Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety
Abstract
:1. Introduction
2. Classification of Hydrogels for Sensors
3. Recent Application in Food Safety
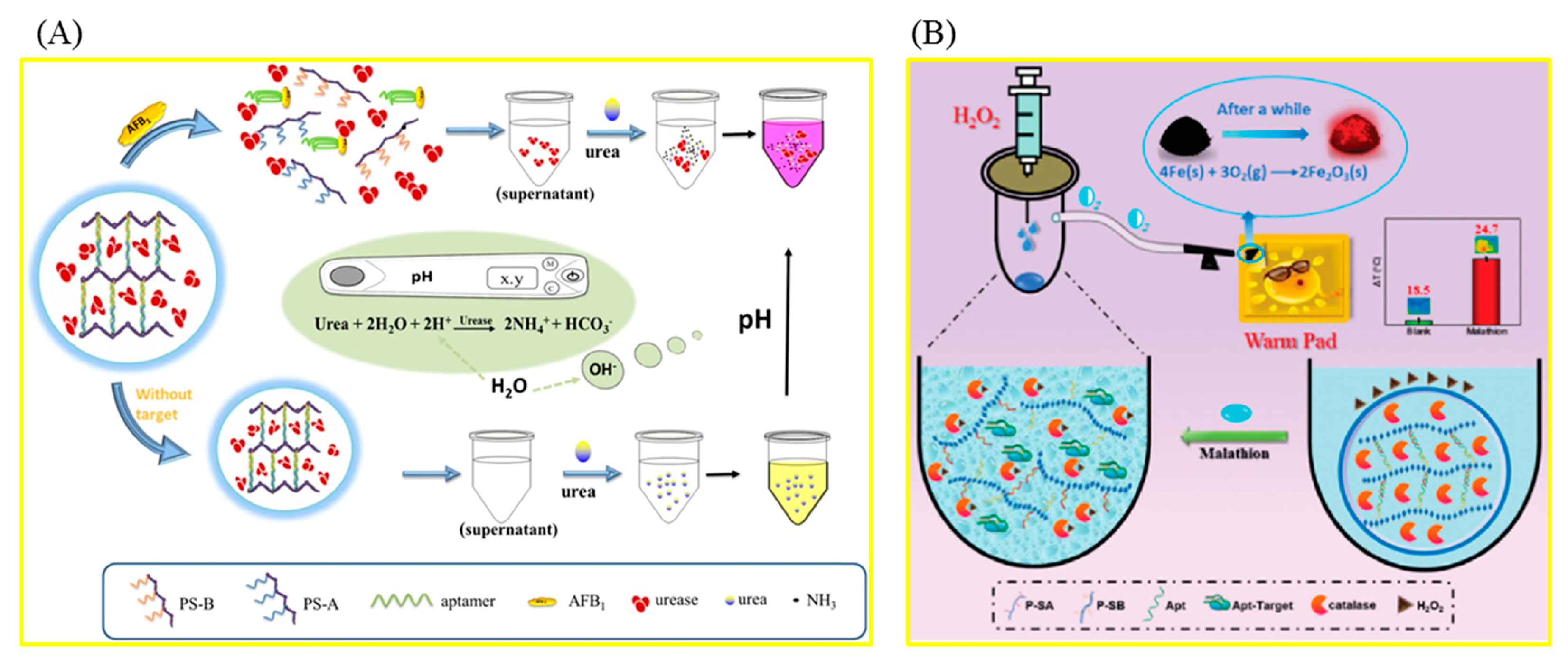
3.1. Biotoxins
3.2. Pesticide Residues
3.3. Antibiotic Residues
3.4. Pathogenic Bacteria
3.5. Heavy Metals
3.6. Food Quality Indication
3.7. Other Applications
| Target | Hydrogel | Function | Method | Detection Range | LOD | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Biotoxins | |||||||
| AFB1 | DNA hydrogel | Encapsulate aptamer, controlled release system | pH | 0.2–20 µmol/L | 0.1 µmol/L | Corn, peanut | [45] |
| OTA | DNA hydrogel | Encapsulate aptamer | Fluorescence | 0.05–100 ng/mL | 0.01 ng/mL | Beer | [47] |
| T-2 toxin | DNA hydrogel | Target-responsive to release HRP | Fluorescence | 0.01–10,000 ng/mL | 0.87 pg/mL | Coffee, corn, soybean | [49] |
| CT | Supramolecular hydrogel | Target-responsive | Color-changing | 0–5 µmol/L | - | Water | [50] |
| OTA | 3D graphene hydrogel | Supporting nanoparticle | Photoelectrochemical | 1–100 ng/mL | 0.29 ng/mL | Corn juice | [51] |
| Pesticide residues | |||||||
| Paraoxon-ethyl | Polydopamin-capped AuPt hydrogel | Immobilize AChE | Electrochemical | 0.5–1000 ng/L | 0.185 ng/L | Tap water, lake water | [55] |
| Chlorpyrifos | Chitosan hydrogel | - | Chemiluminescence | 0.5–1000 ng/mL | 0.21 ng/mL | Pakchoi | [56] |
| Fenthion | AChE-MnO2@HPH | Immobilize AChE | Color information | 4–400 ng/mL | 0.63 ng/mL | Rice, wheat | [59] |
| Paraoxon | DNA hydrogel | Encapsulate CuNPs | Fluorescence | 0.1–1000 ng/mL | 0.0333 ng/mL | Tap water | [60] |
| Malathion | 3D DNA hydrogel | Encapsulate catalase | Thermal | 0.0001–10 ng/mL | 0.032 pg/mL | - | [61] |
| Paraoxon | Alginate hydrogel | Carrier | Colorimetric | 0.397–79.4 ng/mL | 0.115 ng/mL | Six kinds b | [62] |
| Antibiotic residues | |||||||
| Kanamycin | DNA hydrogel | Encapsulate GCNPs | SERS | 1–104 pg/L | 2.3 fmol/L | Milk, honey | [66] |
| Streptomycin | DNA hydrogel | Incorporate DNAzyme, target-responsive | SERS | 0.01–150 nmol/L | 4.85 pmol/L | Milk, honey | [67] |
| Tetracycline | Molecularly imprinted hydrogel | Encapsulate carbon dots, absorb tetracycline | Fluorescence | 0.2–1.0 µg/L | 0.11 µg/L | Tap water, lake water | [68] |
| Pathogenic bacteria | |||||||
| E. coli | PVA/PAA hydrogel | pH-sensitive hydrogel | pH | 102–106 CFU/mL | 102 CFU/mL | Orange juice | [74] |
| E. coli | 3D graphene hydrogel | Encapsulate carbon dots | Photoelectrochemical | 2.9–2.9 × 106 CFU/mL | 0.66 CFU/mL | Milk | [76] |
| Salmonella typhi, E. coli | Cross-linked PEG hydrogel | Conduct LAMP inside | Fluorescence | 1–640 copy/µL | 0.4 copy/µL | Fruit, vegetable | [77] |
| Heavy metals | |||||||
| Pb2+ | PNBC hydrogel a | Embed Fe3O4 | Fluorescence | 10−3–10 mmol/L | - | Water | [81] |
| Pb2+ | DNA hydrogel | Target-responsive | Distance and time | 0.01–50 µmol/L | 10 nmol/L | Tap water | [82] |
| Pb2+ | DNA hydrogel | Target-responsive | Distance | 0–200 nmol/L | 0.3 nmol/L | Lake water, Tap water | [83] |
| Food quality indication | |||||||
| CO2 | Nanocellulose hydrogel | pH indicator | Colorimetric | 0.1–56.5% (v/v) | / | Chicken breast | [85] |
| Total mesophilic counts | Cellulose/chitosan | pH indicator | Colorimetric | 3–7.65 (log10 CFU/mL) | / | Milk | [91] |
| Other applications | |||||||
| Melamine | PEGDA hydrogel micropellet | Encapsulate MNPs | SERS | 10−8–10−3 mol/L | 10 nmol/L | Milk | [99] |
| Wheat gliadin | Conductive hydrogels | Immobilize cell | Electrochemical | 0.1–0.8 ng/mL | 0.036 ng/ml | Gluten-free flour and cookies | [103] |
| Hydrogel | Target | Function | Method | Ref. |
|---|---|---|---|---|
| DNA hydrogel | AFB1, OTA, Paraoxon, Malathion, T-2 toxin, Kanamycin, Streptomycin, Pb2+ | Encapsulate aptamer, controlled release system, target-responsive | pH, fluorescence, thermal, SERS, distance and time | [45,47,49,60,61,66,67,82,83] |
| 3D graphene hydrogel | OTA, E. coli | Supporting nanoparticles, encapsulate carbon dots | Photoelectrochemical | [51,76] |
| Chitosan hydrogel | Chlorpyrifos | pH indicator | Chemiluminescence, colorimetric | [56] |
| Supramolecular hydrogel | CT | Target-responsive | Color-changing | [50] |
| Molecularly imprinted hydrogel | Tetracycline | Encapsulate carbon dots, absorb tetracycline | Fluorescence | [68] |
| Cross-linked PEG hydrogel | Salmonella typhi, E. coli | Conduct LAMP inside | Fluorescence | [77] |
4. Conclusions and Future Prospects
- 1.
- The development of hydrogel-based sensors is limited by the structure and function of polymers, so new cross-linking methods should be used to design and synthesize polymer networks to achieve precise, targeted regulation of functions;
- 2.
- Simulation technology (such as Monte Carlo, molecular dynamics, and multiphysics simulation) can be applied to the structural design and structure–activity relationship interpretation of hydrogel biosensors;
- 3.
- Multiple-target detection platforms should be built to meet the needs of multi-channel fast detection;
- 4.
- In order to optimize the structure of hydrogels and improve the stability and selectivity of hydrogel biosensors, it is necessary to study the interaction mechanism between hydrogels and food substrates;
- 5.
- With the rapid advancement of big data and portable devices, miniaturization and wearable intelligent instruments should be developed to meet the needs of on-site real-time monitoring and rapid government regulation;
- 6.
- The continuous dynamic real-time monitoring device should be developed by making full use of the structural characteristics of hydrogels;
- 7.
- In order to reduce the cost of hydrogel biosensors, new multifunctional hydrogels should be developed;
- 8.
- At present, ethics and privacy are not involved in biosensors in food safety, but in promoting the development of biosensor technology, they will also be included in our future considerations to ensure the proper and safe use of technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, P.; Mohd Noor, N.Q.I.; Shaarani, S.M. Current status of food safety hazards and health risks connected with aquatic food products from Southeast Asian region. Crit. Rev. Food Sci. Nutr. 2022, 62, 3471–3489. [Google Scholar] [CrossRef]
- Griesche, C.; Baeumner, A.J. Biosensors to support sustainable agriculture and food safety. Trends Anal. Chem. 2020, 128, 115906. [Google Scholar] [CrossRef]
- Lin, X.; Yan, H.; Zhao, L.; Duan, N.; Wang, Z.; Wu, S. Hydrogel-integrated sensors for food safety and quality monitoring: Fabrication strategies and emerging applications. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- van Asselt, E.D.; Arrizabalaga-Larrañaga, A.; Focker, M.; Berendsen, B.J.A.; van de Schans, M.G.M.; van der Fels-Klerx, H.J. Chemical food safety hazards in circular food systems: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10319–10331. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.; Palma, P.; Godoy, M.; Vidal, M.; Rivera, A. Co-occurrence and estimation of the risk of total aflatoxins (B1, B2, G1, and G2) and ochratoxin A in agri-food products consumed in Chile. Food Control 2023, 146, 109493. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Q.; Zhang, R.; Zhao, Z.; Leng, Y.; Lam, J.W.Y.; Xiong, Y.; Tang, B.Z. AIEgens: An emerging fluorescent sensing tool to aid food safety and quality control. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2297–2329. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, D.; Bartkevics, V. Recent applications of nano-liquid chromatography in food safety and environmental monitoring: A review. Crit. Rev. Anal. Chem. 2023, 53, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Pérez-Míguez, R.; Montero, L.; Herrero, M. Application of mass spectrometry-based metabolomics approaches for food safety, quality and traceability. Trends Anal. Chem. 2017, 93, 102–118. [Google Scholar] [CrossRef]
- Weng, R.; Lou, S.; Pang, X.; Song, Y.; Su, X.; Xiao, Z.; Qiu, J. Multi-residue analysis of 126 pesticides in chicken muscle by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem. 2020, 309, 125503. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of structures and applications in food science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Payal, A.; Krishnamoorthy, S.; Elumalai, A.; Moses, J.A.; Anandharamakrishnan, C. A review on recent developments and applications of nanozymes in food safety and quality analysis. Food Anal. Methods 2021, 14, 1537–1558. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, Q.; Wu, D.; Yang, Y.; Zhang, Y.; Tang, X. A facile electrochemical method for rapid determination of 3-chloropropane-1,2-diol in soy sauce based on nanoporous gold capped with molecularly imprinted polymer. Food Control 2022, 134, 108750. [Google Scholar] [CrossRef]
- Tan, J.S.; Dai, Z.R.; Zhou, K.M.; Zhang, L.; He, M.; Tan, Y.D.; Zhou, X.H. An ultrasensitive and universal surface plasmonic biosensor for detection of micropollutants in aquatic environments. Environ. Sci. Technol. 2023, 57, 8313–8322. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Lai, K.; Yan, J. Stimulus-responsive DNA hydrogel biosensors for food safety detection. Biosensors 2023, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, L.; Mcclements, D.J.; Qiu, C.; Li, C.; Zhang, Z.; Miao, M.; Tian, Y.; Zhu, K.; Jin, Z. Stimulus-responsive hydrogels in food science: A review. Food Hydrocoll. 2022, 124, 107218. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications-A Review. ACS Appl. Bio. Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef]
- Su, D.; Zhao, X.; Yan, X.; Han, X.; Zhu, Z.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; Liu, X.; et al. Background-free sensing platform for on-site detection of carbamate pesticide through upconversion nanoparticles-based hydrogel suit. Biosens. Bioelectron. 2021, 194, 113598. [Google Scholar] [CrossRef]
- Muthukumaran, P. Application of nanobiosensor in food-A coomprehensive review. J. Pharm. Biol. Sci. 2018, 6, 39–46. [Google Scholar] [CrossRef]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and their applications in detection of organophosphorus pesticides in the environment. Arch. Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Fu, R.; Liu, H.; Zhou, J.; Zhao, Q.; Wang, C.; Jiao, B.; He, Y. A review of portable biosensors for the field detection of mycotoxins. Food Sci. 2022, 43, 234–245. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, G.; Li, Y.; Song, Y.; Wen, Y.; Zhang, X. Development and Application of DNA Hydrogel in Biosensing. Prog. Chem. 2021, 33, 1887–1899. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Zhang, Y.; Liu, Z. Natural biopolymers for flexible sensing and energy devices. Chin. J. Poly Sci. 2020, 38, 459–490. [Google Scholar] [CrossRef]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent progress in natural biopolymers conductive hydrogels for flexible wearable sensors and energy devices: Materials, structures, and performance. ACS Appl. Bio. Mater. 2021, 4, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Ma, L.; Long, T.; Yuan, S.D.; Qi, P.; Han, L.; Hao, J.C. A pH-indicating smart tag based on porous hydrogel as food freshness sensors. J. Colloid. Interface Sci. 2023, 647, 32–42. [Google Scholar] [CrossRef]
- Li, G.S.; Liu, Z.; Feng, J.X.; Zhou, G.Y.; Huang, X.G. Pb2+ fiber optic sensor based on smart hydrogel coated Mach-Zehnder interferometer. Opt. Laser Technol. 2022, 145, 107453. [Google Scholar] [CrossRef]
- Salem, K.S.; Naithani, V.; Jameel, H.; Lucia, L.; Pal, L. Lignocellulosic fibers from renewable resources using green chemistry for a circular economy. Glob. Chall. 2021, 5, 2000065. [Google Scholar] [CrossRef]
- Dhanjai; Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC Trends Anal. Chem. 2019, 118, 488–501. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Liu, D.; Jia, S.; Zhang, H.; Ma, Y.; Guan, Z.; Li, J.; Zhu, Z.; Ji, T.; Yang, C.J. Integrating target-responsive hydrogel with pressuremeter readout enables simple, sensitive, user-Friendly, quantitative point-of-care testing. ACS Appl. Mater. Interfaces 2017, 9, 22252–22258. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.S.S.; Freitas, M.M.; Cerqueira, M.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Haqasif, A.; Karnakar, R.R.; Sreeharsha, N.; Gite, V.V.; Meravanige, G. pH and salt responsive hydrogel based on Guar gum as a renewable material for delivery of curcumin: A natural anti-cancer drug. J. Polym. Environ. 2021, 29, 1978–1989. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent advances in cellulose-based hydrogels: Food applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Joshi, D.J.; Kateshiya, M.R.; Koduru, J.R.; Malek, N.I. Review on the biomedical and sensing applications of nanomaterial-incorporated hydrogels. Mater. Today Chem. 2022, 23, 100746. [Google Scholar] [CrossRef]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing cellulose hydrogels from non-woody biomass. Carbohydr. Polym. 2021, 339, 118036–118045. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Yang, S.; Li, S.; Gao, Z. Design and application of stimuli-responsive DNA hydrogels: A review. Mater. Today. Bio. 2022, 16, 100430. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zuo, J.S.; Yang, C.; Jiang, J.; Zhang, Q.; Ping, J.F.; Li, P.W. Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins):principles, application, and perspective. Trends Anal. Chem. 2023, 165, 117144. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Mwanza, M. Aflatoxins in Maize: Can their occurrence be effectively managed in Africa in the face of climate change and food insecurity? Toxins 2022, 14, 574. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- Ma, Y.; Mao, Y.; Huang, D.; He, Z.; Yan, J.; Tian, T.; Shi, Y.; Song, Y.; Li, X.; Zhu, Z.; et al. Portable visual quantitative detection of aflatoxin B1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab. A Chip 2016, 16, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, P.; Guo, Y.; Wang, L.; Luo, F.; Qiu, B.; Guo, L.; Su, X.; Lin, Z.; Chen, G. Detection of aflatoxin B(1) in food samples based on target-responsive aptamer-cross-linked hydrogel using a handheld pH meter as readout. Talanta 2018, 176, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Huang, Y.; Ma, Y.; Jia, S.; Gao, M.; Li, J.; Zhang, H.; Xu, D.; Wu, M.; Chen, Y.; et al. Design and synthesis of target-responsive aptamer-cross-linked hydrogel for visual quantitative detection of ochratoxin A. ACS Appl. Mater. Interfaces 2015, 7, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, W.; Shen, X.; Wang, S.; Li, Q.; An, F.; Wu, S. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of Ochratoxin A. J. Agric. Food Chem. 2020, 68, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Zhao, S.; Niazi, S.; Mohsin, A.; Shoaib, M.; Duan, N.; Wu, S.; Wang, Z. Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sens. Actuators B Chem. 2018, 277, 328–335. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Chen, R.; Wu, P.; Liang, J. Ultrasensitive and rapid detection of T-2 toxin using a target-responsive DNA hydrogel. Sens. Actuators B Chem. 2020, 311, 127912. [Google Scholar] [CrossRef]
- Biswakarma, D.; Dey, N.; Bhattacharya, S. A thermo-responsive supramolecular hydrogel that senses cholera toxin via color-changing response. Chem. Commun. 2020, 56, 7789–7792. [Google Scholar] [CrossRef]
- Hao, N.; Dai, Z.; Meng, X.; Hua, R.; Lu, J.; Wang, K. A portable solar-driven ratiometric photo-electrochromic visualization biosensor for detection of ochratoxin A. Sens. Actuators B Chem. 2020, 306, 127594. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Cui, H.F.; Zhang, T.T.; Lv, Q.Y.; Song, X.; Zhai, X.J.; Wang, G.G. An acetylcholinesterase biosensor based on doping Au nanorod@SiO2 nanoparticles into TiO2-chitosan hydrogel for detection of organophosphate pesticides. Biosens. Bioelectron. 2019, 141, 111452. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Kong, D.; Yan, X.; Zhao, X.; Li, H.; Liu, F.; Sun, P.; Lin, Y.; Lu, G. Integrating target-responsive hydrogels with smartphone for on-site ppb-level quantitation of organophosphate pesticides. ACS Appl. Mater. Interfaces 2019, 11, 27605–27614. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-capped bimetallic aupt hydrogels enable robust biosensor for organophosphorus pesticide detection. Small 2019, 15, 1900632. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wei, M.; Wang, C.; Wei, W.; Liu, Y. Enhancing hydrogel-based long-lasting chemiluminescence by a platinum-metal organic framework and its application in array detection of pesticides and d-amino acids. Nanoscale 2020, 12, 4959–4967. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Wang, F.; Li, Q.; Yan, X.; Liu, M.; Chen, Y.; Zhou, W.; Gao, H.; Sun, P.; Lu, G. Construction of multienzyme-hydrogel sensor with smartphone detector for on-site monitoring of organophosphorus pesticide. Sens. Actuators B Chem. 2021, 327, 128922. [Google Scholar] [CrossRef]
- Yan, X.; Wang, T.; Li, H.; Zhang, L.; Xin, H.; Lu, G. Flexible aggregation-induced emission-active hydrogel for on-site monitoring of pesticide degradation. ACS Nano 2022, 16, 18421–18429. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, P.; Xu, L.; Wang, M.; Pan, J.; Niu, X. Emulsion-templated construction of enzyme-nanozyme integrated hierarchically porous hydrogels for smartphone-assisted pesticide biosensing. Chem. Eng. J. 2022, 433, 133669. [Google Scholar] [CrossRef]
- Chen, J.; Han, T.; Feng, X.; Wang, B.; Wang, G. A poly(thymine)-templated fluorescent copper nanoparticle hydrogel-based visual and portable strategy for an organophosphorus pesticide assay. Analyst 2019, 144, 2423–2429. [Google Scholar] [CrossRef]
- Tang, J.; Liu, L.; Gao, S.; Qin, J.; Liu, X.; Tang, D. A portable thermal detection method based on the target responsive hydrogel mediated self-heating of a warming pad. Chem. Commun. 2021, 57, 9862–9865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, L.; Hu, P.; Liu, B.; Wang, M.; Yin, X.; Pan, J.; Niu, X. Smartphone-assisted bioenzyme-nanozyme-chromogen all-in-one test strip with enhanced cascade signal amplification for convenient paraoxon sensing. Biosens. Bioelectron. 2022, 215, 114583. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhao, L.; Yan, X.; Han, X.; Lu, G. Lab in hydrogel portable kit: On-site monitoring of oxalate. Biosens. Bioelectron. 2020, 167, 112457. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Yaseri, M.; Faraji, M. Removal of antibiotics from aqueous solutions by nanoparticles: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 8444–8458. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Rathinasabapathi, P. A short review on detection of antibiotics in milk using nanomaterial-based biosensor. Food Anal. Methods 2022, 15, 2181–2192. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, H.; Du, L.; Gan, X.; Quan, X. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens. Bioelectron. 2016, 83, 267–273. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, R.; Liu, G.; Wen, Y.; Bian, X.; Luan, D.; Wang, H.; Lai, K.; Yan, J. Fishing unfunctionalized SERS tags with DNA hydrogel network generated by ligation-rolling circle amplification for simple and ultrasensitive detection of kanamycin. Biosens. Bioelectron. 2022, 207, 114187. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. Ultra-sensitive detection of streptomycin in foods using a novel SERS switch sensor fabricated by AuNRs array and DNA hydrogel embedded with DNAzyme. Food Chem. 2022, 393, 133413. [Google Scholar] [CrossRef]
- Luo, Q.; He, S.; Huang, Y.; Lei, Z.; Qiao, J.; Li, Q.; Xu, D.; Guo, X.; Wu, Y. Non-toxic fluorescent molecularly imprinted hydrogel based on wood-derived cellulose nanocrystals and carbon dots for efficient sorption and sensitive detection of tetracycline. Ind. Crops Prod. 2022, 177, 114528. [Google Scholar] [CrossRef]
- Fengou, L.C.; Spyrelli, E.; Lianou, A.; Tsakanikas, P.; Panagou, E.Z.; Nychas, G.J.E. Estimation of minced pork microbiological spoilage through fourier transform infrared and visible spectroscopy and multispectral vision technology. Foods 2019, 8, 238. [Google Scholar] [CrossRef]
- Xi, S.; Chuang, G.; Li, C.; Yi, X. Hydrogel-based sensing detection of bacteria. Prog. Chem. 2020, 32, 1908–1916. [Google Scholar] [CrossRef]
- Xiong, H.; Zheng, H.; Wang, W.; Liang, J.; Wen, W.; Zhang, X.; Wang, S. A convenient purification method for silver nanoclusters and its applications in fluorescent pH sensors for bacterial monitoring. Biosens. Bioelectron. 2016, 86, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli (E. coli) with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.S.; Dohm, N.; Müller, M.; Jansen, B.; Schönherr, H. Self-reporting hydrogels rapidly differentiate among enterohemorrhagic Escherichia coli (EHEC) and non-virulent Escherichia coli (K12). Eur. Polym. J. 2016, 81, 257–265. [Google Scholar] [CrossRef]
- Hua, R.; Hao, N.; Lu, J.; Qian, J.; Liu, Q.; Li, H.; Wang, K. A sensitive potentiometric resolved ratiometric photoelectrochemical aptasensor for Escherichia coli detection fabricated with non-metallic nanomaterials. Biosens. Bioelectron. 2018, 106, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Fang, M.; Yi, C.Y.; Jiang, Y.; Zhang, C.; Pan, X.; Luo, Z. Functional hydrogel for fast, precise and inhibition-free point-of-care bacteria analysis in crude food samples. Biomaterials 2022, 280, 121278. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Chen, Y.; Wu, X.; Fang, L.; Zhu, Z.; Yang, C.J. Target-responsive DNAzyme cross-linked hydrogel for visual quantitative detection of lead. Anal. Chem. 2014, 86, 11434–11439. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, H.; Xiong, Q.; Zhang, Y.; Zhao, H.; Wang, G. A fluorescent chitosan hydrogel detection platform for the sensitive and selective determination of trace mercury(II) in water. J. Mater. Chem. 2015, 3, 19455–19460. [Google Scholar] [CrossRef]
- Li, W.; Jiang, C.; Lu, S.; Wang, F.; Zhang, Z.; Wei, T.; Chen, Y.; Qiang, J.; Yu, Z.; Chen, X. A hydrogel microsphere-based sensor for dual and highly selective detection of Al3+ and Hg2+. Sens. Actuators B Chem. 2020, 321, 128490. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, H.R.; Wen, J.Y.; Wang, Y.L.; Liang, T.; Cheng, C.J. A novel ion-responsive photonic hydrogel sensor for portable visual detection and timely removal of lead ions in water. Mater. Adv. 2022, 3, 5393–5405. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Wang, H.; Chen, D.; Wen, Y. A portable visual capillary sensor based on functional DNA crosslinked hydrogel for point-of-care detection of lead ion. Sens. Actuators B Chem. 2020, 307, 127625. [Google Scholar] [CrossRef]
- Liu, J.P.; Bi, Y.H.; Tai, W.J.; Wei, Y.; Zhang, Q.; Liu, A.N.; Hu, Q.Z.; Yu, L. The development of a paper-based distance sensor for the detection of Pb2+assisted with the target-responsive DNA hydrogel. Talanta 2023, 257, 124344. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Guo, H.; Sun, X. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens. Bioelectron. 2019, 126, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shen, Q.; Song, T.; Zhao, H.; Zhang, Y.; Ren, A.; Yang, W. Facile fabrication of anthocyanin-nanocellulose hydrogel indicator label for intelligent evaluation of minced pork freshness. Foods 2023, 12, 2602. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cheng, J.; Yang, F.; Hu, Z.; Zheng, Z.; Deng, Y.; Cao, B.; Xie, Y. Visual colorimetric detection of edible oil freshness for peroxides based on nanocellulose. Foods 2023, 12, 1896. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT-Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Tirtashi, F.E.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.Y.; Lu, D.C.; She, Q.T.; You, R.Y.; Feng, S.Y.; Lin, X.L.; Lu, Y.D. Reusable 3D silver superposed silica SERS substrate based on the Griess reaction for the ratiometric detection of nitrite. Anal. Bioanal. Chem. 2021, 413, 4751–4761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhao, C.X.; Yue, G.Z.; Yang, Z.P.; Wang, Y.Y.; Rao, H.B.; Zhang, W.; Jin, B.; Wang, X.X. A highly selective chromogenic probe for the detection of nitrite in food samples. Food Chem. 2020, 317, 126361. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.G.; Teng, P.; Peng, L.; Ji, H.; Qiu, Y.W.; Liu, X.X.; Guo, D.W.; Jiang, S.X. Development and validation of an ultra-performance liquid chromatography-tandem mass spectrometry method to determine maduramicin in crayfish (Procambarus clarkii) and evaluate food safety. Foods 2021, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Ferey, L.; Delaunay, N. Food analysis on electrophoretic microchips. Sep. Purif. Rev. 2016, 45, 193–226. [Google Scholar] [CrossRef]
- Hoysrijan, B.; Sirikulkajorn, A. Colorimetric and fluorogenic detection of nitrite anion in water and food based on Griess reaction of fluorene derivatives. J. Food Compos. Anal. 2023, 117, 105123. [Google Scholar] [CrossRef]
- Nam, J.; Jung, I.B.; Kim, B.; Lee, S.M.; Kim, S.E.; Lee, K.N.; Shin, D.S. A colorimetric hydrogel biosensor for rapid detection of nitrite ions. Sens. Actuators B Chem. 2018, 270, 112–118. [Google Scholar] [CrossRef]
- Xiao, D.; Jiang, Y.; Bi, Y. Molecularly imprinted polymers for the detection of illegal drugs and additives: A review. Mikrochim. Acta 2018, 185, 247. [Google Scholar] [CrossRef]
- Sun, D.; Cao, F.; Wang, H.; Guan, S.; Xu, S. SERS hydrogel pellets for highly repeatable and reliable detections of significant small biomolecules in complex samples without pretreatment. Sens. Actuators B Chem. 2021, 327, 128943. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, C.; Pu, S.; Wang, C.; Cheng, F.; Wang, Y.; Fan, M. Rapid and direct detection of illicit dyes on tainted fruit peel using PVA hydrogel surface enhanced Raman scattering substrate. Anal. Methods 2016, 8, 4816–4820. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.W.; Pu, H.; Wei, Q. A dynamically optical and highly stable pNIPAM @ Au NRs nanohybrid substrate for sensitive SERS detection of malachite green in fish fillet. Talanta 2020, 218, 121188. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Qian, Y.; Zhou, J.; Zheng, L.; Wang, Y. Fluorescence-based quantitative platform for ultrasensitive food allergen detection: From immunoassays to DNA sensors. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Sheng, K.; Jiang, H.; Wang, L. A biomimetic “intestinal microvillus” cell sensor based on 3D bioprinting for the detection of wheat allergen gliadin. Bioelectrochemistry 2021, 142, 107919. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, H.; Qi, Y.; You, C. Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety. Foods 2023, 12, 4405. https://doi.org/10.3390/foods12244405
Li Y, Zhang H, Qi Y, You C. Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety. Foods. 2023; 12(24):4405. https://doi.org/10.3390/foods12244405
Chicago/Turabian StyleLi, Yuzhen, Hongfa Zhang, Yan Qi, and Chunping You. 2023. "Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety" Foods 12, no. 24: 4405. https://doi.org/10.3390/foods12244405
APA StyleLi, Y., Zhang, H., Qi, Y., & You, C. (2023). Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety. Foods, 12(24), 4405. https://doi.org/10.3390/foods12244405