Jing, Y.; Li, X.; Hu, X.; Ma, Z.; Liu, L.; Ma, X. Effect of buckwheat extracts on acrylamide formation and the quality of bread. J. Sci. Food Agric. 2019, 99, 6482–6489. [Google Scholar] [CrossRef]
- Lingnert, H.; Grivas, S.; Jägerstad, M.; Skog, K.; Törnqvist, M.; Åman, P. Acrylamide in food: Mechanisms of formation and influencing factors during heating of foods. Scand. J. Nutr. 2002, 46, 159–172. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Schwadorf, K.; Leiser, W.L.; Würschum, T.; Longin, C.F.H. Assessing the variation and genetic architecture of asparagine content in wheat: What can plant breeding contribute to a reduction in the acrylamide precursor? Theor. Appl. Genet. 2018, 131, 2427–2437. [Google Scholar] [CrossRef]
- Oddy, J.; Chhetry, M.; Awal, R.; Addy, J.; Wilkinson, M.; Smith, D.; King, R.; Hall, C.; Testa, R.; Murray, E.; et al. Genetic control of grain amino acid composition in a UK soft wheat mapping population. Plant Genome 2023, e20335. [Google Scholar] [CrossRef]
- Shewry, P.; Franklin, J.; Parmar, S.; Smith, S.; Miflin, B. The effects of sulphur starvation on the amino acid and protein compositions of barley grain. J. Cereal Sci. 1983, 1, 21–31. [Google Scholar] [CrossRef]
- Wessel, D.; Flugge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; de Godoy, L.M.F.; Li, G.; Macek, B.; Mortensen, P.; Pesch, R.; Makarov, A.; Lange, O.; Horning, S.; Mann, M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell Proteom. 2005, 4, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Ulrich, P.; Cerami, A. Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 2001, 56, 1–21. [Google Scholar] [CrossRef]
- LoPachin, R.M., Jr.; Lehning, E.J. Acrylamide-induced distal axon degeneration: A proposed mechanism of action. Neurotoxicology 1994, 15, 247–259. [Google Scholar]
- Zhang, B.; Zhao, M.; Ji, X.; Xia, Q.; Jiang, L.; Zhao, L. Acrylamide induces neurotoxicity in zebrafish (Danio rerio) via NLRP3-mediated pyroptosis. Sci. Total Environ. 2023, 896, 165208. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Some Industrial Chemicals; IARC: Lyon, France, 1994. [Google Scholar]
- Surdyk, N.; Rosén, J.; Andersson, R.; Åman, P. Effects of asparagine, fructose, and baking conditions on acrylamide content in yeast-leavened wheat bread. J. Agric. Food Chem. 2004, 52, 2047–2051. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of Oxidative Stress in Development of Complications in Diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Structure Elucidation of a Senescence Cross-Link from Human Extracellular Matrix: Implication of Pentoses in the Aging Process*. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [CrossRef] [PubMed]
- Corol, D.I.; Ravel, C.; Rakszegi, M.; Charmet, G.; Bedo, Z.; Beale, M.H.; Shewry, P.R.; Ward, J.L. (1)H-NMR screening for the high-throughput determination of genotype and environmental effects on the content of asparagine in wheat grain. Plant Biotechnol. J. 2016, 14, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Muttucumaru, N.; Halford, N.G.; Elmore, J.S.; Dodson, A.T.; Parry, M.; Shewry, P.R.; Mottram, D.S. Formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 2006, 54, 8951–8955. [Google Scholar] [CrossRef] [PubMed]
- Claus, A.; Schreiter, P.; Weber, A.; Graeff, S.; Herrmann, W.; Claupein, W.; Schieber, A.; Carle, R. Influence of agronomic factors and extraction rate on the acrylamide contents in yeast-leavened breads. J. Agric. Food Chem. 2006, 54, 8968–8976. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Rankenberg, J.; Glomb, M.A.; Nagaraj, R.H. Transient elevation of temperature promotes cross-linking of alpha-crystallin-client proteins through formation of advanced glycation endproducts: A potential role in presbyopia and cataracts. Biochem. Biophys. Res. Commun. 2020, 533, 1352–1358. [Google Scholar] [CrossRef]
- Tian, W.; Zheng, Y.; Wang, W.; Wang, D.; Tilley, M.; Zhang, G.; He, Z.; Li, Y. A comprehensive review of wheat phytochemicals: From farm to fork and beyond. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2274–2308. [Google Scholar] [CrossRef]
- Demirok, E.; Kolsarıcı, N. Effect of green tea extract and microwave pre-cooking on the formation of acrylamide in fried chicken drumsticks and chicken wings. Food Res. Int. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Morales, G.; Jimenez, M.; Garcia, O.; Mendoza, M.R.; Beristain, C.I. Effect of natural extracts on the formation of acrylamide in fried potatoes. LWT -Food Sci. Technol. 2014, 58, 587–593. [Google Scholar] [CrossRef]
- Ou, S.; Shi, J.; Huang, C.; Zhang, G.; Teng, J.; Jiang, Y.; Yang, B. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Gökmen, V. Evaluation of the Maillard reaction in potato crisps by acrylamide, antioxidant capacity and color. J. Food Compos. Anal. 2009, 22, 589–595. [Google Scholar] [CrossRef]
| Wheat Cultivar | Growth Location |
|---|---|
| Glaucus | HOH, EWE, OLI |
| Akteur | HOH, EWE, OLI |
| Tobak | HOH, EWE, OLI |
| Pionier | HOH, EWE, OLI |
| Xerxes | HOH, EWE, OLI |
| Lear | HOH, EWE, OLI |
| Julius | HOH, EWE, OLI |
| Tengri | HOH, EWE, OLI |
| Altigo | HOH, EWE, OLI |
| Grain | Dough Resting | Baking Time | Top Heat | Bottom Heat |
|---|---|---|---|---|
| wheat | 30 min | 50–60 min | 250 | 230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wächter, K.; Longin, C.F.H.; Winterhalter, P.R.; Bertsche, U.; Szabó, G.; Simm, A. The Antioxidant Potential of Various Wheat Crusts Correlates with AGE Content Independently of Acrylamide. Foods 2023, 12, 4399. https://doi.org/10.3390/foods12244399
Wächter K, Longin CFH, Winterhalter PR, Bertsche U, Szabó G, Simm A. The Antioxidant Potential of Various Wheat Crusts Correlates with AGE Content Independently of Acrylamide. Foods. 2023; 12(24):4399. https://doi.org/10.3390/foods12244399
Chicago/Turabian StyleWächter, Kristin, Carl Friedrich H. Longin, Patrick R. Winterhalter, Ute Bertsche, Gábor Szabó, and Andreas Simm. 2023. "The Antioxidant Potential of Various Wheat Crusts Correlates with AGE Content Independently of Acrylamide" Foods 12, no. 24: 4399. https://doi.org/10.3390/foods12244399
APA StyleWächter, K., Longin, C. F. H., Winterhalter, P. R., Bertsche, U., Szabó, G., & Simm, A. (2023). The Antioxidant Potential of Various Wheat Crusts Correlates with AGE Content Independently of Acrylamide. Foods, 12(24), 4399. https://doi.org/10.3390/foods12244399







